Abstract
The biosorption and bioaccumulation of gadolinium by Arthospira platensis in batch experiments was examined. In biosorption experiments, the influence of pH, gadolinium concentration, time of contact and temperature on Arthospira platensis sorption capacity was investigated. The maximum biosorption capacity of 101 mg/g was attained at a pH of 3.0 and temperature of 20 °C. A pseudo-first-order model was applicable to describe the kinetics of the biosorption and the Freundlich model to explain the equilibrium of the process. In bioaccumulation experiments, besides the examination of the gadolinium uptake by Arthospira platensis, its effect on biomass productivity as well as the content of proteins, lipids, carbohydrates and pigments was assessed. The addition of gadolinium in the cultivation medium resulted in the increase in biomass productivity and the content of MDA and, at the same time, in the reduction in the amount of proteins and carbohydrates. The content of other monitored parameters did not change significantly. The water extracts obtained from Arthospira platensis showed a higher antiradical activity against the ABTS cation radical in comparison with ethanolic extracts. Arthospira platensis is of interest for the development of the technology of gadolinium-contaminated wastewater remediation.
1. Introduction
In recent years, the recovery of rare earth elements has gained special attention due to their wide application in different areas of industry and medicine and their high price [1,2]. Gadolinium compounds are extensively applied in nuclear technologies, nuclear medicine, ceramics, metallurgy, in luminescent material, magnet and superconductor production, and in magnetic resonance imaging [1,2,3]. Gadolinium, due to its high thermal neutron absorption cross-section, is used in reactors as neutron poison during start up and shutdown, as well as for neutron flux management during reactor operation [4].
As result of this extensive usage, gadolinium in stable and radioactive forms is released in the environment, affecting the quality of water. The toxicity of gadolinium for living organisms is mainly due to its chemical similarity with calcium; therefore, it can act as an inorganic blocker to several voltage-channel gates [5]. It was reported that gadolinium ions may exhibit toxicity towards different organs through the inhibition of sarcoplasmic reticulum activities in skeletal muscles and damage to DNA [5,6]. Consequently, the removal of gadolinium from spent nuclear fuel and biomedical waste is critically important due to environmental concerns [7].
Commonly applied wastewater treatment technologies, cloud point extraction, solvent extraction, nanofiltrations and solid−liquid extraction are not very efficient for gadolinium ions’ removal owing to their good water solubility and tendency to form stable complexes [2]. Therefore, biological techniques can be regarded as a new opportunity in gadolinium ion removal.
Among the mechanisms of microorganisms’ interaction with metal ions and radionuclides, biosorption, bioaccumulation and biotransformation can be highlighted [8]. Biosorption is more attractive for industrial application due to the use of dead biomass. Non-living biomass does not require nutrients and is not affected by metals’ toxicity. In addition, it can be stored for long periods, reused for several cycles and allows work on a greater range of environmental variables [9,10,11]. Bioaccumulation, which combines both biosorption and intracellular uptake, is a process mainly occurring in nature. In the case of its application for industrial purposes, it is necessary to ensure conditions for microorganisms’ growth, which would raise the cost of the process. It is also important to select microbial species that are resistant to high concentrations of pollutants and do not possess mechanisms protecting them from the excessive accumulation of xenobiotics inside the cell [12]. At the same time, the selection of resistant strains allows the biotransformation or biodegradation of pollutants, increasing their removal from the environmental objects [11].
According to Dev and co-authors’ review [13], microorganisms have mainly been applied for lanthanum and cerium removal, whereas information about gadolinium recovery from waste using biological objects is very scarce. Thus, Breuker et al. [9] applied bacteria, yeast, fungi and alga for lanthanum, cerium, neodymium, gadolinium, dysprosium, erbium, ytterbium and lutetium biosorption removal in acidic conditions. Wet microorganisms, Bacillus subtilis, Pseudomonas aeruginosa, Ralstonia metallidurans CH34, Mycobacterium smegmatis and Saccharomyces cerevisiae, were applied for gadolinium biosorption at a fixed pH value [8]. The impact of gadolinium compounds on Nitrosomonas europaea activity, bioaccumulation capacity and growth was reported in [14]. Three living marine macroalgae, Ulva lactuca, Fucus spiralis and Gracilaria sp., showed high gadolinium uptake capacity—up to 85% of metal ions were removed from the solution [15]—while dry baker’s yeast was able to remove no more than 60% of gadolinium ions from the solution [16]. According to our current knowledge, there are no studies devoted to cyanobacteria application for gadolinium removal from wastewater, though they are actively used for heavy metal removal from batch solutions and real wastewater [17,18,19,20].
Previously, cyanobacteria Arthospira platensis was applied for europium, erbium and yttrium removal from batch systems [21,22,23]. The Arthospira platensis biosorption and bioaccumulation capacity toward the studied rare earth elements changed in the following order Eu (80–99%) > Y (60–80%) > Er (45–78%). We can hypothesize that Arthospira platensis will show high removal capacity toward gadolinium ions as well. It should be mentioned that the sorption capacity of inorganic sorbents applied for gadolinium ion removal usually is not very high [2,24,25].
