1. Introduction
Limited availability of water resources and a number of complex water supply issues affecting key economic sectors remain difficult problems for countries in the Global South, particularly for Africa, which is the most water-scarce region. According to the World Health Organization, one in three people is affected by water scarcity. Experts predict that, if the situation does not change, 75% of the continent’s population will live in regions with water scarcity by 2050 [
1].
Obstacles which prevent the implementation of specific measures for the elimination of water scarcity include the poor infrastructure of the states concerned, the lack of clear environmental plans and a shortage of qualified personnel. Significant population growth, climate change, lack of infrastructure, and underdeveloped water treatment systems and wastewater disposal systems in poor countries of the Global South lead to pollution of rivers, lakes and groundwater. In such conditions, traditional methods of water treatment cannot ensure the purification of water to a safe level [
2]. In addition, the hot climate and the lack of necessary disinfectants lead to a rapid proliferation of pathogenic bacteria in the water.
To solve these problems, the governments of African states rely on the help of the international community, individual countries and organisations, which have finances, highly qualified personnel and the necessary technical equipment.
In the countries of the Middle East and North Africa, positive changes have been observed due to the implementation of desalination of seawater by reverse osmosis; however, as mentioned before, other countries of the Global South with access to the sea cannot apply this experience due to the lack of financial resources for the construction and maintenance of such large-scale production facilities. The lack of infrastructure in hard-to-reach remote regions of individual countries also significantly affects the use of chemical technologies for water purification.
Thus, the countries of the Global South are extremely interested in new highly efficient and environmentally friendly innovative technologies. Such technologies should have a low cost, should allow improvement in the quality of drinking water, without the use of chemical reagents, and should not require significant capital expenditures. The development of appropriate modern technologies and equipment can serve as one of the key launching grounds for the development of comprehensive cooperation with friendly countries in the context of the formation of a new world order and a shift in the economic centre of development from the West to the East and South.
Water treatment is a complex process that includes several stages of preparation. One of the key stages of traditional treatment methods is the addition of chemicals to suppress the microorganisms remaining after multi-stage aeration and separation of solid suspensions by settling [
3,
4,
5,
6]. However, reagent methods have a number of significant disadvantages. For example, the use of the most common chlorine-based chemicals in water treatment processes [
7,
8,
9,
10] leads to the formation of organochlorine compounds, such as chloroform, dichlorobromomethane, chlorobromomethane and tribromomethane, and hard-to-remove compounds with metal ions (iron, zinc, manganese, cobalt, lead, copper and cadmium). These substances accumulate in the human body and spread through the circulatory system, causing various diseases of the respiratory tract and digestion and significantly increasing the risk of developing cancerous tumours [
11]. If these substances enter the soil or return to the consumer, they pose a major threat to human health and the environment [
12,
13,
14,
15,
16]. In addition, pathogenic microorganisms that remain or re-enter treated water can develop resistance due to constant interaction with chlorine and form strains more resistant to antiseptics [
17,
18].
Reagent-free treatment methods are a promising alternative in the field of water treatment methods and will help to minimise the risks associated with the use of hazardous reagents. As the name suggests, these methods do not add any chemical reagents directly to wastewater. Active substances are produced directly in treated volumes due to physical and physical–chemical processes, with virtually zero formation of dangerous reaction by-products [
19,
20]. A special place among these techniques is occupied by advanced oxidation processes (AOPs), which are based on the generation of large amounts of hydroxide radical and other reactive oxygen species, which, due to the high reaction rates, manage both to react with pollutants and recombine into environmentally friendly substances [
21,
22,
23,
24,
25].
The development of plasma treatment harbours great potential in this area. However, despite having positive laboratory results, most developments cannot be applied on an industrial scale [
26,
27,
28,
29,
30,
31,
32,
33]. Previously, we carried out work in which we were able to propose a treatment mechanism and develop a design for a flow-through plasma discharge water treatment system, which was called sonoplasma [
34,
35,
36,
37,
38]. This study demonstrated that plasma treatment in a flow of cavitating liquid can be an effective method of purification from both microbiological contaminants and complex organic pollutants.
Herein, we present the results of a study on possible mechanisms of pollutant decomposition carried out to optimise the plasma combustion process and improve the efficiency of the equipment. After optimising the sonoplasma setup, industrial tests were carried out with wastewater under real conditions at the site of the biological wastewater treatment plant of a health resort (Republic of Tatarstan, Almetyevsk, Russian Federation).
