Abstract
Per- and polyfluoroalkyl substances (PFASs) have been under intense investigation by the scientific community due to their persistence in the environment and potentially hazardous effects on living organisms. In order to tackle the presence of these compounds in water, to date, the research has been strongly focused on the evaluation of the effectiveness of different types of technologies. Considering the extreme complexity of the matter of PFASs and our relatively low knowledge in this topic, the following question arises: is the “chemical only” approach that is followed for evaluating the effectiveness of technologies for PFAS removal from water reliable enough? In this work, some limitations of the present approach are discussed, highlighting the reasons why it cannot be considered a reliable tool to correctly estimate the effectiveness of technology when referring to emerging compounds such as PFASs. Bioassays can play a key role in moving towards an integrated bio-chemical evaluation (chemical analysis and ecotoxicological evaluation), which is strongly encouraged. This represents the only way to completely characterize a water matrix and fully evaluate the impact of technologies when dealing with micropollutants in water, such as PFASs. Future research should focus on defining an optimal battery of bioassays that specifically fit to best represent changes in water quality in terms of short- and long-term impacts on living organisms.
1. PFASs in the Water: The Background
Due to their chemical properties, since the 1940s, per- and polyfluoroalkyl substances (PFASs) have been largely used in several industrial sectors [1] for building and construction, the production and storage of energy, the manufacturing of metal, the production of chemicals and pharmaceuticals, and also in food industries, textile manufacturing, and personal care product production [1,2]. However, the same stable C-F bond(s), which promote their usage, allow them to persist in the environment, posing a serious risk to living organisms [3]. Some PFASs have been targeted as potential hazardous pollutants due to their negative effects on human health. For instance, perfluorooctane sulfonic acid (PFOS) has been classified by the International Agency for Research on Cancer as “possibly carcinogenic to humans” [4]. The same agency will also soon update the classification of PFOS and perfluorooctanoic acid (PFOA) (which is not yet classified) [5].
However, this only represents the “tip of the iceberg”, with the exact number of different existing PFASs currently unknown. For this same reason, the global amount of PFASs produced each year is unknown. Thanks to even more specific and detailed studies, the number of known PFASs is constantly rising, with more than 4000 fluorinated compounds having already been ascertained according to recent data [6,7]. Despite the efforts made, this number is suspected to be a serious underestimation when considering that several perfluoroalkyl molecules remain the property of private industries, with the practical impossibility of being researched and analyzed in environmental media and their molecular structures themselves remaining undeclared [8].
The knowledge among the different categories of PFASs remains heterogeneous, with most of the studies focused on long-chain compounds (more than C7), while few data are available for short (C5-C6) and ultra-short compounds (C2-C3) [9]. Some alternative PFASs (a-PFASs) have been developed to replace compounds targeted as hazardous and/or persistent (especially long-chain ones), but in some cases, these molecules also show huge drawbacks, such as (i) a lower performance in the industrial sector, which means a higher amount of a-PFAS consumption, and (ii) the possible formation of recalcitrant and persistent compounds, which override the advantages in using these alternative molecules [10]. Wang et al. [11] carefully reviewed the potential environmental implications of a-PFASs, highlighting that some of the most used alternative compounds (e.g., hexafluoropropylene oxide dimer (HFPO-DA), 6:2 chlorinated polyfluorinated ether sulfonic acid (6:2 Cl-PFESA), and hexafluoropropylene trimer acids (HFPO-TA)) showed comparable or even higher potential toxicity with respect to the “conventional” ones. Therefore, the path to overcoming the usage of PFASs with new alternative molecules is still long.
Due to the limited amount of available data, knowledge about the possible health implications of short- and long-term compounds is also quite limited. Cui et al. [12] reported a possible correlation between PFAS exposure and the development of urinary bladder cancer, leukemia, pancreatic cancer, and non-Hodgkin’s lymphoma in the population of Florida, U.S.A. However, as stated by the same authors, the factors of risk that could affect the probability of developing cancer vary (e.g., lifestyle, other environmental pollutants, etc.), and developing an extensive investigation (as a sort of case-control study), remains difficult. In Veneto, an industrial region in northern Italy in which PFASs have been detected in drinking water sources [13], the presence of PFASs in exposed populations has been proved [14]. However, despite results that seem to highlight a relationship between PFAS exposure and a higher mortality level for some causes of death [15], the development of a massive epidemiological study among the population to better quantify the problem remains a priority.
Due to the absence of robust literature regarding the health implications of short- and long-term exposure to PFASs, the legislation about the presence of these contaminants in water differs strongly depending on the country and type of water [16]. For this reason, the monitoring of these contaminants in water is not homogeneous in all countries but mainly depends on the presence of regulations in force or public concern for this issue. However, when comparing data from different regions of the world, PFASs appear to be distributed regardless of the level of economic/industrial development [17].