Considering the increasing application of gadolinium in technological processes, and the presence of this element in the environment, including its radioactive form, the present study aimed to examine the removal capacity of cyanobacteria Arthospira platensis toward gadolinium ions, both in biosorption and bioaccumulation experiments. For the first time, the effect of several parameters, such as pH, metal concentration, time of contact and temperature, on gadolinium biosorption was assessed, and the kinetics, thermodynamics and equilibrium of the biosorption were evaluated. In the bioaccumulation experiments, the effect of gadolinium on biomass amount and its accumulation capacity, as well as the content of main biomolecules and antioxidant activity, was evaluated.
2. Materials and Methods
2.1. Object of Study
Arthospira platensis CNMN-CB-02 (A. platensis, spirulina) obtained from the collection of non-pathogenic microorganisms (IMB TU, Chisinau, Moldova) was grown in the cultivation medium described in [26]. The amount of inoculum was 0.4–0.45 g/L. The biomass was cultivated at temperature 28–32 °C, pH—9–10 and with continuous illumination of approximately 7 µM photons/m2/s. For biosorption experiments, biomass was grown for six days, then separated from the cultivation medium, dried and homogenized.
2.2. Biosorption Experiment
To prepare the gadolinium solutions, Gd(NO3)3·6H2O (Sigma Aldrich, Darmstadt, Germany) was dissolved in distillated water. Experiments were carried out in Erlenmeyer flasks of 50 mL volume, where 20 mL of gadolinium solution of the desired concentration was mixed with 0.1 g of spirulina biomass. The solutions with different pHs ranging from 2.0 to 6.0 were prepared using 0.1 mol HCl or NaOH. Kinetic parameters of the biosorption were collected with the contact time of 3–120 min. Adsorption isotherms were obtained with gadolinium concentrations of 10–100 mg/L. In order to understand the nature of the biosorption process, experiments were conducted by varying the temperature of the solution from 20 to 50 °C. Gadolinium concentrations in the initial solutions and solutions after biosorption were determined using an ICP-OES PlasmaQuant PQ 9000 Elite spectrometer (Analytik Jena, Jena, Germany). All experiments were carried out in triplicate.
The sorption capacity of A. platensis q (mg/g) and efficiency of gadolinium removal R (%) were evaluated using Equations (1) and (2):
where V is the volume of the gadolinium solution in ml, Ci and Cf are the initial and final gadolinium concentrations in mg/L and m is the mass of spirulina in g.
2.3. Bioaccumulation Experiment
In the bioaccumulation experiment, gadolinium in concentrations of 10–30 mg/L by metal was added to the cultivation medium described in Section 2.1 on the first day of biomass cultivation. Biomass cultivation in gadolinium-supplemented medium was performed in Erlenmeyer flasks with a working volume of 100 mL for six days. Biomass obtained after the experiment was used for biochemical tests, while the concentration of gadolinium in the medium was determined using ICP-OES. Biomass cultivated in the standard conditions described in Section 2.1 was used as a control.
2.4. Biomass Productivity and Biochemical Tests
The biomass productivity, in g/L, was determined according to [26]. The content of protein was determined according to the Lowry method and that of carbohydrates was determined spectrophotometrically based on the formation of hydroxymethylfurfural through the interaction of carbohydrates with the Anthon reagent (C14H10O) in acid medium. In the case of phycobiliproteins, the absorbance of the water extract obtained from biomass was measured at 620 nm for c-phycocyanin and 650 nm for allophycocyanin. The lipid content was determined spectrophotometrically using phosphovanilinic reagent. The degree of lipid oxidation was determined based on the reactive products of thiobarbituric acid. The absorbance of chlorophyll a was determined at 665 nm with the extinction coefficient 0.8 × 105 M−1 cm−1, and that of β-carotene was determined at 450 nm with the extinction coefficient 1.5 × 105 M−1 cm−1. More details about biochemical parameter determination can be found in [22,23].
2.5. Antioxidant Activity
The antioxidative activity of the ethanolic and water extracts was determined through the application of non-biological tests with radicals ABTS (2.2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) [27].
2.6. Statistical Analysis
All experiments were performed in triplicate, and values are presented as the mean of three experiments ± standard deviation. To elucidate the difference between experimental and control values, Student’s t-test was applied.
3. Results and Discussion
3.1. Biosorption of Gadolinium on A. platensis
The pH of the solution significantly influenced the adsorption capacity of different sorbents. Gadolinium biosorption on spirulina was shown to be a pH-dependent process (Figure 1a). The increase in the pH from 2.0 to 3.0 resulted in the increase in the spirulina removal capacity from 45 to 63% and achievement of the maximum gadolinium ion removal. A further increase in pH value led to a decrease in gadolinium removal to 26% at pH 6.0.
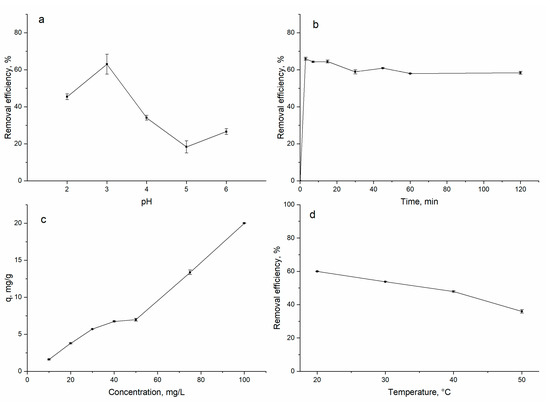
Figure 1.
Impact of (a) pH, (b) time, (c) gadolinium concentration and (d) temperature on gadolinium biosorption by A. platensis.
