Influence of the Presence of Poly(butylene succinate) in the Poly(ethylene terephthalate) Recycling Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Miscibility between the Components and Microstructural Characterization
2.3. Mechanical Characterization
2.4. Thermal Characterization
2.5. Disintegration under Composting Conditions
3. Results and Discussion
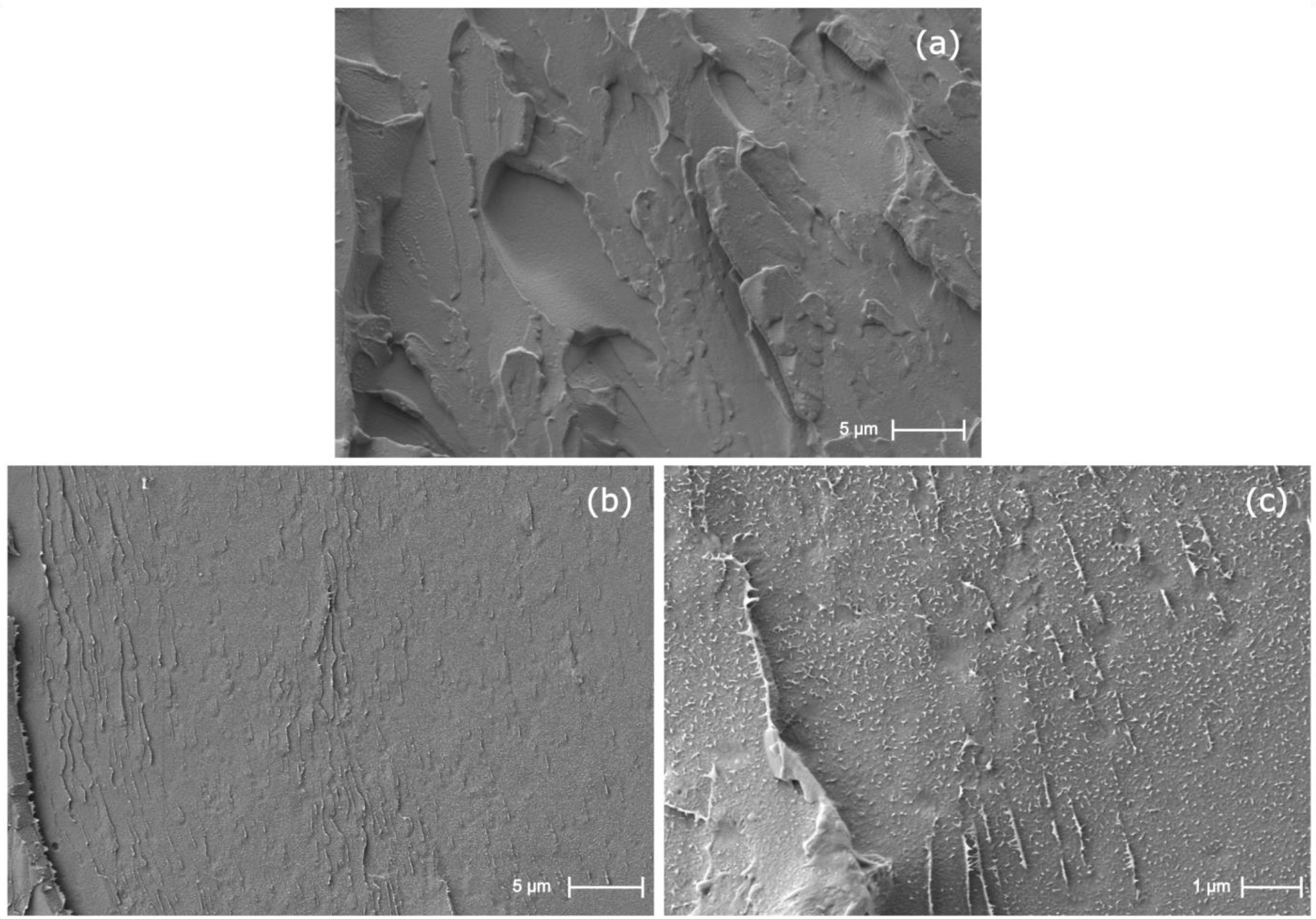
3.1. Miscibility between PET and PBS and Microstructural Characterization
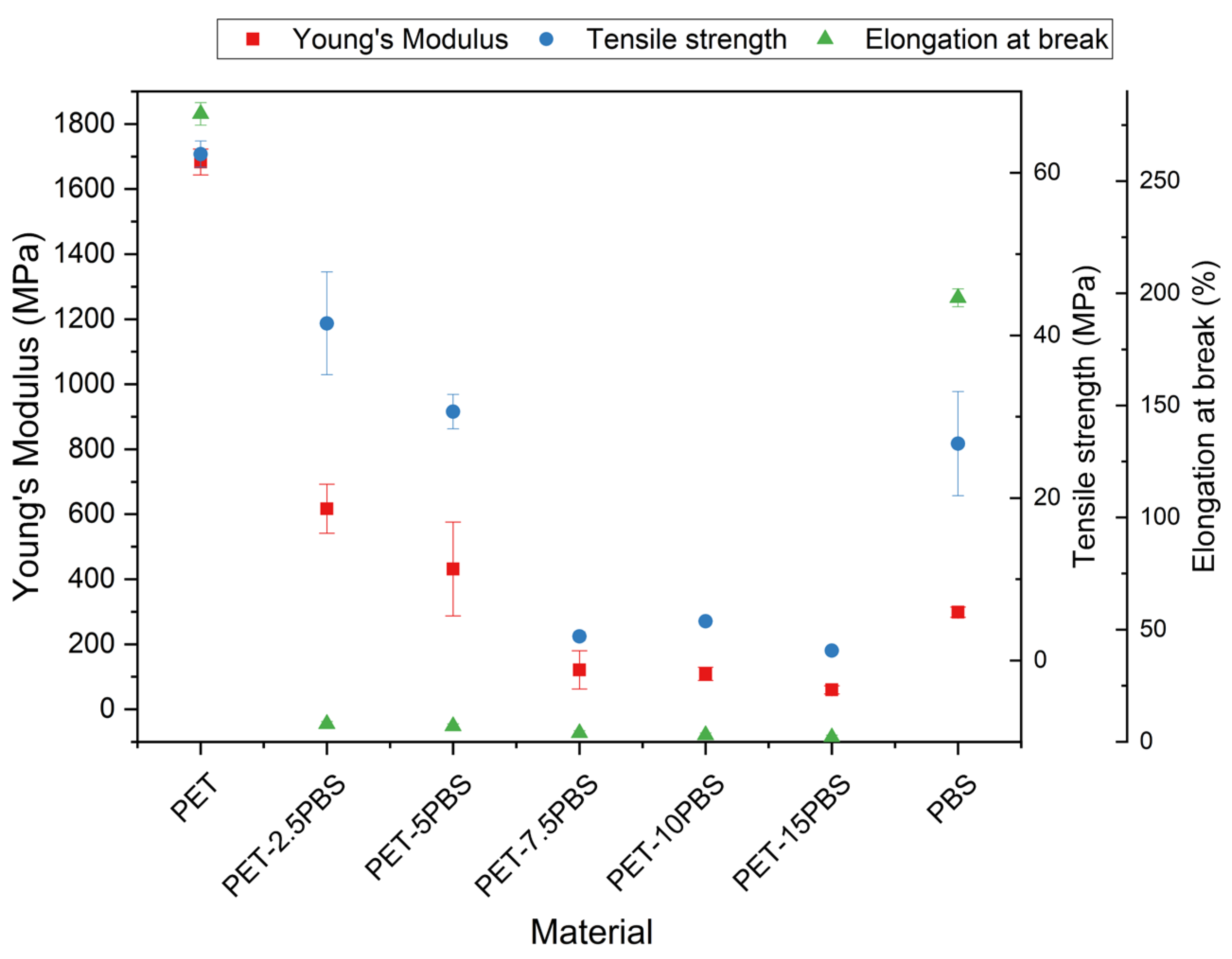
3.2. Mechanical Characterization
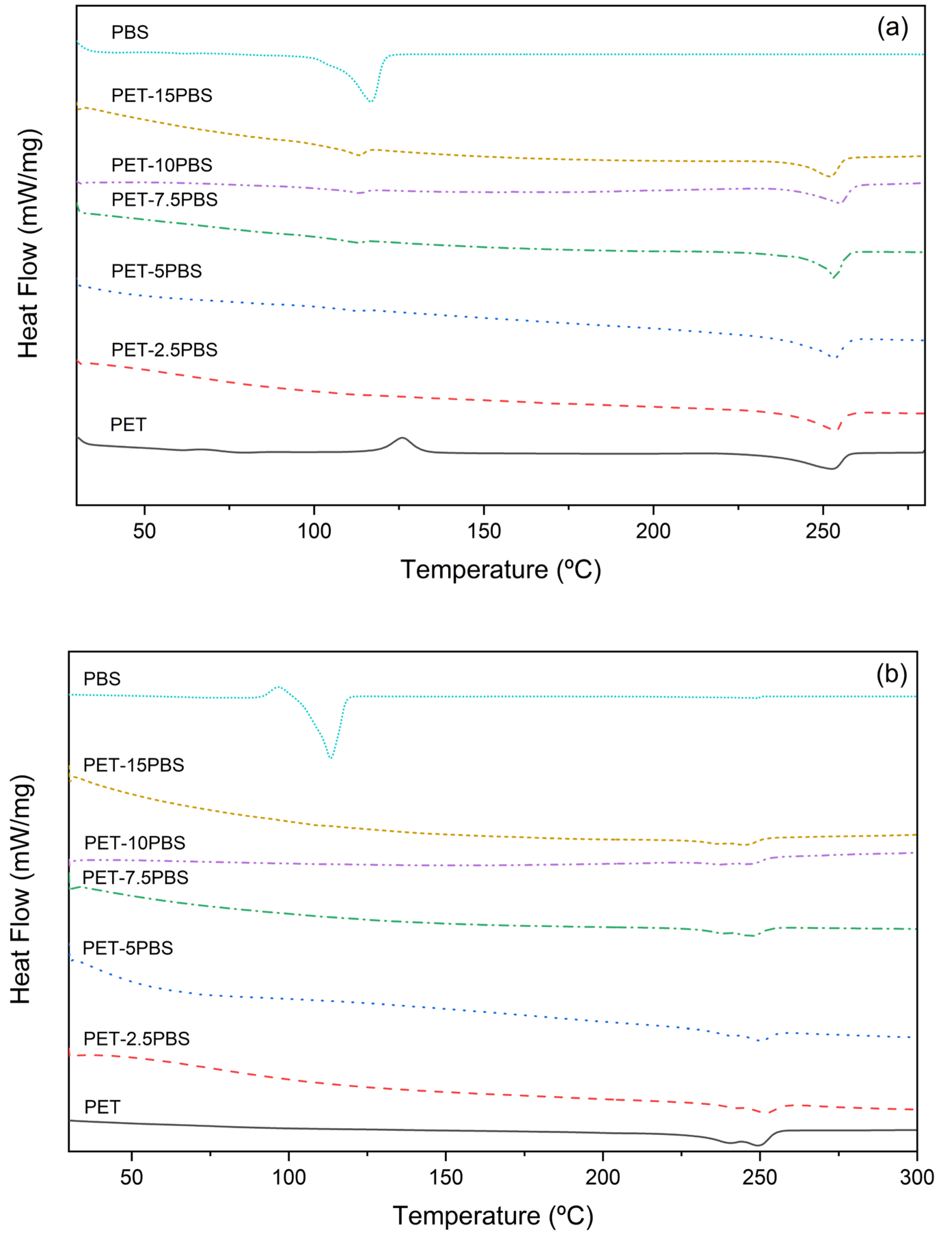
3.3. Thermal Characterization
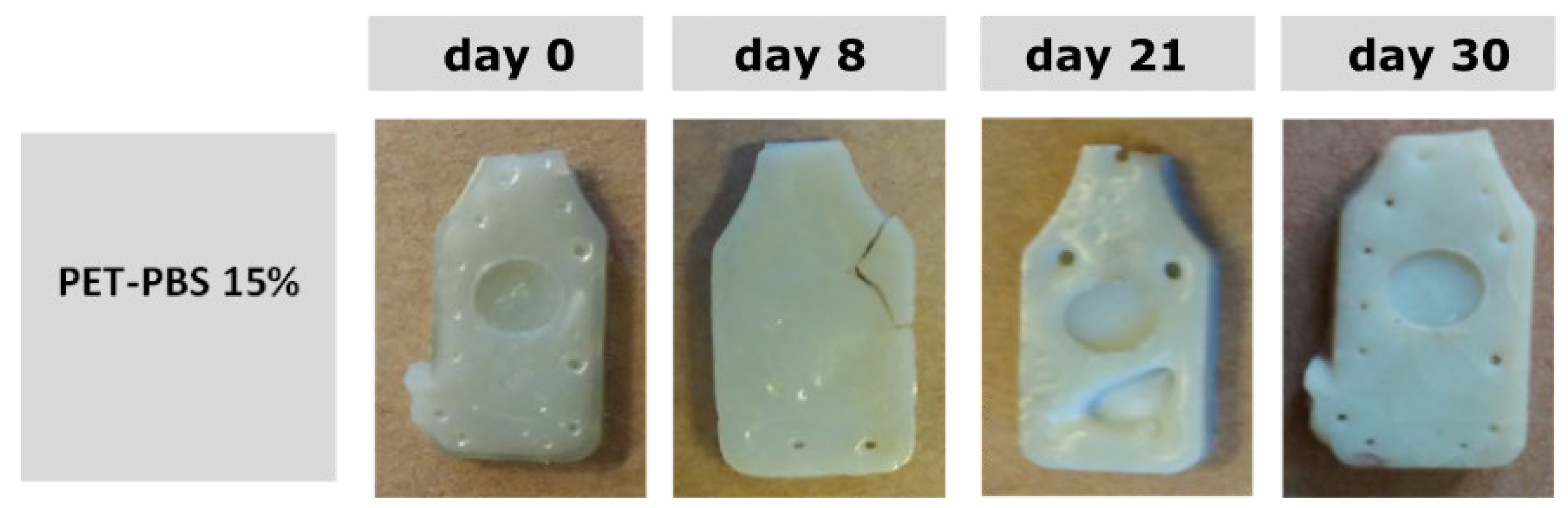