2. Materials and Methods
A detailed description of the sonoplasma reactor and the methodology for the generation of the discharge were published in our early work [
34]. Sonoplasma treatment has advantages over other plasma discharge treatment technologies, which include high efficiency and cost-effectiveness in treating large volumes of water.
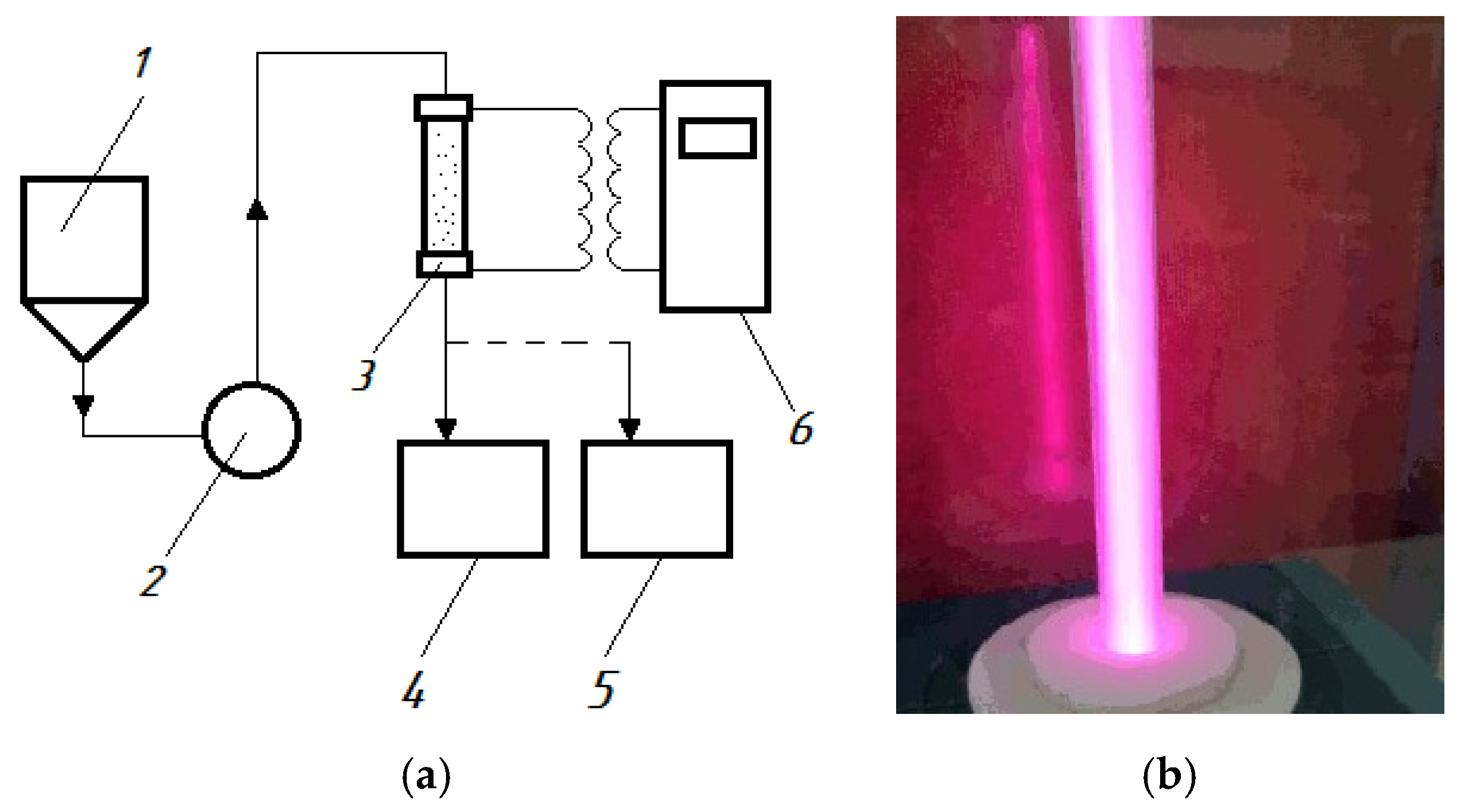
The experimental setup with a capacity of 1 m
3/h used in the current research was a flow-through reactor connected to a generator of high-frequency current pulses equipped with a hydroacoustic emitter and electrodes. A diagram and real photo of the setup is shown in
Figure 1 and
Figure 2.