The process of gadolinium removal was studied in the pH range 2.0–6.0 since gadolinium precipitates at pH values higher than 6.5 [4]. At pHs 1.0–4.0, gadolinium is present in solution in the form of Gd3+, and at pHs above 5, the formation of hydroxo complex species such as Gd(OH)2+, Gd(OH)+2 and Gd(OH)4+ starts [1]. The highest gadolinium sorption at pH 3.0 can be explained by the creation of optimal conditions for sorption, since at low pHs, gadolinium ions compete with H+ for binding sites and at pHs above 4.0, competition with OH groups may take place [21].
The pH 3.0 was found to be optimal for europium, erbium and yttrium sorption on spirulina biomass [21,22,23]. The gadolinium removal using Dowex HCR-S/S resin was not dependent on the pH of the solution [1]. Gadolinium biosorption by Bacillus subtilis, Pseudomonas aeruginosa, Ralstonia metallidurans CH34, Mycobacterium smegmatis and Saccharomyces cerevisiae was studied at pH 5.0 [8]. Gadolinium sorption on multi-walled carbon nanotubes was studied at pH 5–5.5. The highest gadolinium biosorption on Pichia sp. was achieved at pH 3.0 [9].
The equilibrium time played an important role in the adsorption process. Gadolinium removal was shown to be a very quick process; three minutes was enough for maximum metal removal (66%), and then equilibrium was achieved (Figure 1b). The process of gadolinium sorption occurred in two stages: fast sorption in the first 3 min of sorbate interaction with sorbent, explained by a large number of unoccupied binding sites, replaced by a slow stage and equilibrium attainment. In the case of gadolinium removal with Dowex-HCR S/S resin, equilibrium was achieved in 40 min [1]. Andres et al. [8] reported equilibrium attainment in 2 h when microorganisms were used for gadolinium removal. Gadolinium sorption on multi-walled carbon nanotubes reached saturation in approximately 60 min [2].
The increase in gadolinium concentration in solution was accompanied by the increase in biosorption capacity: from 1.6 mg/g at a gadolinium concentration of 10 mg/L to 20 mg/g at a concentration of 100 mg/L (Figure 1c). It was reported that the affinity of the metal ions to biomass shows a tendency to increase with an increase in the maximum metal uptake [28].
The effect of temperature on the biosorption process was studied in order to understand the thermodynamics of the process. The increase in the temperature of solutions from 20 to 50 °C was associated with the decrease in biomass sorption capacity from 60 to 36% (Figure 1d). This behavior proved the exothermic nature of the biosorption. In contrast, gadolinium removal with Dowex-HCR S/S resin increased with the temperature increase [1]. The same pattern was noted when manganese oxide nanoparticles were used for gadolinium sorption [24].
3.2. Equilibrium, Kinetics and Thermodynamic Studies
Equilibrium and kinetic data provide essential physicochemical data for the estimation of the applicability of a sorption process [2]. They were described using well-known models:
Pseudo-first-order model:
Pseudo-second-order models:
where qe and qt are the content of gadolinium (mg/g) and k2 (g/mg·min) and k1 (1/min) are the pseudo-first-order and pseudo-second-order constants, respectively.
Langmuir equilibrium model:
Freundlich equilibrium model:
where qe and qm are the content of gadolinium adsorbed at equilibrium and maximum adsorption capacity in mg/g and b, KF and n are the Langmuir and Freundlich constants, respectively.
The non-linear fitting of the applied models is illustrated in Figure 2, and parameters are given in Table 1.
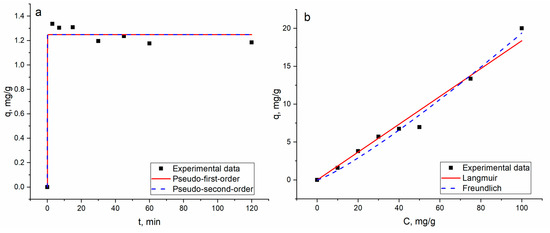
Figure 2.
(a) Kinetics and (b) isotherms of gadolinium biosorption on A. platensis biomass.

Table 1.
Values of the kinetics and isotherm parameters related to gadolinium biosorption on A. platensis.
In case the of kinetic studies, for both models, the values of the calculated and experimentally obtained sorption capacity matched well (Table 1). However, the negative value of the rate constant of the pseudo-second-order model indicates its inapplicability for the explanation of the experimental data. Gadolinium biosorption fitted the pseudo-first-order model better, suggesting the physical nature of biosorption [23]. The Akaike Information Criterion test proved the applicability of the pseudo-first-order model for the description of kinetic data.
According to the coefficient of determination values, the pseudo-second-order kinetic model was shown to be more adequate for the description of Gd(III) sorption on multi-walled carbon nanotubes [2].
Regarding equilibrium studies, the coefficient of determination for the Freundlich model was higher than that for the Langmuir one (Table 1), indicating its suitability for the explanation of the process. The Freundlich model is usually applicable for the description of sorption on a heterogeneous surface. Spirulina surfaces include a wide number of functional groups, C=O, C-C, C-O-C, P=O, COO- (νas(COO-) and νs(COO-)), CH2 and NHC(O)amid, which may participate in gadolinium ion binding [29]. According to the modelling studies, gadolinium biosorption is equally good with carboxyl or phosphate sites [9].