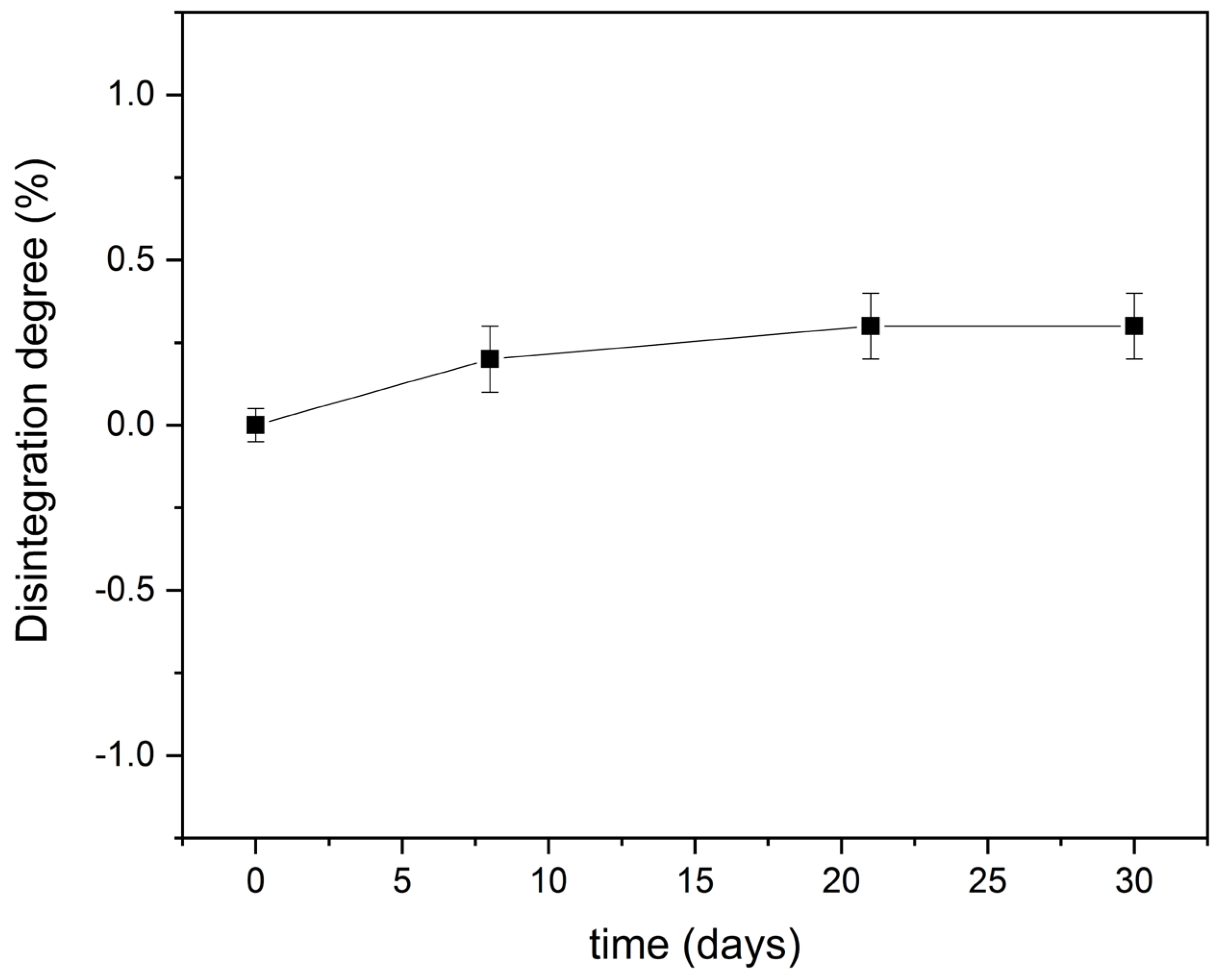
3.4. Disintegration under Composting Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janczak, K.; Hrynkiewicz, K.; Znajewska, Z.; Dąbrowska, G. Use of Rhizosphere Microorganisms in the Biodegradation of PLA and PET Polymers in Compost Soil. Int. Biodeterior. Biodegrad. 2018, 130, 65–75. [Google Scholar] [CrossRef]
- Montava-Jorda, S.; Lascano, D.; Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Martinez-Sanz, A.V.; Ferrandiz-Bou, S.; Torres-Giner, S. Mechanical Recycling of Partially Bio-Based and Recycled Polyethylene Terephthalate Blends by Reactive Extrusion with Poly(Styrene-Co-Glycidyl Methacrylate). Polymers 2020, 12, 174. [Google Scholar] [CrossRef]