PFASs have been detected in all types of water (surface and groundwater, industrial and domestic wastewater, urban stormwater, tap water, and even bottled water) [18,19,20,21,22,23], and thousands of published documents deal with this issue. In water, these compounds can be released during the entire lifecycle of consumer goods, from the production phase to their usage (e.g., in the case of firefighting foams) and final disposal (e.g., in landfills) [24]. Water practically acts as a carrier for PFASs, helping them to reach food, such as terrestrial animals, fish, and vegetables [25,26,27]. For this reason, the ingestion of contaminated water or food represents the main route of exposure in humans [28,29,30].
An intense investigation is currently being carried out by the scientific community, especially in order to estimate the effectiveness of different types of treatments in the removal of these compounds. When combining the keywords “PFAS”, “removal”, and “water” in Google Scholar©, almost 26,800 scientific documents are available (≃16,900 only from the last 4 years) (searched on 9 June 2023), demonstrating the huge interest on this topic. To date, there has been no unique solution for PFAS removal from water found given that the effectiveness of the treatments depends on many factors, primarily the type of target compound.
Generally, biological processes are not effective to degrade PFASs, so the most-used approach is to separate them from water flux with techniques that have given promising results in sorption (with activated carbon, ion exchange resins, and nanomaterials), membrane filtration (nanofiltration and reverse osmosis), and foam fractionation [31,32]. Other techniques, such as advanced oxidation processes, are currently under study, but they represent a source of concern due to the possible formation of by-products. For instance, Wang et al. [33] highlighted that the UV-based advanced oxidation of PFOA and PFOS can generate short-chain perfluorocarboxylic acid compounds (PFCAs) as by-products.
On the contrary, some non-destructive techniques, like adsorption and ion exchange, seem to give very promising results without the formation of by-products [34]. Especially in regard to adsorption, to overcome the high cost of virgin materials, novel bio-adsorbents were studied [35], but the question of how to stabilize and manage the exhausted material remains open [32].
Currently, the main approach used to evaluate the effectiveness of a treatment on PFAS removal is based on a comparison between the concentration (or load, in case of continuous-flow conditions) of the target compound(s) before and after the process. This “chemical only” assessment is essentially focused on detecting and quantifying the amount of target substance(s) that the process is able to remove from the water.
Keeping this background in mind, some questions remain about this type of assessment: is this approach the most reliable? Are we moving in the right direction? In this work, we discuss the main limitations, suggesting a paradigm shift towards an alternative type of evaluation.
2. Method and Approach
The literature has been screened on Scopus® and Google Scholar© using the keywords “PFAS AND removal AND water” and “Bioassays AND emerging contaminants”. As a criterion of exclusion, only works written in English and subjected to peer-review before publication have been considered. Then, the documents were screened to avoid presenting papers that are not relevant to the current discussion.
This work has been separated into three main sections: (i) Section 3 presents the main gaps of the current studies regarding the removal of PFASs from water, (ii) Section 4 focuses on the reasons why we need a paradigm shift from the current “chemical only” approach, and (iii) Section 5 discusses why the bioassays could play a key role in evaluating the performances of technologies for PFAS removal and presents some examples where an integrated bio-chemical approach has been applied.
3. Some Limitations of the Current Studies
As discussed in Section 1, thousands of scientific documents are focused on evaluating the feasible strategies for PFAS removal from water. However, one of the first questions that could be raised is the following: are the results of the currently available studies representative of a real scenario?
To answer this question, some aspects should be considered. Firstly, to date, most of the works have been carried out with initial conditions that were far from what is observed in real case studies. Recently, Phong Vo et al. [23] reviewed the presence of PFASs in different types of water and wastewater, highlighting that, despite some differences based on the country, the concentration of PFASs in surface water, groundwater, and wastewater generally varies from ng L−1 to µg L−1, with even higher concentration in case of some type of industrial wastewater (e.g., leachates). Drinking water generally exhibits lower concentrations (in the order of ng L−1) depending on several factors, such as the effectiveness of the treatment plant, the contamination of the untreated water, and the type of perfluoroalkyl compound [23].
However, most of the studies on new removal strategies were carried out on synthetic water with an initial concentration of the target PFAS up to several order of magnitude higher than the concentration detected in the “real” waters. This aspect has been also pointed out by Gagliano et al. [36], who reviewed the results of adsorption techniques on both short- and long-chain PFASs and found that most of the previous works were carried out under operating conditions that were not realistic (e.g., high concentrations of PFASs or high dosage of adsorbent materials). Lei et al. [37] also reviewed the current approaches and applications for PFAS removal from water solutions and highlighted that nearly all studies were carried out in conditions that were far from real ones, representing a serious issue in the upscaling from laboratory to commercial applications.