The maximum sorption capacity of A. platensis was compared with the values obtained for other sorbents (Table 2). As can be seen, A. platensis has the highest sorption capacity toward gadolinium ions compared to other sorbents.

Table 2.
Comparison of A. platensis sorption capacity with the literature data.
To describe the thermodynamics of the biosorption process, the Gibbs energy (∆G°), enthalpy (∆H°) and entropy (∆S°) changes were calculated (Equations (7)–(9)). The values of ∆S° and ∆H° were obtained from the slope and intercept of the plot of ln Kd vs. t 1/T r (Figure S1).
where Kd is the distribution coefficient:
Negative values of ∆G° (Table 3) indicate the spontaneous nature of the biosorption process, while the negative ∆H° values of −14.1 kJ/mol indicate the exothermic character of the biosorption. The negative values of ∆S° (−7.8 J/mol·K) suggest a reduction in the randomness at the solid/solution interface during the biosorption of gadolinium on A. platensis [30].

Table 3.
Thermodynamics parameters for gadolinium biosorption on A. platensis.
3.3. Gadolinium Bioaccumulation Using A. platensis and Assessment of Biochemical Changes in Biomass
Gadolinium in concentrations of 10, 20 and 30 mg/L did not inhibit A. platensis biomass accumulation but, on the contrary, even intensified its growth (Figure 3).
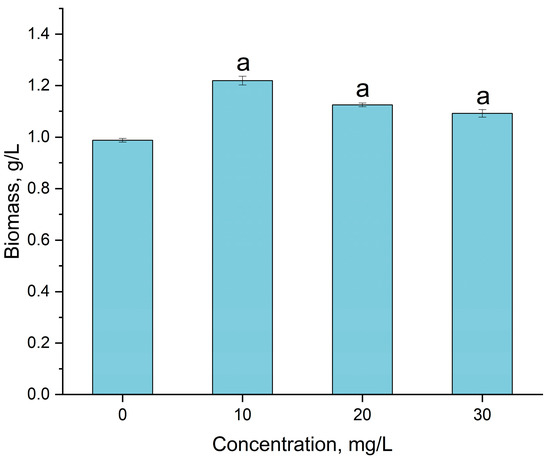
Figure 3.
The effect of gadolinium applied in concentrations of 10, 20 and 30 mg/L on the Arthrospira platensis biomass (a—p < 0.0005 for difference between sample and control, n = 3).
Thus, at the addition of 10 mg/L gadolinium, the amount of spirulina biomass was 1.22 g/L, which is 23.4% higher than the control (0.99 g/L). With the increase in gadolinium concentration in the cultivation medium, the amount of accumulated biomass decreased, but still remained significantly higher than in the control. Thus, at a gadolinium concentration of 20 mg/L, the biomass increased by 13.9%, and at its concentration of 30 mg/L, it increased by 10.5% compared to the control. It should be mentioned that A. platensis accumulated 96–98% of gadolinium ions present in the cultivation medium.
It has been demonstrated that rare earth elements (REEs) can have various effects on spirulina culture. In the majority of cases, the presence of REEs did not significantly modify the amount of biomass. At the same time, some of the REEs can ensure an increase in the values of this parameter. Thus, as was shown previously, Dy and La, depending on the applied concentration, can ensure an increase in the amount of biomass by up to 19.3% compared to the control, mainly at a metal concentration of 30 mg/L [31]. Additionally, Y at a concentration of 20 mg/L provoked a statistically significant increase (by 9.3%) in biomass with respect to the control [23].
The effect of gadolinium on other photosynthetic microorganisms has also been studied. For example, in the case of Dunaliella salina, gadolinium in the form of Gd2O3 nanoparticles in concentrations up to 1 mg/L led to a slight decrease in the amount of biomass during the first 15 days of cultivation, after which a significant increase in biomass productivity followed [32]. Additionally, the element’s uptake and biomass productivity can be influenced by growing conditions. Thus, light intensity modified the effects caused by REEs, including gadolinium, on the microalgae Trachydiscus minutus and Parachlorella kessleri [33]. At the same time, the concentration of gadolinium nitrate 30 µM caused a 50% reduction in the growth rate of the microalga Skeletonema costatum [34]. Thus, the effects generated by the presence of gadolinium in the cultivation medium can differ depending on the species of microorganism, concentration of the element and other cultivation factors.
The biochemical composition of the spirulina biomass was subjected to changes under the influence of gadolinium ions. In Figure 4, data related to the amount of protein and carbohydrates in biomass grown under standard conditions and in the medium containing gadolinium ions at different concentrations are shown.
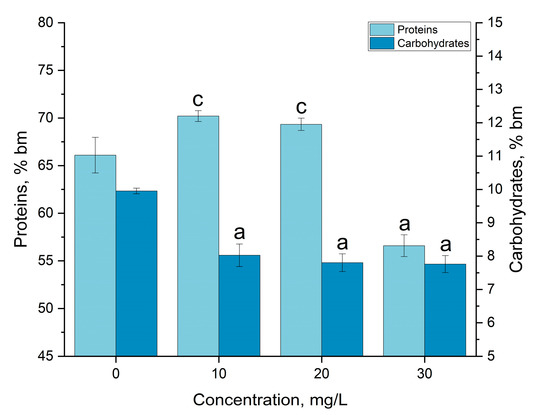
Figure 4.
