From Fruit Waste to Hydrogels for Agricultural Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
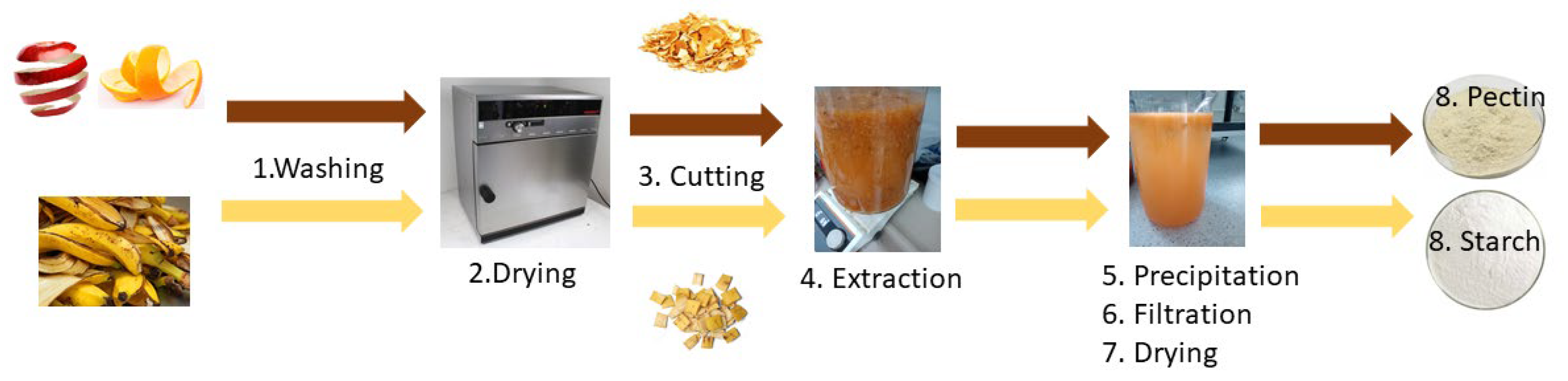
2.2. Extraction of Pectin from Apples and Oranges Peel
2.3. Extraction of Starch from Banana Peel
2.4. Preparation of Pectin-and-Starch-Based Hydrogels
2.4.1. Starch Preparation by Thermal Approach
2.4.2. Pectin–Starch Hydrogels
2.5. Mechanical Properties of the Hydrogels
2.6. Surface Morphology and Inner Structure of the Hydrogels
2.7. FTIR Analysis
2.8. Methylation Degree of Extracted Pectin
2.9. Measurement of Water Absorbency
2.10. Soil Moisture Content
2.11. Determination of Water Retention Efficiency
2.12. Porosity
2.13. Retention Rate of Picloram in Soil
2.14. Biodegradation in Soil
3. Results
3.1. Extraction Yield
3.2. Hydrogels’ Characterization
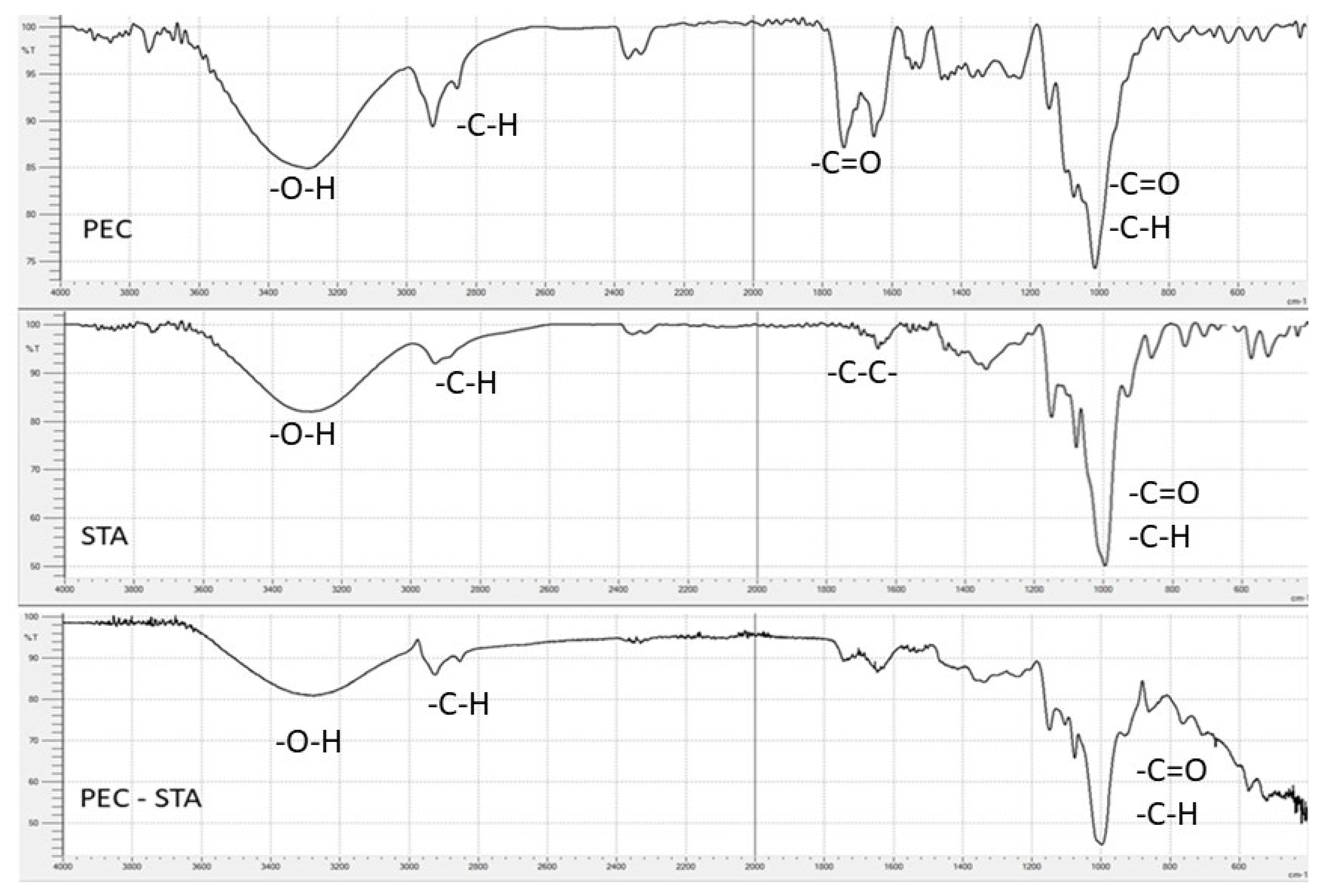
3.2.1. FTIR Spectra
3.2.2. Mechanical Properties
3.2.3. Hydrogel Porosity
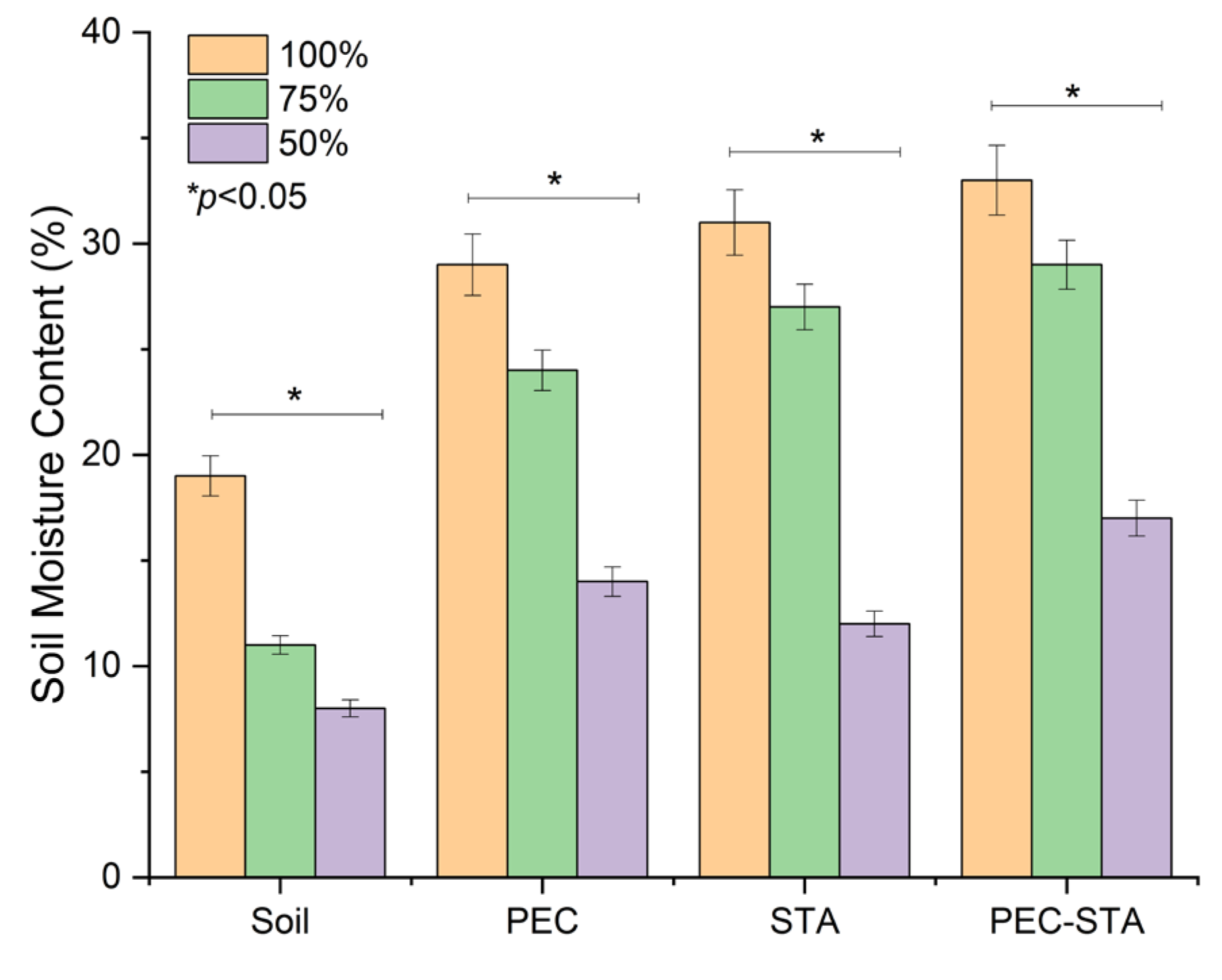
3.2.4. Swelling and Soil Water Retention
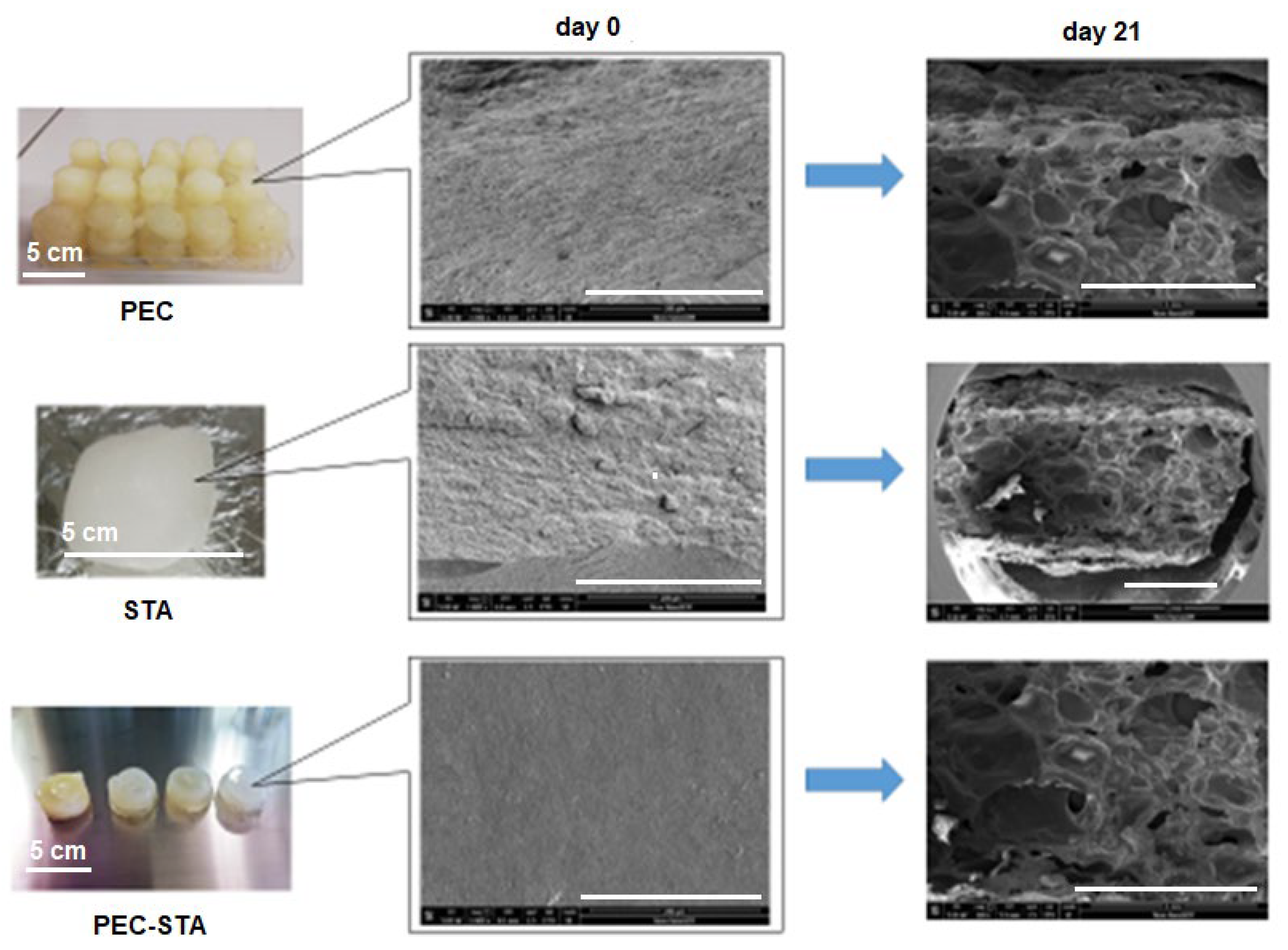
3.3. Biodegradation in Soil
- (a)
- Mechanism of de-esterification by pectin esterase and pectin methyl esterase—for example, a multi-attack mechanism—which is then followed by the decomposition of the oligomers to release the products;
- (b)
- Mechanism of hydrolytic cleavage in polygalacturonases and pectin lyases.
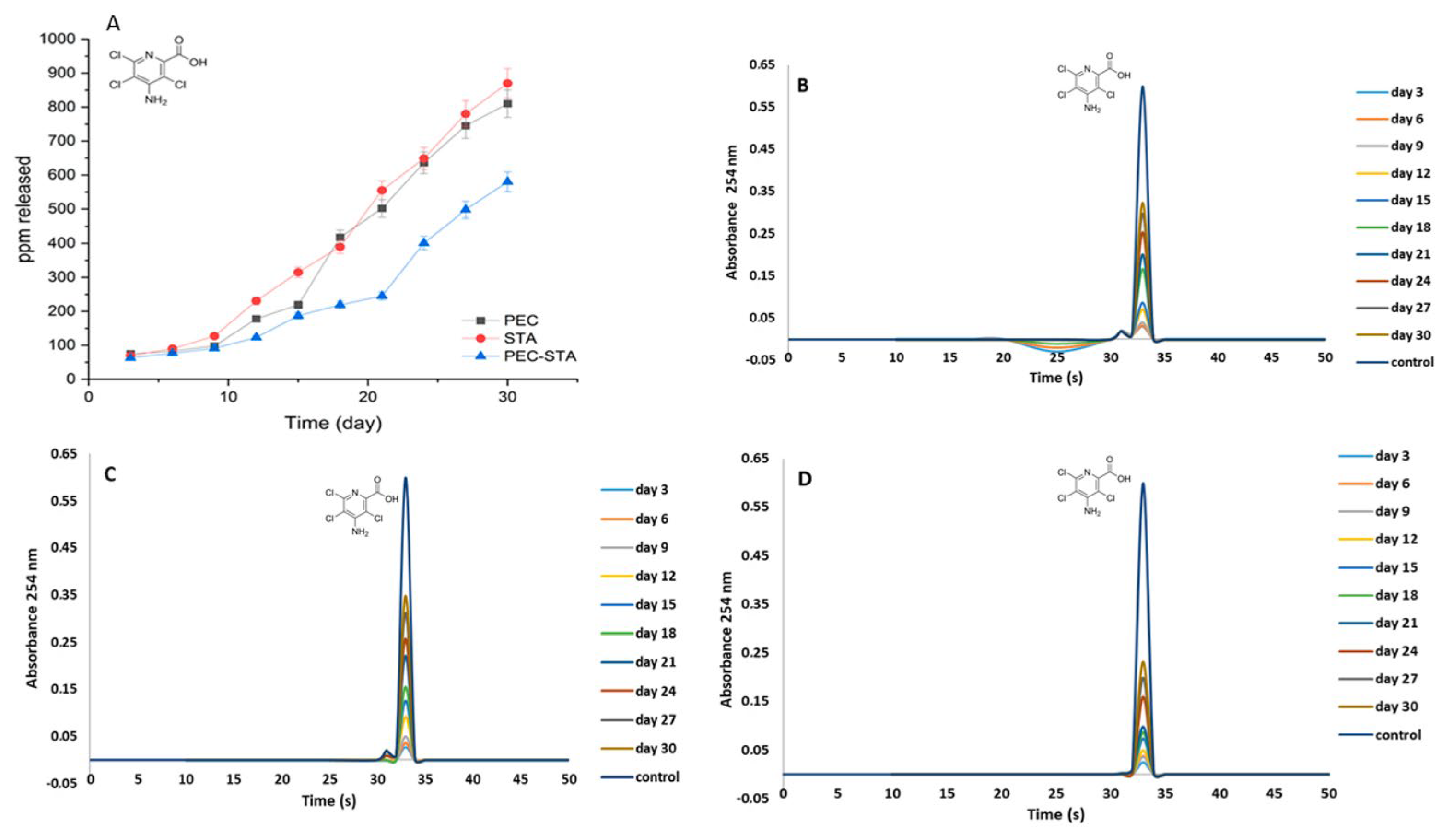
3.4. Retention Rate of Picloram in Sandy Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majeed, A. Application of agrochemicals in agriculture: Benefits, risks and responsibility of stakeholders. J. Food Sci. Toxicol. 2018, 2, 3. [Google Scholar]