Some inefficiencies of the current methods in detecting and quantifying PFASs in drinking water have been pointed out by Winchell et al. [38], who also suggest that focus should be placed on the optimization of analytical procedures. This could represent a reason why most of the studies have been designed for simulating a higher concentration of PFASs, making the removal phenomenon more appreciable. However, in recent years, the determination and quantification of perfluoro-alkyl compounds has been seriously improved. For instance, by coupling chromatographic system with ultra-high resolution orbitrap mass spectrometry, it is possible to detect PFASs in concentrations up to 5 ng L−1 [39,40]. Moreover, the choice to test PFAS removal in conditions far that are from real ones could cause misinterpretations of the effectiveness of the same treatment, such as in the case of full-scale application, in which the operational conditions are different.
So far, the co-presence of other chemical pollutants (as in the real waters) has also often been neglected, with focus instead being placed on the performance of the process on a single PFAS, following the one-factor-at-a-time approach instead of applying more realistic conditions. In fact, several recent studies proved that the co-presence of other per- and polyfluoroalkyl compounds can lead to a competitive effect on the removal of target molecules, especially in treatments involving adsorption [41].
4. The Need of a Paradigm Shift from the “Chemical Only” Approach
To date, more than 4000 PFAS compounds have been detected in the environment (which is an underestimated number). Assuming the current limitations (see Section 3) are surmountable, the following question arises: can a “chemical only” assessment be considered an effective and reliable approach to evaluate the effectiveness of a technology on PFAS removal?
To answer this question, the issue of precursors needs to be considered. In the currently available studies dealing with removal strategies, these compounds are often neglected. However, some PFASs can change their structure, forming intermediates and new, more stable terminal products [42], making it difficult to understand the effective performance of a treatment. For instance, fluorotelomer alcohols (FTOHs) have been used as a replacement for other common PFASs in the industrial production of goods, but previous works have proved that FTOHs can degrade in the environment into more stable compounds such as PFOA [43]. To date, only some of the pathways of PFAS transformation have been fully defined, while most remain unknown [44]. Moreover, although the conversion seems to be stimulated in an oxidative environment, the conditions that lead to the transformation of the precursors are not fully understood [45].
To make this concept clearer, a Sankey diagram of a fictional case study, in which the effectiveness of a technology is evaluated, is reported in Figure 1. In this example, four fictional PFASs were considered as known substances and investigated in this potential work (PFAS-a, PFAS-b, PFAS-c, and PFAS-d), two PFASs symbolize the per- and polyfluoroalkyl compounds known but not currently under investigation (PFAS-e, and PFAS-f), and the other two PFASs represent compounds that are present in water but are still unknown (PFAS-x and PFAS-y). The fictional hypothesis (not known by the potential investigator) are (i) PFAS-b has two precursors (PFAS-a and PFAS-c), (ii) PFAS-x can be produced from the transformation of four other compounds (PFAS-c, PFAS-d, PFAS-f, and PFAS-y), (iii) PFAS-e and PFAS-f act as precursors of PFAS-d, and (iv) PFAS-c can transform into PFAS-y. Please, note that the interactions between PFASs (both precursors and final products) in this fictional case study have been voluntarily emphasized in order to better highlight the difference between the deductions that can be made from a “chemical only” approach and a real situation. However, this is not an unrealistic case considering that hundreds of compounds are known to be precursors of perfluoroalkyl acids and perfluoroalkylether acids (PFAAs) (e.g., perfluoroalkane sulfonyl fluorides, perfluoroalkanoyl fluorides, perfluoroalkyl chetones, etc.), and many others are unknown either because the pathways of transformation are still unclear or the compounds themselves have not yet been discovered [46,47]. This scenario can also be emphasized in case of the adoption of oxidation processes due to the formation of intermediate perfluoroalkyl by-products [33].
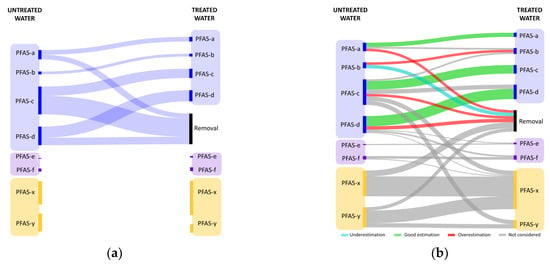
Figure 1.
Sankey diagram of a fictional case study, in which the effectiveness of a technology is evaluated. Blue indicates known and investigated PFASs, violet represents known but not investigated PFASs, while yellow indicates unknown PFASs. Fluxes that can be estimated in case of (a) the “chemical only” assessment are compared to (b) a real situation. The legend refers to the comparison of the current approach with respect to the real condition.
Due to the practical and economic impossibility of carrying out analyses on all compounds and considering that some PFASs remain unknown, the “chemical only” assessment intends to evaluate effectiveness based on a limited number of target compounds (Figure 1a). However, the results could cause an overestimation and/or underestimation of the fluxes and therefore of the real effectiveness of the process (Figure 1b). The “chemical only” approach fails to fully take into account the precursors of other known (including the several pathways that have not yet been identified) and unknown PFAS compounds.