- Welle, F. Twenty Years of PET Bottle to Bottle Recycling—An Overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Silva Spinacé, M.A.; de Paoli, M.A. Characterization of Poly(Ethylene Terephthalate) after Multiple Processing Cycles. J. Appl. Polym. Sci. 2001, 80, 20–25. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Wongnarat, C.; Intolo, W.; Suato, S.; Kulsetthanchalee, C. Effect of TiO2 and ZnO on Thin Film Properties of PET/PBS Blend for Food Packaging Applications. Energy Procedia 2014, 56, 102–111. [Google Scholar] [CrossRef]
- Plastics Europe Market Research Group (PEMRG). Plastics-The Facts 2022, An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Dorigato, A. Recycling of Polymer Blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Greene, J.P. Bio-Based and Biodegradable Plastics. In Automot. Plast. Compos: Mat Proc; William Andrew Publishing: Cambridge, MA, USA, 2021; pp. 149–174. [Google Scholar] [CrossRef]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(Butylene Succinate) (PBS) Bioplastic and Biofuel Co-Products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Samper, M.D.; Arrieta, M.P.; Ferrándiz, S.; López-Martínez, J. Influence of Biodegradable Materials in the Recycled Polystyrene. J. Appl. Polym. Sci. 2014, 131, 41161. [Google Scholar] [CrossRef]
- Aldas, M.; Pavon, C.; de La Rosa-Ramírez, H.; Ferri, J.M.; Bertomeu, D.; Samper, M.D.; López-Martínez, J. The Impact of Biodegradable Plastics in the Properties of Recycled Polyethylene Terephthalate. J. Polym. Environ. 2021, 29, 2686–2700. [Google Scholar] [CrossRef]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J.; Bartomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S. Can Biodegradable Plastics Solve Plastic Solid Waste Accumulation? Plast. Energy Fuel Chem. Sustain. Implic. 2019, 403–423. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 2 February 2022).