The effect of gadolinium applied in concentrations of 10, 20 and 30 mg/L on the amount of proteins and carbohydrates in Arthrospira platensis biomass (a—p < 0.0005, c—p < 0.05; for difference between sample and control in case of carbohydrates, n = 3).
In the case of the first two concentrations of gadolinium, a slight increase in the content of protein in the biomass was observed—from 66.1% of the dry biomass in the control to 70.2 and 69.3%, respectively, at concentrations of 10 and 20 mg/L. However, at a gadolinium concentration of 30 mg/L, the protein amount significantly decreased, by 14.4% compared to the control, and constituted 56.6% of the dry biomass. The phenomenon of the decrease in the content of proteins in spirulina biomass was also observed in the case of other REEs: by 16% for Nd [31], by 14.5% for Y [23] and by 17.7% for Eu [22].
The change in the content of carbohydrates is presented in Figure 4. At all applied concentrations, their content was 19.4–22.0% lower than in the control. However, there were no statistically significant difference between the experimental variants.
Other REEs also provoking a reduction in the carbohydrate content in Arthrospira platensis biomass was also observed in the presence of Thus. At the same concentrations of Y, the content of carbohydrates in A. platensis biomass decreased by up to 16.25% compared to the control [23]; in the presence of Eu, the reduction was by approximately 27% [22]; in the presence of Er, by 36.7% [21] and in the case of Nd and Yb, by 15.8–21.9% [31]. In contrast, Sm, Tb, La and Dy stimulated an increase in carbohydrate content in biomass [31].
The change in the content of pigment under the influence of gadolinium is shown in Figure 5. The content of total phycobiliproteins in the control dry biomass was 14.2%, and in the experimental variants, this value significantly decreased. The observed effect was strongly dose-dependent; the amount of phycobiliproteins decreased progressively with the increase in the concentration of gadolinium in the medium: by 11.2% at a concentration of 10 mg/L and by 27.9% at a concentration of 30 mg/L.
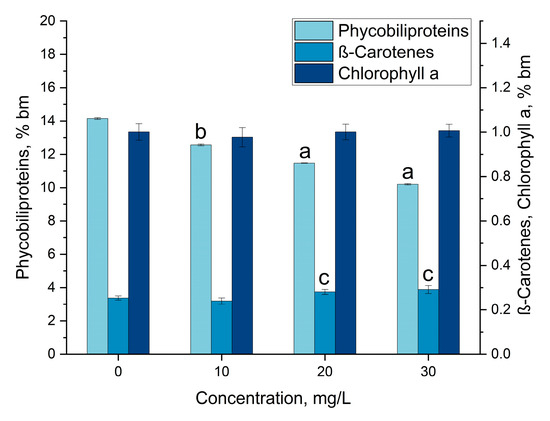
Figure 5.
The effect of gadolinium applied in concentrations of 10, 20 and 30 mg/L on the amount of phycobiliproteins, chlorophyll α and β-carotene in Arthrospira platensis biomass. (a—p < 0.0005, b—p < 0.001, c—p < 0.05 for difference between sample and control, n = 3).
The reduction in phycobiliprotein content in spirulina in the presence of an REE is a common phenomenon. It is well known that a decrease in the amount of phycobiliproteins in the biomass of cyanobacteria is an indicator of stress, and the presence of an REE seems to be a stress factor for the spirulina culture, even if often no inhibition of the culture growth process is observed. Thus, Nd and Yb in a concentration of 30 mg/L reduced the content of phycobiliproteins by 10.7–19.0% [31], and Y by 18.2–27.5% depending on its concentration in the medium [23]. In the presence of 30 mg/L of La, Dy, Sm and Tb, the content of phycobiliproteins was reduced by 50% with respect to the control. Only some REEs applied in concentrations up to 30 mg/L, for example, Eu and Er, did not provoke a decrease in the pigment in the spirulina biomass [21,22].
The amount of chlorophyll α in the spirulina biomass did not change in the presence of gadolinium, and the value of this parameter was in the range of 0.98–1.0% of the dry biomass. The constant level of chlorophyll α is characteristic for spirulina biomass grown in the presence of an REE, and only some of them can significantly modify it. Thus, in the presence of Y in concentrations of 10 and 20 mg/L, the amount of this pigment increased by 13.34 and 19.92%, respectively [31]. Supplementation of biomass with Sm [31] and Eu led to a considerable decrease in the content of chlorophyll α in the biomass [22].
The content of β-carotene in the control biomass and experimental variants was 0.25% and 0.24–0.29%, respectively. At gadolinium concentrations of 20 and 30 mg/L, the amount of β-carotene increased compared to the control by 10.7 and 14.9%, respectively. Among REEs, only Eu in high concentrations provoked a slight decrease in the amount of β-carotene [22], while other elements, such as Sm, La, Dy, Nd, Yb, Y and Er, did not modify it [21,23,31].
The effect of gadolinium on photosynthetic pigments in Trachydiscus minutus and Parachlorella kessleri was studied by Goecke et al. [33]. They established that under the influence of gadolinium, the content of chlorophyll α in Trachydiscus minutus increased, and in Parachlorella kessleri, the proportion of carotenoids changed significantly in favor of oxy forms. The increase in carotenoid content under the influence of gadolinium oxide nanoparticles was also observed in Dunaliella salina [32].