The average statistical productivity of typical water treatment facilities, depending on the purpose of the installations, ranges from 1 m3 to 100 m3 per hour. The experimental setup presented in this article can be scaled up by increasing the number of reactors and/or increasing the dimensions of a single reactor. Thus, the experimental installation can be scaled for a specific task.
The processing time in the reactor is 0.01–0.02 s. However, processing occurs not only in the reactor, but also partially at the outlet of the installation. The full cycle of treatment of 10 L of a sample takes about 36 s.
From the feed tank (1), wastewater is pumped (2) into the discharge chamber of the reactor (3), where a discharge is formed in the liquid flow using a generator of high-frequency current pulses (6). As a result, plasma is generated in the cavitation field and the water is processed and drains into the receiving tank (5). During the processing, samples are taken into the storage tank (4).
The analysis of the effects of sonoplasma on the pollutants was carried out by treating model solutions of titanium oxysulphate (TiOSO4) (Sigma-Aldrich. Co., St. Louis, MO, USA) and potassium bichromate (K2Cr2O7) (LLC «Your workshop», Saint Petersburg, Russia) in distilled water. Concentrations of 1 g/L and 0.01 g/L were used in the experiment. The obtained solutions were tested using an SF-2000 spectrophotometer (LLC Special Design Bureau «SPECTRE», Saint Petersburg, Russia) in the wavelength range from 200 nm to 1000 nm; as a result, light absorption spectra with characteristic spikes were obtained.
The analysis of the efficiency of removal of microbiological contaminants was carried out on real wastewater. The wastewater was taken from the tertiary decantation tank with a capacity of up to 10 m
3/h (depending on the time of day, season, weather conditions and discharge peaks), which is located on the premises of the biological water treatment facilities of a health resort in the Republic of Tatarstan, Almetyevsk, Russian Federation (
Figure 3). Before the wastewater entered the tertiary decantation tank, it passed through several filtration stages and was then decanted and aerated in two successive stages. The final disinfection stage—treatment with sodium hypochlorite—was replaced by the sonoplasma process. The wastewater treatment was carried out at room temperature (18–21 °C).
Before starting the experiments, the feed tank, the storage tank, the pump and all the connecting tubes were pre-filled with three-percent hydrogen peroxide solution and stored for at least 2 h. After exposure, all the elements were washed with clean distilled water to prevent distortion of the results.
Sampling was carried out through the drainpipe of the experimental unit. Water for processing was poured into the receiving tank through the neck. Clean plastic canisters with a volume of 10 L were used as containers for the processed samples. The sample volume was also 10 L. Control samples were taken after water had passed through the experimental setup, which made it possible to compare the effect of acoustic cavitation without plasma discharge treatment and obtain a clean effect comparison.
During the experiment, 7 samples were taken, including a control sample of the wastewater. The treatment process was carried out at three frequencies of electrical pulses (40 kHz, 55 kHz and 68 kHz), with two complete processing cycles for each. A list of the obtained samples is presented in
Table 1. The characteristics of the electrical pulses generated during the treatment are shown in
Figure 4.
Microbiological analysis was carried out by culturing bacteria in Petri dishes, followed by counting the bacteria that formed colonies by microscopic analysis.
The analysis of the contents of hydrogen sulphide, chlorides and iron was carried out by photometric methods using a photocolorimeter (KFK-2, OJSC “Zagorsk Optical and Mechanical Plant” (ZOMZ), Sergiev Posad, Russia) at wavelengths of 670 ± 25 nm, 450 ± 25 nm and 500 ± 25 nm. The hydrogen index was measured using the AMTAST AMT03 water quality meter (Amtast USA Inc., Lakeland, FL, USA) with an accuracy of ±0.01. The density was determined using a set of aerometers AON-1 (PJSC “Khimlaborpribor”, Moscow, Russia) with an accuracy of 1 g/cm3. The mass concentration of ions was measured using the titrimetric method.
All reagents used in the experiments had a degree of purity not lower than “PURE”.
3. Results
When water containing 1 g/L of titanium oxysulphate in plasma was treated, an increase in absorbance was observed over the entire visible wavelength range, with the solution acquiring a slight yellow tinge (
Figure 5 and
Figure 6). When dissolving the same amount of titanium oxysulphate in water pre-treated with plasma, the result was similar, but the optical dissipation was higher and the yellow colouration more pronounced. A clear increase in the wavelength range from 200 to 400 nm could also be observed in the dilution sample.