- Jeschke, P. Propesticides and their use as agrochemicals. Pest Manag. Sci. 2015, 72, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.M.; Santos, M.; Silva, P.; Cagide, F.; Garrido, J.; Borges, F. Host-guest complexes of phenoxy alkyl acid herbicides and cyclodextrins. MCPA and β-cyclodextrin. J. Environ. Sci. Health Part B 2012, 47, 869–875. [Google Scholar] [CrossRef]
- Işıklan, N. Controlled release study of carbaryl insecticide from calcium alginate and nickel alginate hydrogel beads. J. Appl. Polym. Sci. 2007, 105, 718–725. [Google Scholar] [CrossRef]
- Tay, J.-W.; Choe, D.-H.; Mulchandani, A.; Rust, M.K. Hydrogels: From Controlled Release to a New Bait Delivery for Insect Pest Management. J. Econ. EÈntomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Nikiema, J.; Mateo-Sagasta, J.; Asiedu, Z.; Saad, D.; Lamizana, B. Water Pollution by Plastics and Microplastics: A Review of Technical Solutions from Source to Sea; United Nations Environment Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Qamruzzaman; Ahmed, F.; Mondal, I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Di Martino, A.; Khan, Y.A.; Durpekova, S.; Sedlarik, V.; Elich, O.; Cechmankova, J. Ecofriendly renewable hydrogels based on whey protein and for slow release of fertilizers and soil conditioning. J. Clean. Prod. 2021, 285, 124848. [Google Scholar] [CrossRef]
- Khan, Y.A.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 2020, 61, 102126. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Shinde, R.; Siddiqui, M.Z.; Jha, U. Guar gum based hydrogel as controlled micronutrient delivery system: Mechanism and kinetics of boron release for agricultural applications. Biopolymers 2021, 112, e23418. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Rosseto, M.; Krein, D.D.; Balbé, N.P.; Dettmer, A. Starch–gelatin film as an alternative to the use of plastics in agriculture: A review. J. Sci. Food Agric. 2019, 99, 6671–6679. [Google Scholar] [CrossRef] [PubMed]