The modification of the content of lipids and MDA under gadolinium influence is presented in Figure 6. The content of lipids in the biomass grown in the presence of 10 and 20 mg/L of gadolinium did not differ significantly from that in the control samples, where the lipid content was 4.80 ± 0.13% of dry biomass. A gadolinium concentration of 30 mg/L caused an increase in the content of lipids in the biomass up to the level of 5.42 ± 0.17% of the dry biomass, which was 112.89% higher.
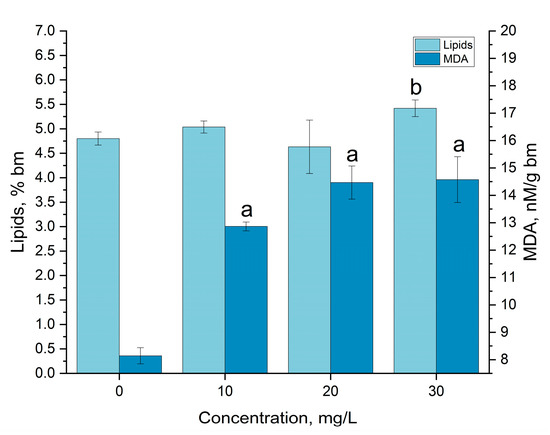
Figure 6.
The effect of gadolinium applied in concentrations of 10, 20 and 30 mg/L on the amount of lipids and MDA in Arthrospira platensis biomass. (a—p < 0.0005, b—p < 0.001 for difference between sample and control, n = 3).
The comparison of the obtained results with those reported for other REEs showed a good correlation between the obtained data and data reported for La and Y. Only in the case of these two REEs was an increase in the content of lipids observed (in the case of La, by up to, 29.1%, and in the case of Y up to 30.7% compared to the control) [23,31]. An increase in total lipid content under the influence of gadolinium oxide nanoparticles was also observed in the microalga Dunaliella salina [32].
Most of the REEs caused a reduction in the content of lipids in the spirulina biomass or did not change it significantly. Thus, Tb, Dy, Yb and Eu were able to reduce lipid content in spirulina biomass by up to 35%, while Sm and Nd did not affect it [22,31]. A decrease in the content of lipids from 27.3 to 21.1% in Trachydiscus minutus and from 15.7 to 14.0% in Parachlorella kessleri under gadolinium influence was reported in [33].
The content of MDA experimental variants was 58.0–79.0% higher than in the control. It increased with the increase in gadolinium concentration in the medium, but the dependence was not directly proportional—at concentrations of 20 and 30 mg/L, the MDA values were very close, but significantly higher than at a concentration of 10 mg/L.
The increase in the content of MDA in spirulina biomass grown in the presence of an REE was reported in other studies. Thus, the content of MDA in spirulina biomass increased upon the addition of La, Dy, Sm, Nd, Yb, Eu, Er and Y to the medium [21,22,23,31]. The most significant increase—more than two times—was observed under the influence of Er and Y, and the values obtained for gadolinium were the closest to those reported for Eu [21,22,23].
The MDA values obtained for gadolinium and other REEs support the high adaptation capacity of A. platensis compared to other species of cyanobacteria, for which the doubling of the content of MDA occurred under the influence of REEs at much lower concentrations. At a Y concentration of 1 mg/L, the MDA content in Microcystis aeruginosa doubled [35].
The activity of extracts (ethanolic and water) from spirulina biomass grown on gadolinium-supplemented medium can be seen in Figure 7. The water extracts obtained from the spirulina biomass, both the experimental and control samples, showed a higher antiradical activity against the ABTS cation radical compared to the ethanolic extracts. The extracts of both types obtained from experimental biomass were more active than those from the control biomass. The highest increase in the activity of the water extract compared to the control was observed at a gadolinium concentration of 10 mg/L (57.2% higher compared to the control) and for the ethanolic extract, at 30 mg/L (32.1% compared to the control).
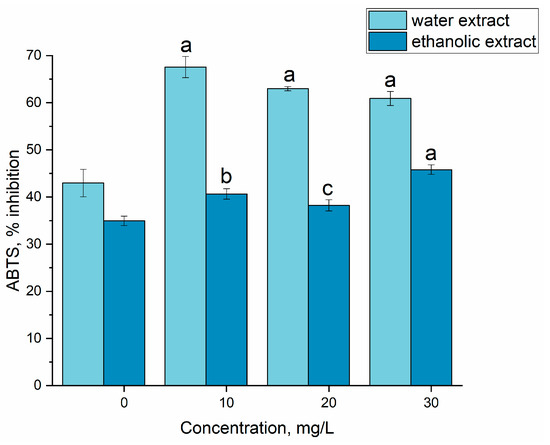
Figure 7.
The effect of gadolinium applied in concentrations of 10, 20 and 30 mg/L on the antioxidant activity of Arthrospira platensis biomass. (a—p < 0.0005, b—p < 0.001, c—p < 0.05 for difference between sample and control, n = 3).
In the previously performed research, the addition of Y into the cultivation medium resulted in the increase in water extract activity by up to 41.6%, and in the ethanolic one by 25.5% [23]. Extracts from spirulina biomass grown on Eu-supplemented medium had activity 37.9% higher than in the control [22]. Additionally, La, Dy, Sm and Tb increased the antioxidant activity of extracts from spirulina biomass. However, some of the REEs, such as Nd and Yb, cause a significant reduction in the inhibition activity of the ABTS radical [31].