No significant changes were observed in the solution either when the solution was treated with high-frequency electrical pulses without plasma discharge or when the solution was treated only with hydroacoustic cavitation. The visual observations were confirmed by photospectrometric data. A photograph of the samples and the spectra of light absorption intensity in the wavelength range from 200 nm to 1000 nm are shown in
Figure 5 and
Figure 6. The spectra were obtained with a preliminary subtraction of the initial light absorption and reflect the changes in the samples after processing.
The treatment of potassium bichromate with a concentration of 1 g/L showed the following results: despite the fact that the samples virtually did not differ visually, when potassium bichromate was dissolved in water after plasma treatment to a concentration of 1 g/L, the optical density in the range from 200 to 350 nm increased significantly relative to the control sample. At the same time, treatment with electric pulses without plasma formation increased the intensity of light absorption at the same wavelengths as the initial substance. When treating a solution of potassium bichromate in plasma or after treatment with hydroacoustic cavitation, the intensity of light absorption virtually did not change. A photo of the samples and the spectra are shown in
Figure 7 and
Figure 8.
To reduce the effect of light scattering, a similar experiment was conducted but with the concentration of the starting substances reduced 100 times (up to 0.01 g/L). At these concentrations, the solutions became much more transparent, and, consequently, the light absorption over the entire visible range became lower. However, when processing samples of both titanium oxysulphate and potassium bichromate, the results did not change on the whole and repeated the experimental results at concentrations of 1 g/L. The results are shown in
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13.
The microbiological analysis conducted showed that the concentration of coliform bacteria after the plasma discharge treatment of wastewater decreased by 68.75% compared to the control sample, which is 10 times less than the maximum permissible values for wastewater discharge in the territory of the Russian Federation. A summary table with the results of the microbiological study is shown in
Table 2.
These results were observed with any treatment mode. In this regard, subsequent experiments were carried out at a frequency of 68 kHz, this being the least energy-intensive mode of operation of the equipment. The power consumption of the sonoplasma unit in this mode was 2.5 kW·h per cubic meter of purified water, which is significantly less compared with traditional energy-intensive purification methods, such as ozonation, where a similar consumption indicator is from 13.2 to 30.2 kW·h [
39]. Due to the fact that electricity costs make up the largest share in the cost of water purification, the efficiency of sonoplasma purification exceeds ozonation by 5–10 times.
The second treatment cycle resulted in 100% lethality to the microorganisms, which allowed the conclusion that sequential treatment of the liquid flow with two plasma reactors can significantly increase the efficiency of microbiological purification.
When analysing the concentration of coliphages and Escherichia coli in all the treated samples, the above-mentioned microorganisms were not detected, which allowed us to conclude that there was 100% efficiency after one treatment cycle.
Viable eggs of helminths (ascaris, whipworm, threadworm, toxocara, fasciola, taeniidae and dwarf tapeworm) as well as viable cysts of pathogenic intestinal protozoa were not found in either the control sample or the treated samples.
Chemical analysis of treated wastewater (which was taken from an industrial site nearby) showed a significant decrease in the concentration of harmful inorganic substances. The results of the chemical analysis of the treated water are presented in
Table 3.
During the first treatment cycle, the amount of hydrogen sulphide was reduced by 61.53%, and chlorides were completely removed. The second treatment cycle allowed the residual concentration of hydrogen sulphide to be completely removed. Thus, the plasma discharge water treatment method can be used not only for microbiological contamination, but also as a method for removing chemical contaminants.
The chemical oxygen demand was decreased by 38%.
4. Discussion
On the basis of the spectra obtained, it can be assumed that the chemical reactions at the time of plasma treatment in the reactor and the chemical reactions at the outlet of the reactor are different, yet they are similar in nature and are linked to each other. For example, in the light absorption spectrum of titanium oxysulphate, an increase in the intensity of light absorption relative to the initial sample and a slight deviation in wavelength was detected, and when diluting titanium oxysulphate in plasma-treated water, a similar increase in intensity occurred relative to the initial sample, but the intensity of light absorption was noticeably higher, and the peak became more pronounced and was shifted more towards short wavelengths. At the same time, the effect on the spectrum of electric pulses without initialisation of an electric discharge and the effect on the spectrum of hydroacoustic cavitation was excluded, since under such processing modes the spectra did not differ from the control one. This result was observed both when processing a solution with a concentration of 1 g/L of titanium oxysulphate and when processing a solution with a concentration of 0.01 g/L.