- Vinti, G.; Vaccari, M. Solid Waste Management in Rural Communities of Developing Countries: An Overview of Challenges and Opportunities. Clean Technol. 2022, 4, 1138–1151. [Google Scholar] [CrossRef]
- Masoud, M.R.; Masry, E.; Fatma, H.; Hareedy, L.A. Utilization of Orange Wastes for Production of Value Added Products. Egypt. J. Agric. Res. 2017, 95, 221–231. [Google Scholar] [CrossRef]
- Babalola, B.A.; Akinwande, A.I.; Gboyega, A.E.; Otunba, A.A. Extraction, purification and characterization of papain cysteine-proteases from the leaves of Carica papaya. Sci. Afr. 2023, 19, e01538. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Wesslén, K.B.; Wesslén, B. Synthesis of amphiphilic amylose and starch derivatives. Carbohydr. Polym. 2002, 47, 303–311. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; Halal, S.L.d.M.E.; Lim, L.-T.; Dias, Á.R.G.; Zavareze, E.d.R. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, G.H.A.; Ibrahim, A.S.; Fahmy, A.H. Using Environmentally Friendly Hydrogels to Alleviate the Negative Impact of Drought on Plant. Open J. Appl. Sci. 2022, 12, 111–133. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sengoku, K.; Fujioka, R. Pectin-based surperabsorbent hydrogels crosslinked by some chemicals: Synthesis and characterization. Polym. Bull. 2005, 55, 123–129. [Google Scholar] [CrossRef]
- Konstantakos, S.; Marinopoulou, A.; Papaemmanouil, S.; Emmanouilidou, M.; Karamalaki, M.; Kolothas, E.; Saridou, E.; Papastergiadis, E.; Karageorgiou, V. Preparation of model starch complex hydrogels. Food Hydrocolloids 2019, 96, 365–372. [Google Scholar] [CrossRef]