Considering that the current methods for the targeted determination of PFASs allows for obtain values to refer only to a limited number of compounds [48], which is very different from the more than 4000 substances known so far, the attention of this research has been recently focused on the analytical evaluation of “total PFAS”.
So, can the total PFAS quantification overcome the limitations of the targeted analysis in evaluating the performance of a technology on PFAS removal? The answer is negative for two main reasons: (i) one of the currently most challenging aspect in the non-targeted determination of PFASs is the need to balance selectivity and inclusivity (i.e., achieving sufficient detection limits to have reliable results but at the same time avoiding considering fluorinated compounds other than PFASs) [49]; and (ii) not all PFASs show the same level of toxicity [50]. Therefore, the knowledge of the removal efficiency on an aggregated number of compounds does not properly represent the real effectiveness of the process.
5. Bioassays Could Play a Key Role
Ecotoxicology deals with the evaluation of the response to contaminants and external conditions on individuals, populations, communities, and ecosystems [51]. Bioassays represent a tool that can provide important data about the short- and long-term effect of contaminants by measuring the ecotoxicity at a lab scale [52].
A huge number of bioassays is currently available. For instance, testing the inhibition of natural luminescent bacteria (e.g., Vibrio fischeri) is a common approach for evaluating the cytotoxicity of water. Ames test, Comet assay, micronuclei test (on the cells of Allia cepa), the reporter gene assay, the Green Screen assay (on the cells of Saccharomyces cerevisiae) are examples of tests used to quantify genotoxicity and mutagenicity [53,54]. Also, neurotoxicity can be evaluated with tests such as the acetylcholinesterase inhibition assay [55].
As suggested by Pedrazzani et al. [54] and Campana and Wlodkowic [56], the selection of the bioassay to be used is generally based on criteria such as the aim (acute/chronic effect), the duration of the tests, the operational requirements (volume of samples, need of skilled operators, etc.), and the costs.
To date, several authors have already proposed and applied bioassays to assess the ecotoxicological quality of different types of water. For instance, in Sweden, Oskarsson et al. [57] recently used this tool for evaluating the quality of source and drinking water. Although the routine chemical target analysis did not detect any chemicals, bioassays highlighted the activity of aryl hydrocarbon, estrogen, and androgen in some samples of surface water. These interesting results suggest that bioassays can be very helpful in detecting the presence of trace contaminants in water more effectively than performing chemical analyses alone.
In case of the use of advanced oxidation processes, bioassays can also be helpful to check if the unwanted by-products of the treatment are more aggressive than the initial compounds [58,59]. For instance, Kienle et al. [60] evaluated the effect of ozonation, used for removing micropollutants, on the quality of treated effluents of wastewater treatment plants. They applied in vitro bioassays for the quantification of endocrine, genotoxic, and mutagenic effects, as well as in vivo bioassays (e.g., with oligochaetes). Their results concluded that ozonation increased the ecotoxicity of treated wastewater, except for mutagenicity, which can be reduced by the post-treatments [60].
The question that can raise is the following: can bioassays be considered “mature” enough to be used in evaluating the performance of PFAS removal technologies? Several previous works have already shown the advantages of using a combined approach (chemical analysis + bioassays) for evaluating the performance of a technology, especially when dealing with emerging and trace contaminants.
For instance, in order to evaluate the effectiveness of tertiary wastewater treatment on organic micropollutants, Papa et al. [61] combined chemical analysis with bioassays to investigate estrogenicity and mutagenicity. Interestingly, their work pointed out that bioassays were more sensitive to the overall toxicity of the effluent, even when the chemical analysis showed a very good performance of the process on micropollutant removal [61]. Similarly, Bertanza et al. [62] proposed an integrated bio-chemical monitoring of ozonation of WWTP effluents for the removal of endocrine disruptor compounds. Based on their results, while chemical analysis showed an effectiveness of the process of up to 70%, the bioassays showed that the process was not able to significantly reduce the estrogenicity of the sample and even enhanced the genotoxicity of the treated water [62]. The same approach has also been applied for testing photocatalytic treatments for the removal of 17-β-Estradiol from drinking water. In this case, toxicity tests using Cyprinus carpio confirmed the ability of photocatalysis to remove this estrogen [63].
Neale et al. [64] combined the chemical analysis of WW for more than 400 compounds with bioassays for 13 different endpoints (e.g., mutagenicity with Ames test, cytotoxicity combining algae assay with Pseudokirchneriella subcapitata, mortality with the Zebrafish embryo toxicity test, etc.). They proved that the integrated bio-chemical approach represents a valid solution to characterize water with micropollutants. The two analyses were complementary: chemical analysis provided data about the contaminants in the water, while the bioassays gave information about the effects of the mixture in case of several endpoints [64].
Regarding drinking water, on several points of a plant treating water containing PFASs, Alias et al. [65] applied a battery of tests on Daphnia magna, Pseudokirchneriella subcapitata, Salmonella typhimurium, and Allium cepa in combination with conventional chemical analysis prescribed by the legislation in force. Their results highlighted that an integrated bio-chemical approach is essential to make considerations about the risk involved in the daily intake of water by the population, especially in the case of micropollutant contamination [65].