In the case of potassium bichromate, the main change in the light absorption spectrum occurs when it is diluted in plasma-treated water, while treatment directly in plasma did not result in an obvious change in the light absorption spectrum relative to the control sample. This means that there is no reaction in the reactor, that the reaction products do not affect the overall light absorption spectrum or that the peak of light absorption is beyond the wavelength measurement range; for example, when processing a solution with a concentration of potassium bichromate 0.01 g/L after plasma treatment, a deviation in the intensity of light absorption at very short wavelengths was detected. This spectral behaviour also indicates the difference between the chemical reactions that occur in the reactor and outside the reactor. A slight-intensity episode during the electric pulse treatment of solutions can be explained by a measurement error or impurities, since no such episode was observed at a concentration of 0.01 g/L. The difference between the spikes at dilutions of 1 g/L and 0.01 g/L in water after plasma can be explained by high light scattering, which does not allow the distinguishment of individual peaks spikes.
Thus, both experimental results indicate that during plasma treatment, one active substance is formed in the cavitation field, which, as it moves in the reactor, recombines into a more stable active form and continues to interact or remains in dissolved form. Such active forms can be hydroxide ions and the hydrogen peroxide formed from them.
In our previous research, an analysis of a radiation spectrum (
Figure 14) indicated the formation of hydrogen and a large number of hydroxide ions [
33,
35]. At the same time, in our other studies on the effect of plasma on antibiotics, it was found that the residual active forms of oxygen are most likely hydrogen peroxide [
34].
Thus, it can be assumed that at the moment of formation of a cavitation bubble and at the moment of electrical breakdown, bubbles filled with a vapor–gas mixture consisting mainly of water vapor, hydroxide ions and atomic hydrogen are formed. Based on our data, the amount of released hydrogen that was detected in the exhaust gases was about 55%. In the presence of model pollutant solutions, the hydrogen content dropped to 40%.
Firstly, water vapor is filled and further converted into atomic hydrogen and hydroxide radicals through the dissociation process.
Then, the conversion of hydroxide radicals occurs. Since the reaction time of hydroxyl groups is very short, even compared to the lifetime of bubbles [
40], it can be assumed that this process occurs even before the process of diffusing reaction products through the phase boundary begins, that is, a proportion of the hydroxyl groups form hydroxyl radicals, which combine into hydrogen peroxide [
41], while atomic hydrogen passes into a diatomic gas only after the collapse of the cavitation bubble.
This process can proceed even faster when the surfaces of metal oxides are exposed to ultraviolet rays [
42,
43]. For example, titanium oxysulphate does not enter the reactor in its pure form but as a suspension of nanoscale particles.
On this basis, it can be assumed that the main chemical reagent at the time of plasma burning is the hydroxyl group, while the residual active oxygen forms are predominantly hydrogen peroxide.
Provided that cavitation treatment and chemical interaction due to the dissociation process and the products formed during it do not significantly affect the light absorption spectra, it can be assumed that the hydrogen formed participates in the reduction of sulphuric acid or is released as a gas, while the hydroxide group completely reacts with the substance or is converted into hydrogen peroxide. Concomitant substances such as ozone or radicals formed as a result of recombination of hydrogen peroxide in simple reactions make an insignificant contribution and are formed in very small concentrations, but they can still exert a synergistic effect during the decomposition of complex organic compounds, initiating the beginning of chemical interaction.
Based on the above assumption, the effect of plasma on inorganic compounds, in particular on chlorides and hydrogen sulphide, is possible due to the powerful oxidative effect of OH radicals produced in large quantities. Given the large amounts of sulphate ions (SO
42−) found in the samples of treated water, it can be assumed that the hydrogen sulphide molecules also dissociate into atomic hydrogen and a sulphur atom under the influence of an electric current, after which sulphur continues to oxidise in a large excess of hydroxyl radicals. At the same time, only a small proportion of the sulphate ions form sulphuric acid with free hydrogen, while the bulk of the hydrogen is released as a gas. Some of the hydrogen sulphide may react with the resulting hydrogen peroxide to form sulphur.
After release, the sulphate ions with great probability react with free trivalent iron to form iron sulphate.
This assumption is supported by a drop in the concentration of iron (III) in the samples after treatment.
However, other possible reactions involving iron, for example, the formation of iron (III) oxide and hydroxide, are not excluded.