- Cury, B.; Klein, S.; Evangelista, R. Modeling a system of phosphated cross-linked high amylose for controlled drug release. Part 1: Synthesis and polymer characterization. React. Funct. Polym. 2008, 68, 1200–1206. [Google Scholar] [CrossRef]
- Markov, P.A.; Khramova, D.S.; Shumikhin, K.V.; Nikitina, I.R.; Beloserov, V.S.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Mechanical properties of the pectin hydrogels and inflammation response to their subcutaneous implantation. J. Biomed. Mater. Res. Part A 2019, 107, 2088–2098. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Sringam, J.; Pankongadisak, P.; Trongsatitkul, T.; Suppakarn, N. Improving Mechanical Properties of Starch-Based Hydrogels Using Double Network Strategy. Polymers 2022, 14, 3552. [Google Scholar] [CrossRef]
- Kozioł, A.; Środa-Pomianek, K.; Górniak, A.; Wikiera, A.; Cyprych, K.; Malik, M. Structural Determination of Pectins by Spectroscopy Methods. Coatings 2022, 12, 546. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Undabeytia, T.; Torres Sanchez, R.M.; dos Santos Afonso, M. Slow-release formulations of the herbicide picloram by using Fe–Al pillared montmorillonite. Environ. Sci. Pollut. Res. 2017, 24, 10410–10420. [Google Scholar] [CrossRef] [PubMed]
- Shirbin, S.J.; Karimi, F.; Chan, N.J.-A.; Heath, D.E.; Qiao, G.G. Macroporous Hydrogels Composed Entirely of Synthetic Polypeptides: Biocompatible and Enzyme Biodegradable 3D Cellular Scaffolds. Biomacromolecules 2016, 17, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Krzyszowska, A.J.; Vance, G.F. Solid-Phase Extraction of Dicamba and Picloram from Water and Soil Samples for HPLC Analysis. J. Agric. Food Chem. 1994, 42, 1693–1696. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Twinomuhwezi, H.; Godswill, A.C.; Kahunde, D. Extraction and Characterization of Pectin from Orange (Citrus sinensis), Lemon (Citrus limon) and Tangerine (Citrus tangerina). Am. J. Phys. Sci. 2020, 1, 17–30. [Google Scholar] [CrossRef]
- Wang, P.; Fei, P.; Zhou, C.; Hong, P. Stearic acid esterified pectin: Preparation, characterization, and application in edible hydrophobic pectin/chitosan composite films. Int. J. Biol. Macromol. 2021, 186, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Thanyapanich, N.; Jimtaisong, A.; Rawdkuen, S. Functional properties of banana starch (Musa spp.) and its utilization in cosmetics. Molecules 2021, 26, 3637. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; El Achaby, M.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Wei, Y.; Durian, D.J. Rain water transport and storage in a model sandy soil with hydrogel particle additives. Eur. Phys. J. E 2014, 37, 97. [Google Scholar] [CrossRef]
- Han, B.; Benner, S.G.; Flores, A.N. Evaluating impacts of climate change on future water scarcity in an intensively managed semi-arid region using a coupled model of biophysical processes and water rights. Hydrol. Earth Syst. Sci. Discuss. 2018, 1–53. [Google Scholar] [CrossRef]
- Choudhary, M.; Shalaby, A.; Al-Omran, A. Water holding capacity and evaporation of calcareous soils as affected by four synthetic polymers. Commun. Soil Sci. Plant Anal. 1995, 26, 2205–2215. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, S.; Singh, V.; Kumar, M.; Kumar, M. Hydrogel and its effect on soil moisture status and plant growth: A review. J. Pharmacogn. Phytochem. 2020, 9, 1746–1753. [Google Scholar]