However, the application of this approach in studies dealing with the removal of PFASs from water is not currently widespread. The main reasons could be various, but the main ones could be (i) the higher costs of this type of assessment, which requires the integration of the chemical analysis with ecotoxicological tests; (ii) the need for more time to obtain the final results (the preparation and execution of bioassays can be longer than chemical analysis), and (iii) the absence of a unique protocol that defines the battery of bioassays tests that should be performed.
Which battery of the ecotoxicological assays is most convenient to perform to fully characterize the water before and after treatment for PFAS removal? To date, data on this aspect are limited, and a definitive answer is not yet available. However, some preliminary results suggest how to direct future research. Neale et al. [66] studied the development of a battery of bioassays to representatively characterize water with micropollutants, such as pharmaceuticals and PFOA. Their results pointed out that the exact type of bioassay is not important, but rather that a diverse panel of bioassays that includes different endpoints is present [66]. In any case, further studies are needed to expand the battery of available bioassays in order to better define and quantify their potential effect on the environment and on the human health [53]. This is a crucial aspect considering that even residual PFASs in water can represent a risk for human safety due to their persistence when this water is reused.
Adopting a “chemical only” assessment to define the effectiveness of treatments for PFAS removal not only cannot be considered sufficient to obtain representative results, but can also lead to a misinterpretation of the real impact of the process on the environment and on human health. Therefore, an integrated approach combining chemical analysis and bioassays is strongly encouraged as the main solutions to overcome the limitations of the current “chemical only” one.
6. Conclusions
- “Chemical only” assessment cannot be considered reliable to evaluate the effectiveness of treatments for PFAS removal due to the presence of precursors (in many cases with unknown pathways of transformation). Moreover, to date, many PFASs remain unknown, and the only knowledge of the concentration of per- and polyfluoroalkyl compounds cannot provide an exhaustive characterization of the water.
- Focusing the evaluation only on total PFASs could skew the results since (i) the need to balance selectivity and inclusivity is a current challenge, and (ii) the level of toxicity among the different PFASs is heterogeneous.
- Bioassays are already effectively used to assess the ecotoxicological quality of different types of water. To better define the effectiveness of removal technologies in the case of trace and emerging pollutants, some authors have suggested integrating chemical analysis with a bio-chemical assessment.
- Although the use of an integrated bio-chemical approach (chemical analysis + bioassays) in the evaluation of the effectiveness of technologies in PFAS removal is not currently widespread, a paradigm shift towards this approach is strongly suggested to overcome the main limitations of an “chemical only” approach.
- A unique protocol with an optimal battery of bioassays that are useful for fully characterizing water before and after the application of processes for the removal of PFASs has not yet been defined. Previous works have suggested that the most important aspect is to evaluate different bioassays that include different endpoints. In any case, further research on this topic is strongly suggested by the authors.
Author Contributions
Conceptualization, M.C.M. and P.H.; methodology, M.C.M.; validation, J.R. and P.H.; writing—original draft preparation, M.C.M.; writing—review and editing, T.H., T.M. and J.R.; visualization, M.C.M.; supervision, P.H.; funding acquisition, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated/used in the study are presented in the published manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per-and Polyfluoroalkyl Substances (PFAS). Environ. Sci Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Gallen, C.; Bignert, A.; Taucare, G.; O’Brien, J.; Braeunig, J.; Reeks, T.; Thompson, J.; Mueller, J.F. Temporal Trends of Perfluoroalkyl Substances in an Australian Wastewater Treatment Plant: A Ten-Year Retrospective Investigation. Sci. Total Environ. 2022, 804, 150211. [Google Scholar] [CrossRef] [PubMed]
- IARC Perfluorooctanoic Acid, Tetrafluoroethylene, Dichloromethane, 1,2-Dichloropropane, and 1,3-Propane Sultone. Available online: https://publications.iarc.fr/547 (accessed on 9 June 2023).
- IARC Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). Available online: https://monographs.iarc.who.int/iarc-monographs-volume-135/ (accessed on 9 June 2023).