The decomposition of chlorides also occurs in several stages, where free chlorine is partially released as a gas and part of it reacts with the iron present, forming a salt. This assumption may be supported by a decrease in the sodium content after the first treatment cycle and its increase after the second treatment cycle.
The decrease in the concentration of carbonates can be explained by the formation of carbon dioxide with the formation of hydrocarbonate as a product of intermediate reactions.
The increase in some indicators after the second cycle of plasma discharge treatment can be explained by accumulated reactive oxygen species (in particular, hydrogen peroxide), as well as the formation of small, difficult-to-remove hydrogen bubbles [
44,
45,
46,
47], which, during the repeated dissociation process, react with intermediate or final products of the chemical interaction of plasma with wastewater.
The process of exposure of a plasma discharge in a cavitation field to microbiological contamination takes place in several stages. The first stage is the mechanical action on cells by hydroacoustic cavitation. At the moment a microorganism enters a cavitation bubble, it stretches due to the pressure difference (
Figure 15), which leads to a violation of its protective layer [
48]. Some cells can be completely destroyed at the moment of collapse of the bubble. However, this exposure factor is not always fatal for the cell. Even after breaking the outer layer, the cell can remain viable.
The main stage of exposure to cells involves the physico-chemical and chemical processes occurring at the time of discharge formation in cavitation bubbles.
In part, the lethality to microorganisms is due to the high temperature and pressure that are formed when an electric discharge penetrates the bubbles [
49]. However, due to the fact that the nature of the process is local, thermal exposure is more an auxiliary cleaning mechanism than the main factor.
One of the factors in the physical–chemical effect on microorganisms is the interaction of current with cells, which leads to chemical transformations of the elements of which they consist (chemical reactions with carbon, hydrogen, oxygen and nitrogen) [
50,
51]. Since the main chemical substance of the cell is water, first of all, the process of dissociation of a water molecule occurs with the release of a powerful oxidiser, a hydroxide ion, which continues to destroy the internal organs of the cell.
If a cell has not been exposed to current, then the main chemical effect is a chemical reaction with the active substances formed in the treated medium. Since the main chemical in the treated effluents is also water, due to a similar dissociation process, water molecules formed in large quantities of OH radicals and hydrogen peroxide tend to come from the outside into the cell, also causing a powerful antibacterial effect. This process is facilitated by the previously described mechanism of pressure increase in the bubble, which allows active substances to penetrate the cell through the damaged membrane.
Other chemical agents, such as released oxygen and the resulting ozone, can also be involved in the process of chemical exposure to cells.
The last stage of the exposure process is the formation of stable reactive oxygen species, which continue to react with microorganisms for a certain time. Our previous research showed that hydrogen peroxide formed with varying degrees of contamination can persist for up to several days [
35]. The antibacterial effect of treated water lasts from 24 to 48 h, and in some cases even longer.
5. Conclusions
Currently, there are many conventional technologies for water purification and treatment that are offered to countries in the Global South. It is obvious that in the conditions of intense competition for emerging markets, it is necessary to offer something innovative that has undeniable advantages over traditional methods and has been developed taking into account the specifics of the problems characteristic of the countries of the Global South (difficulties in the supply of chemicals, lack of access to uninterrupted power supplies and necessary financing, etc.).
During the study, it was found that the main active chemicals formed from treated water during sonoplasma treatment are hydroxide radicals and hydrogen peroxide. Hydroxide is formed only in bubbles and is partially converted to peroxide even before diffusing through the phase boundary. The effect of prolonged exposure is mainly achieved by the chemical action of hydrogen peroxide. Both substances have a high reaction rate and pose no chemical hazard when released into water.
Decomposition of 100% of chlorides and more than 60% of hydrogen sulphide was observed. Such results indicate that plasma in a cavitation field can be used as a method for treating chemical contaminants.
The installation for the treatment of wastewater with flow plasma made it possible to reduce the values of indicators of microbiological contamination to 68%, which in general allows us to conclude that the developed method has been successfully applied in real conditions of wastewater treatment. In view of the results obtained, the possibility of replacing the existing stage of reagent treatment with sonoplasma treatment should be investigated.
The next studies will be devoted to conducting field tests of the sonoplasma water purification setup in African countries with the involvement of local organisations dealing with water treatment. Research will also be conducted to expand the capabilities of the sonoplasma water purification technology, in particular to adjust the technology for the removal of complex organic pollutants with high concentrations, such as antibiotics, organic dyes, etc.