- Laxmi, S.; Chanu, P.H.; Rani, P.; Rai, S.; Prasad, S.K.; Singh, R.K. Effect of hydrogel on soil moisture stress. J. Pharmacogn. Phytochem. 2019, 8, 316–320. [Google Scholar]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2018, 112, 678–687. [Google Scholar] [CrossRef]
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer Hydrogel Based on Acid Whey and Cellulose Derivatives for Enhancement Water Retention Capacity of Soil and Slow Release of Fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Reimer, M.; Maier, M.; Zollfrank, C. Biodegradation of polysaccharides, polyesters and proteins in soil based on the determination of produced carbon dioxide. Polym. Degrad. Stab. 2023, 217, 110538. [Google Scholar] [CrossRef]
- Cheshire, M.V.; Greaves, M.P.; Mundie, C.M. Decomposition of soil polysaccharide. Eur. J. Soil Sci. 1974, 25, 483–498. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Zamir, S.S.; Fathi, B.; Ajji, A.; Robert, M.; Elkoun, S. Biodegradation of modified starch/poly lactic acid nanocomposite in soil. Polym. Degrad. Stab. 2022, 199, 109902. [Google Scholar] [CrossRef]
- Khattab, A.M. The microbial degradation for pectin. In Pectins: The New-Old Polysaccharides; IntechOpen: London, UK, 2022; pp. 83–108. [Google Scholar]
- Martin, J.P.; Haider, K.; Kassim, G. Biodegradation and stabilization after 2 years of specific crop, lignin, and polysaccharide carbons in soils. Soil Sci.Soc. Am. J. 1980, 44, 1250–1255. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Q.; Shi, M.; Zhang, Y.; Tao, W.; Li, M. Effect of Pore Size on the Biodegradation Rate of Silk Fibroin Scaffolds. Adv. Mater. Sci. Eng. 2015, 2015, 315397. [Google Scholar] [CrossRef]