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide Trends in Tracing Poly- and Perfluoroalkyl Substances (PFAS) in the Environment. TrAC Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Hernandéz, J.A.; González-González, R.B.; Macias-Garbett, R.; Martínez-Ruiz, M.; Reyes-Pardo, H.; Hernández Martínez, S.A.; Parra-Arroyo, L.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; et al. Detection and Tertiary Treatment Technologies of Poly-and Perfluoroalkyl Substances in Wastewater Treatment Plants. Front. Environ. Sci 2022, 10, 864894. [Google Scholar] [CrossRef]
- Gaines, L.G.T. Historical and Current Usage of Per-and Polyfluoroalkyl Substances (PFAS): A Literature Review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Sadia, M.; Nollen, I.; Helmus, R.; ter Laak, T.L.; Béen, F.; Praetorius, A.; van Wezel, A.P. Occurrence, Fate, and Related Health Risks of PFAS in Raw and Produced Drinking Water. Environ. Sci Technol. 2023, 57, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The Overlooked Short-and Ultrashort-Chain Poly- and Perfluorinated Substances: A Review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, W.; Wang, L.; Zhang, Y.; Zhang, Y.; Wang, M.; Wang, Y.; Li, P. A Review of Sources, Multimedia Distribution and Health Risks of Novel Fluorinated Alternatives. Ecotoxicol. Environ. Saf. 2019, 182, 109402. [Google Scholar] [CrossRef]
- Cui, D.; Li, X.; Quinete, N. Occurrence, Fate, Sources and Toxicity of PFAS: What We Know so Far in Florida and Major Gaps. TrAC Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- VR/WHO. Keeping Our Water Clean: The Case of Water Contamination in the Veneto Region, Italy; WHO Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Zare Jeddi, M.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 027007. [Google Scholar] [CrossRef]
- Mastrantonio, M.; Bai, E.; Uccelli, R.; Cordiano, V.; Screpanti, A.; Crosignani, P. Drinking Water Contamination from Perfluoroalkyl Substances (PFAS): An Ecological Mortality Study in the Veneto Region, Italy. Eur. J. Public Health 2018, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per-and Polyfluoroalkyl Substances in Water and Wastewater: A Critical Review of Their Global Occurrence and Distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and Their Substitutes in Groundwater: Occurrence, Transformation and Remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Houtz, E.F.; Sedlak, D.L. Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol. 2012, 46, 9342–9349. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Son, Y. Perfluoroalkyl Substances (PFAS) in Surface Water and Sediments from Two Urban Watersheds in Nevada, USA. Sci. Total Environ. 2021, 751, 141622. [Google Scholar] [CrossRef]
- Jurikova, M.; Dvorakova, D.; Pulkrabova, J. The Occurrence of Perfluoroalkyl Substances (PFAS) in Drinking Water in the Czech Republic: A Pilot Study. Environ. Sci. Pollut. Res. 2022, 29, 60341–60353. [Google Scholar] [CrossRef]
- Chow, S.J.; Ojeda, N.; Jacangelo, J.G.; Schwab, K.J. Detection of Ultrashort-Chain and Other per- and Polyfluoroalkyl Substances (PFAS) in U.S. Bottled Water. Water Res. 2021, 201, 117292. [Google Scholar] [CrossRef]
- Phong Vo, H.N.; Ngo, H.H.; Guo, W.; Hong Nguyen, T.M.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Hang Nguyen, T.A. Poly-and Perfluoroalkyl Substances in Water and Wastewater: A Comprehensive Review from Sources to Remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications, Environmental Release, and Remediation Technologies of Per- and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef]
- Christensen, K.Y.; Raymond, M.; Blackowicz, M.; Liu, Y.; Thompson, B.A.; Anderson, H.A.; Turyk, M. Perfluoroalkyl Substances and Fish Consumption. Environ. Res. 2017, 154, 145–151. [Google Scholar] [CrossRef]
- Piva, E.; Fais, P.; Ioime, P.; Forcato, M.; Viel, G.; Cecchetto, G.; Pascali, J.P. Per- and Polyfluoroalkyl Substances (PFAS) Presence in Food: Comparison among Fresh, Frozen and Ready-to-Eat Vegetables. Food Chem. 2023, 410, 135415. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and Polyfluoroalkyl Substances (PFAS) in Livestock and Game Species: A Review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- Vendl, C.; Pottier, P.; Taylor, M.D.; Bräunig, J.; Gibson, M.J.; Hesselson, D.; Neely, G.G.; Lagisz, M.; Nakagawa, S. Thermal Processing Reduces PFAS Concentrations in Blue Food—A Systematic Review and Meta-Analysis. Environ. Pollut. 2022, 304, 119081. [Google Scholar] [CrossRef] [PubMed]