4. Conclusions
For the first time, cyanobacteria, namely Arthrospira platensis, were applied for gadolinium ion removal from batch solutions. In biosorption experiments, the effect of the most important parameters, such as gadolinium concentration, time, pH and temperature, on the biomass biosorption capacity was investigated. The maximum biosorption of gadolinium of 101 mg/g was attained at pH 3.0, and it was significantly higher than the values reported in the literature. The kinetics of the biosorption was better described by pseudo-first-order kinetic model, while equilibrium data were better presented by the Freundlich model, suggesting biosorption on the heterogeneous surface. From a thermodynamic point of view, the process of gadolinium biosorption was spontaneous and exothermic in nature.
In the bioaccumulation experiments, gadolinium ions were almost completely accumulated from the cultivation medium and stimulated biomass growth. The accumulation of gadolinium in biomass resulted in the decrease in the content of phycobiliproteins and carbohydrates in biomass, but did not affect the content of chlorophyll α and β-carotene. The content of proteins and lipids in biomass changed only at a gadolinium concentration of 30 mg/L; the changes were expressed by the decrease in the content of proteins and increase in the content of lipids. Maintaining an adequate level of biomass productivity was ensured by maintaining the physiological level of photosynthetic pigments and high level of antioxidant activity of the biomass.
The obtained data showed that cyanobacteria Arthrospira platensis can be applied for gadolinium removal from wastewater through biosorption and/or bioaccumulation processes. The accumulated information, along with the data obtained for other rare earth elements, can be used for the development of the technology for the efficient treatment of effluents containing several rare earth elements.
Data obtained for stable gadolinium ions can also serve as an analogue for the treatment of radioactive curium.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cleantechnol5020032/s1. Figure S1. lnKd versus 1/T.
Author Contributions
Conceptualization, N.Y., I.Z. and L.C.; methodology, N.Y., T.C. and L.R.; software, D.G.; formal analysis, D.G.; investigation, N.Y., I.Z., L.C., T.C. and L.R.; data curation, I.Z. and L.C.; writing—original draft preparation, I.Z. and L.C.; writing—review and editing, all authors; visualization, I.Z. and N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamed, M.M.; Rizk, S.E.; Nayl, A.A. Adsorption Kinetics and Modeling of Gadolinium and Cobalt Ions Sorption by an Ion-Exchange Resin. Part. Sci. Technol. 2016, 34, 716–724. [Google Scholar] [CrossRef]
- Maksin, D.; Vukčević, M.; Đurkić, T.; Stanišić, I.; Bakić, T.; Radomirović, M.; Onjia, A. Gadolinium sorption on multi-walled carbon nanotubes. Contemp. Mater. 2019, 10, 1. [Google Scholar] [CrossRef]
- Siew, E.L.; Farris, A.F.; Rashid, N.; Chan, K.M.; Rajab, N.F. In Vitro Toxicological Assessment of Gadolinium (III) Chloride in V79-4 Fibroblasts. Genes Environ. 2020, 42, 22. [Google Scholar] [CrossRef]
- Sasikumar, P.; Narasimhan, S.V.; Velmurugan, S. Development of a Modified Ion Exchange Resin Column for Removal of Gadolinium From the Moderator System of PHWRs. Sep. Sci. Technol. 2013, 48, 1220–1225. [Google Scholar] [CrossRef]
- Port, M.; Idée, J.M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, Thermodynamic and Kinetic Stability of Marketed Gadolinium Chelates and Their Possible Clinical Consequences: A Critical Review. BioMetals 2008, 21, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Yongxing, W.; Xiaorong, W.; Zichun, H. Genotoxicity of Lanthanum (III) and Gadolinium (III) in Human Peripheral Blood Lymphocytes. Bull. Environ. Contam. Toxicol. 2000, 64, 611–616. [Google Scholar] [CrossRef]
- Sappidi, P.; Boda, A.; Ali, S.M.; Singh, J.K. Adsorption of Gadolinium (Gd3+) Ions on the Dibenzo Crown Ether (DBCE) and Dicyclo Hexano Crown Ether (DCHCE) Grafted on the Polystyrene Surface: Insights from All Atom Molecular Dynamics Simulations and Experiments. J. Phys. Chem. C 2019, 123, 12276–12285. [Google Scholar] [CrossRef]
- Andrès, Y.; Thouand, G.; Boualam, M.; Mergeay, M. Factors Influencing the Biosorption of Gadolinium by Micro-Organisms and Its Mobilisation from Sand. Appl. Microbiol. Biotechnol. 2000, 54, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Breuker, A.; Ritter, S.F.; Schippers, A. Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions. Metals 2020, 10, 954. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, L.K.; Gaur, J.P. Metal sorption by algal biomass: From batch to continuous system. Algal Res. 2016, 18, 95–109. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and Bioaccumulation—The Prospects for Practical Applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of Biological Recovery of Rare-Earth Elements from Industrial and Electronic Wastes: A Review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Fujita, Y.; Walton, M.; Das, G.; Dohnalkova, A.; Vanzin, G.; Anderko, A. Impacts of Anthropogenic Gadolinium on the Activity of the Ammonia Oxidizing Bacterium Nitrosomonas Europaea. Chemosphere 2020, 257, 127250. [Google Scholar] [CrossRef]