- Dumancas, G.G.; Rodney, T.L., Jr.; Subong BJ, J.; Koralege RS, H.; Perera UD, N.; Pham-Bugayong, P.J.; Cobbinah, E. Picloram; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Tomadoni, B.; Casalongué, C.; Alvarez, V.A. Biopolymer-based hydrogels for agriculture applications: Swelling behavior and slow release of agrochemicals. In Polymers for Agri-Food Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–125. [Google Scholar]
- Pang, L.; Gao, Z.; Feng, H.; Wang, S.; Wang, Q. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. J. Control. Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Renewable Mixed Hydrogels Based on Polysaccharide and Protein for Release of Agrochemicals and Soil Conditioning. Sustainability 2021, 13, 10439. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P. Starch-Chitosan Hydrogels for the Controlled-Release of Herbicide in Agricultural Applications: A Study on the Effect of the Concentration of Raw Materials and Crosslinkers. J. Polym. Environ. 2022, 30, 2448–2461. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Singha, A.; Singh, N.; Parmar, B.S.; Kumar, A. Effect of Addition of Superabsorbent Hydrogels on Sorption and Mobility of Metribuzin in Sandyloam Soil. Pestic. Res. J. 2012, 24, 138–143. [Google Scholar]
- Agaba, H.; Orikiriza, L.J.B.; Esegu, J.F.O.; Obua, J.; Kabasa, D.J.; Hüttermann, A. Effects of hydrogel amendment on plant accessible water and tree life under drought conditions. Clean-Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Zhang, S.; He, F.; Fang, X.; Zhao, X.; Liu, Y.; Yu, G.; Zhou, Y.; Feng, Y.; Li, J. Enhancing soil aggregation and acetamiprid adsorption by ecofriendly polysaccharides hydrogel based on Ca2+-amphiphilic sodium alginate. J. Environ. Sci. 2022, 113, 55–63. [Google Scholar] [CrossRef]