- Schwanz, T.G.; Llorca, M.; Farré, M.; Barceló, D. Perfluoroalkyl Substances Assessment in Drinking Waters from Brazil, France and Spain. Sci. Total Environ. 2016, 539, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Post, G.B. Recent US State and Federal Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2021, 40, 550–563. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.S.; Dasgupta, S.; Burdick, J. A Review of Emerging Technologies for Remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of Currently Available PFAS Remediation Technologies in Water: A Review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Wang, Y.; Sun, W. A Review on Degradation of Perfluorinated Compounds Based on Ultraviolet Advanced Oxidation. Environ. Pollut. 2021, 291, 118014. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Recent Progress and Challenges on the Removal of Per- and Poly-Fluoroalkyl Substances (PFAS) from Contaminated Soil and Water. Environ. Sci. Pollut. Res. 2022, 29, 58405–58428. [Google Scholar] [CrossRef]
- Garg, S.; Wang, J.; Kumar, P.; Mishra, V.; Arafat, H.; Sharma, R.S.; Dumée, L.F. Remediation of Water from Per-/Poly-Fluoroalkyl Substances (PFAS)—Challenges and Perspectives. J. Environ. Chem. Eng. 2021, 9, 105784. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Poly- and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A Review of PFAS Adsorption from Aqueous Solutions: Current Approaches, Engineering Applications, Challenges, and Opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef] [PubMed]
- Winchell, L.J.; Wells, M.J.M.; Ross, J.J.; Fonoll, X.; Norton, J.W.; Kuplicki, S.; Khan, M.; Bell, K.Y. Analyses of Per- and Polyfluoroalkyl Substances (PFAS) through the Urban Water Cycle: Toward Achieving an Integrated Analytical Workflow across Aqueous, Solid, and Gaseous Matrices in Water and Wastewater Treatment. Sci. Total Environ. 2021, 774, 145257. [Google Scholar] [CrossRef]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent Advances in the Analysis of Per- and Polyfluoroalkyl Substances (PFAS)—A Review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Liu, Y.; Pereira, A.D.S.; Martin, J.W. Discovery of C5–C17 Poly- and Perfluoroalkyl Substances in Water by In-Line SPE-HPLC-Orbitrap with In-Source Fragmentation Flagging. Anal. Chem. 2015, 87, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Bertanza, G.; Capoferri, G.U.; Carmagnani, M.; Icarelli, F.; Sorlini, S.; Pedrazzani, R. Long-Term Investigation on the Removal of Perfluoroalkyl Substances in a Full-Scale Drinking Water Treatment Plant in the Veneto Region, Italy. Sci. Total Environ. 2020, 734, 139154. [Google Scholar] [CrossRef] [PubMed]
- Coggan, T.L.; Moodie, D.; Kolobaric, A.; Szabo, D.; Shimeta, J.; Crosbie, N.D.; Lee, E.; Fernandes, M.; Clarke, B.O. An Investigation into Per- and Polyfluoroalkyl Substances (PFAS) in Nineteen Australian Wastewater Treatment Plants (WWTPs). Heliyon 2019, 5, e02316. [Google Scholar] [CrossRef] [PubMed]
- Barisci, S.; Suri, R. Removal of Polyfluorinated Telomer Alcohol by Advanced Oxidation Processes (AOPs) in Different Water Matrices and Evaluation of Degradation Mechanisms. J. Water Process Eng. 2021, 39, 101745. [Google Scholar] [CrossRef]
- Lin, H.; Lao, J.-Y.; Wang, Q.; Ruan, Y.; He, Y.; Lee, P.K.H.; Leung, K.M.Y.; Lam, P.K.S. Per- and Polyfluoroalkyl Substances in the Atmosphere of Waste Management Infrastructures: Uncovering Secondary Fluorotelomer Alcohols, Particle Size Distribution, and Human Inhalation Exposure. Environ. Int. 2022, 167, 107434. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Chiang, D.; Cashman, M.; Acheson, C. Total Oxidizable Precursor (TOP) Assay─Best Practices, Capabilities and Limitations for PFAS Site Investigation and Remediation. Environ. Sci. Technol. Lett. 2023, 10, 292–301. [Google Scholar] [CrossRef]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; DeWitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf, S.; Filipovic, M.; Lewis, J.; Sparrenbom, C.J.; Barthel, R. A Review of Contamination of Surface-, Ground-, and Drinking Water in Sweden by Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). Ambio 2017, 46, 335–346. [Google Scholar] [CrossRef]
- EPA. PFAS Analytical Methods Development and Sampling Research. Available online: https://www.epa.gov/water-research/pfas-analytical-methods-development-and-sampling-research (accessed on 14 June 2023).