- Ferreira, N.; Ferreira, A.; Viana, T.; Lopes, C.B.; Costa, M.; Pinto, J.; Soares, J.; Pinheiro-Torres, J.; Henriques, B.; Pereira, E. Assessment of Marine Macroalgae Potential for Gadolinium Removal from Contaminated Aquatic Systems. Sci. Total Environ. 2020, 749, 141488. [Google Scholar] [CrossRef]
- Ojima, Y.; Kosako, S.; Kihara, M.; Miyoshi, N.; Igarashi, K.; Azuma, M. Recovering Metals from Aqueous Solutions by Biosorption onto Phosphorylated Dry Baker’s Yeast. Sci. Rep. 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, A.; Parvin, F.; Chakraborty, J.; Kim, Y.I. Cyanobacteria Mediated Heavy Metal Removal: A Review on Mechanism, Biosynthesis, and Removal Capability. Environ. Technol. Rev. 2021, 10, 44–57. [Google Scholar] [CrossRef]
- Kulal, D.K.; Loni, P.C.; Dcosta, C.; Some, S.; Kalambate, P.K. Cyanobacteria: As a Promising Candidate for Heavy-Metals Removal. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 291–300. ISBN 9780128193112. [Google Scholar]
- Sarup, R.; Behl, K.; Joshi, M.; Nigam, S. Heavy Metal Removal by Cyanobacteria. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Elsevier: Amsterdam, The Netherlands, 2021; pp. 441–466. ISBN 9780128229651. [Google Scholar]
- Ghorbani, E.; Nowruzi, B.; Nezhadali, M.; Hekmat, A. Metal Removal Capability of Two Cyanobacterial Species in Autotrophic and Mixotrophic Mode of Nutrition. BMC Microbiol. 2022, 22, 1–15. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Application of Cyanobacteria Arthospira Platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials 2022, 15, 6101. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Biosorption and Bioaccumulation Capacity of Arthospira Platensis toward Europium Ions. Water 2022, 14, 2128. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Biosorption and Bioaccumulation Capacity of Arthrospira Platensis toward Yttrium Ions. Metals 2022, 12, 1465. [Google Scholar] [CrossRef]
- Sayed, M.A.; Helal, A.I.; Abdelwahab, S.M.; Mahmoud, H.H.; Aly, H.F. Sorption and Possible Preconcentration of Europium and Gadolinium Ions from Aqueous Solutions by Mn3O4 Nanoparticles. Chem. Pap. 2020, 74, 619–630. [Google Scholar] [CrossRef]
- Abass, M.R.; El-Kenany, W.M.; Eid, M.A. Sorption of Cesium and Gadolinium Ions onto Zirconium Silico Antimonate Sorbent from Aqueous Solutions. Appl. Radiat. Isot. 2022, 192, 110542. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Rotari, I.; Turchenko, V.; Djur, S. Effects of PEG-Coated Silver and Gold Nanoparticles on Spirulina Platensis Biomass during Its Growth in a Closed System. Coatings 2020, 10, 717. [Google Scholar] [CrossRef]
- Cepoi, L.; Rudi, L.; Miscu, V.; Cojocari, A.; Chiriac, T.; Sadovnic, D. Antioxidative Activity of Ethanol Extracts from Spirulina Platensis and Nostoc Linckia Measured by Various Methods. An. Univ. Din Oradea Fasc. Biol. 2009, 16, 43–48. [Google Scholar]
- Lee, H.S.; Suh, J.H.; Kim, I.B.; Yoon, T. Effect of Aluminum in Two-Metal Biosorption by an Algal Biosorbent. Miner. Eng. 2004, 17, 487–493. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Tregubova, V.; Ilin, V.; Cepoi, L.; Chiriac, T.; Rudi, L.; Frontasyeva, M.V. Uptake of Metals from Single and Multi-Component Systems by Spirulina Platensis Biomass. Ecol. Chem. Eng. S 2016, 23, 401–412. [Google Scholar] [CrossRef]
- Sarada, B.; Krishna Prasad, M.; Kishore Kumar, K.; Murthy, C.V.R. Biosorption of Cd+2 by Green Plant Biomass, Araucaria Heterophylla: Characterization, Kinetic, Isotherm and Thermodynamic Studies. Appl. Water Sci. 2017, 7, 3483–3496. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Grozdov, D.; Pavlov, S.; Djur, S. Accumulation of Dysprosium, Samarium, Terbium, Lanthanum, Neodymium and Ytterbium by Arthrospira Platensis and Their Effects on Biomass Biochemical Composition. J. Rare Earths 2021, 39, 1133–1143. [Google Scholar] [CrossRef]
- Makhi, V.V.; Ahmad, A.; Chaugule, B.B. Effect of Bismuth, Gadolinium, and Cadmium Nanoparticles on Biomass, Carotenoid, and Lipid Content of Dunaliella Salina (Dunal) Teodoresco. Curr. Microbiol. 2022, 79, 30. [Google Scholar] [CrossRef]
- Goecke, F.; Vítová, M.; Lukavský, J.; Nedbalová, L.; Řezanka, T.; Zachleder, V. Effects of Rare Earth Elements on Growth Rate, Lipids, Fatty Acids and Pigments in Microalgae. Phycol. Res. 2017, 65, 226–234. [Google Scholar] [CrossRef]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological Toxicity of Lanthanide Elements on Algae. Chemosphere 2010, 80, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Luo, X.; Ren, Y.; Gao, E.; Gao, H. Effects of Yttrium and Phosphorus on Growth and Physiological Characteristics of Microcystis Aeruginosa. J. Rare Earths 2018, 36, 781–788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).