| Soil Properties | Value |
|---|---|
| Sand (%) | 95 |
| Silt (%) | 6 |
| Clay (%) | 4 |
| Bulk Density (mg/m3) | 1.55 |
| PWP (%) | 7.35 |
| Conductivity (ds/m) | 2.4 |
| pH | 7.8 |
| CEC (cmol/kg) | 4.27 |
| OM (%) | 0.09 |
| CaCO3 (%) | 4.16 |
| Biomass | Wet Weight (g) | Moisture Content in the Biomass (%) | Dry Weight (g) | Polysaccharide Yield (g)/(%) |
|---|---|---|---|---|
| Orange peel | 800 ± 10 | 82 ± 1 | 144 ± 1 | Pectin 87/26% |
| Apple peel | 800 ± 10 | 76 ± 2 | 192 ± 1 | |
| Banana peel | 1600 ± 10 | 85 ± 1 | 240 ± 1 | Starch 31/13% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adi Sulianto, A.; Adiyaksa, I.P.; Wibisono, Y.; Khan, E.; Ivanov, A.; Drannikov, A.; Ozaltin, K.; Di Martino, A. From Fruit Waste to Hydrogels for Agricultural Applications. Clean Technol. 2024, 6, 1-17. https://doi.org/10.3390/cleantechnol6010001
Adi Sulianto A, Adiyaksa IP, Wibisono Y, Khan E, Ivanov A, Drannikov A, Ozaltin K, Di Martino A. From Fruit Waste to Hydrogels for Agricultural Applications. Clean Technologies. 2024; 6(1):1-17. https://doi.org/10.3390/cleantechnol6010001
Chicago/Turabian StyleAdi Sulianto, Akhmad, Ilham Putra Adiyaksa, Yusuf Wibisono, Elena Khan, Aleksei Ivanov, Aleksandr Drannikov, Kadir Ozaltin, and Antonio Di Martino. 2024. "From Fruit Waste to Hydrogels for Agricultural Applications" Clean Technologies 6, no. 1: 1-17. https://doi.org/10.3390/cleantechnol6010001
APA StyleAdi Sulianto, A., Adiyaksa, I. P., Wibisono, Y., Khan, E., Ivanov, A., Drannikov, A., Ozaltin, K., & Di Martino, A. (2024). From Fruit Waste to Hydrogels for Agricultural Applications. Clean Technologies, 6(1), 1-17. https://doi.org/10.3390/cleantechnol6010001