- McDonough, C.A.; Guelfo, J.L.; Higgins, C.P. Measuring Total PFASs in Water: The Tradeoff between Selectivity and Inclusivity. Curr. Opin. Environ. Sci. Health 2019, 7, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for Grouping Per- and Polyfluoroalkyl Substances (PFAS) to Protect Human and Environmental Health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Newman, M.C.; Unger, M.A. Fundamentals of Ecotoxicology, 2nd ed.; Lewis Publishers (CRC Press): Boca Raton, FL, USA, 2003. [Google Scholar]
- Xu, J.; Wei, D.; Wang, F.; Bai, C.; Du, Y. Bioassay: A Useful Tool for Evaluating Reclaimed Water Safety. J. Environ. Sci. 2020, 88, 165–176. [Google Scholar] [CrossRef]
- Barceló, D.; Žonja, B.; Ginebreda, A. Toxicity Tests in Wastewater and Drinking Water Treatment Processes: A Complementary Assessment Tool to Be on Your Radar. J. Environ. Chem. Eng. 2020, 8, 104262. [Google Scholar] [CrossRef]
- Pedrazzani, R.; Bertanza, G.; Brnardić, I.; Cetecioglu, Z.; Dries, J.; Dvarionienė, J.; García-Fernández, A.J.; Langenhoff, A.; Libralato, G.; Lofrano, G.; et al. Opinion Paper about Organic Trace Pollutants in Wastewater: Toxicity Assessment in a European Perspective. Sci. Total Environ. 2019, 651, 3202–3221. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a Tool for Evaluating Advanced Oxidation Processes in Water and Wastewater Treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef]
- Campana, O.; Wlodkowic, D. Ecotoxicology Goes on a Chip: Embracing Miniaturized Bioanalysis in Aquatic Risk Assessment. Environ. Sci. Technol. 2018, 52, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, A.; Rosenmai, A.K.; Mandava, G.; Johannisson, A.; Holmes, A.; Tröger, R.; Lundqvist, J. Assessment of Source and Treated Water Quality in Seven Drinking Water Treatment Plants by in Vitro Bioassays—Oxidative Stress and Antiandrogenic Effects after Artificial Infiltration. Sci. Total Environ. 2021, 758, 144001. [Google Scholar] [CrossRef]
- Finlayson, K.A.; van de Merwe, J.P.; Leusch, F.D.L. Review of Ecologically Relevant in Vitro Bioassays to Supplement Current In Vivo Tests for Whole Effluent Toxicity Testing—Part 2: Non-Apical Endpoints. Sci. Total Environ. 2022, 851, 158094. [Google Scholar] [CrossRef]
- Song, M.; McKenna, E.; Ferrer, I.; Thurman, E.M.; Taylor-Edmonds, L.; Hofmann, R.; Ishida, K.P.; Roback, S.L.; Plumlee, M.H.; Hanigan, D. Comparison of Oxidants Used in Advanced Oxidation for Potable Reuse: Non-Target Analysis and Bioassays. ACS EST Water 2023, 3, 690–700. [Google Scholar] [CrossRef]
- Kienle, C.; Werner, I.; Fischer, S.; Lüthi, C.; Schifferli, A.; Besselink, H.; Langer, M.; McArdell, C.S.; Vermeirssen, E.L.M. Evaluation of a Full-Scale Wastewater Treatment Plant with Ozonation and Different Post-Treatments Using a Broad Range of In Vitro and In Vivo Bioassays. Water Res. 2022, 212, 118084. [Google Scholar] [CrossRef] [PubMed]
- Papa, M.; Paredes, L.; Feretti, D.; Viola, G.; Mazzoleni, G.; Steimberg, N.; Pedrazzani, R.; Lema, J.; Omil, F.; Carballa, M. How Should Ecohazard of Micropollutants in Wastewater Be Gauged? Using Bioassays to Profile Alternative Tertiary Treatments. Environ. Eng. Res. 2020, 26, 200153. [Google Scholar] [CrossRef]
- Bertanza, G.; Papa, M.; Pedrazzani, R.; Repice, C.; Mazzoleni, G.; Steimberg, N.; Feretti, D.; Ceretti, E.; Zerbini, I. EDCs, Estrogenicity and Genotoxicity Reduction in a Mixed (Domestic + Textile) Secondary Effluent by Means of Ozonation: A Full-Scale Experience. Sci. Total Environ. 2013, 458, 160–168. [Google Scholar] [CrossRef]
- Orozco-Hernández, L.; Gómez-Oliván, L.M.; Elizalde-Velázquez, A.; Natividad, R.; Fabian-Castoño, L.; SanJuan-Reyes, N. 17-β-Estradiol: Significant Reduction of Its Toxicity in Water Treated by Photocatalysis. Sci. Total Environ. 2019, 669, 955–963. [Google Scholar] [CrossRef]
- Neale, P.A.; Munz, N.A.; Aït-Aïssa, S.; Altenburger, R.; Brion, F.; Busch, W.; Escher, B.I.; Hilscherová, K.; Kienle, C.; Novák, J.; et al. Integrating Chemical Analysis and Bioanalysis to Evaluate the Contribution of Wastewater Effluent on the Micropollutant Burden in Small Streams. Sci. Total Environ. 2017, 576, 785–795. [Google Scholar] [CrossRef]
- Alias, C.; Feretti, D.; Benassi, L.; Zerbini, I.; Zani, C.; Sorlini, S. Tools for Monitoring Toxicological and Genotoxicological Changes in a Drinking Water Treatment Plant in Northeast Italy. Water Environ. J. 2023, 37, 81–94. [Google Scholar] [CrossRef]
- Neale, P.A.; Altenburger, R.; Aït-Aïssa, S.; Brion, F.; Busch, W.; de Aragão Umbuzeiro, G.; Denison, M.S.; Du Pasquier, D.; Hilscherová, K.; Hollert, H.; et al. Development of a Bioanalytical Test Battery for Water Quality Monitoring: Fingerprinting Identified Micropollutants and Their Contribution to Effects in Surface Water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).