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-Based Monomers and Polymers Using Microbes for a Sustainable Bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef]
- Liminana, P.; Quiles-Carrillo, L.; Boronat, T.; Balart, R.; Montanes, N. The Effect of Varying Almond Shell Flour (ASF) Loading in Composites with Poly(Butylene Succinate (PBS) Matrix Compatibilized with Maleinized Linseed Oil (MLO). Materials 2018, 11, 2179. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Arevalo, J.; Gomez-Caturla, J.; Garcia-Garcia, D.; Torres-Giner, S. Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(Butylene Succinate) and Its Compatibilizing Effect on Poly(Butylene Succinate)/Pistachio Shell Flour Composites. Molecules 2021, 26, 5927. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Ferri, J.M.; Bertomeu, D.; Pawlak, F.; Samper, M.D. Identification of Biodegradable Polymers as Contaminants in the Thermoplastics Recycling Process. Dyna 2021, 96, 415–421. [Google Scholar] [CrossRef]
- Odelius, K.; Ohlson, M.; Höglund, A.; Albertsson, A.C. Polyesters with Small Structural Variations Improve the Mechanical Properties of Polylactide. J. Appl. Polym. Sci. 2013, 127, 27–33. [Google Scholar] [CrossRef]
- Carraher, C.E. Polymer Chemistry, 6th ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; ISBN 0824708067. [Google Scholar]
- ISO 527-1:2012; Plastics—Determination of Tensile Properties—Part 1: General Principles 2012. International Standards Organization: Geneva, Switzerland, 2012.
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Standards Organization: Geneva, Switzerland, 2003.
- Hongsriphan, N.; Dangmanee, P.; Mankrut, M.; Jantaraka, W. Mechanical and Thermal Properties of Eucalyptus Fiber Composites from Blend between Biodegradable Poly(Butylene Succinate) and Recycled PET. J. Met. Mater. Miner. 2017, 27, 13–23. [Google Scholar]
- ISO 20200; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. International Standards Organization: Geneva, Switzerland, 2016.
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under Composting Conditions of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Santulli, C.; Sarasini, F.; Kenny, J.M. Biodegradation of Phormium Tenax/Poly(Lactic Acid) Composites. J. Appl. Polym. Sci. 2012, 125, 562–572. [Google Scholar] [CrossRef]
- Chemical Retrieval on the Web (CROW) Plastic Library. Available online: https://polymerdatabase.com/polymer%20classes/Intro.html (accessed on 3 August 2022).
- Arribada, R.G.; Behar-cohen, F.; de Barros, A.L.B.; Silva-cunha, A. The Use of Polymer Blends in the Treatment of Ocular Diseases. Pharmaceutics 2022, 14, 1431. [Google Scholar] [CrossRef] [PubMed]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Thermal Degradation Mechanism of Poly(Ethylene Succinate) and Poly(Butylene Succinate): Comparative Study. Thermochim. Acta 2005, 435, 142–150. [Google Scholar] [CrossRef]
- Aldas, M.; Pavon, C.; Ferri, J.M.; Arrieta, M.P.; López-Martínez, J. Films Based on Mater-Bi® Compatibilized with Pine Resin Derivatives: Optical, Barrier, and Disintegration Properties. Polymers 2021, 13, 1506. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. Handb. Environ. Chem. 2019, 78, 71–92. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Benito-Kaesbach, A.; Sánchez-García, N.; Sanz-Lázaro, C. Degradation of Conventional and Biobased Plastics in Soil under Contrasting Environmental Conditions. Sci. Total Environ. 2021, 787, 147678. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic Degradation and Its Environmental Implications with Special Reference to Poly(Ethylene Terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend under Soil Conditions. Polym. Test 2013, 32, 918–926. [Google Scholar] [CrossRef]

| Group | F (cal1/2 cm3/2/mol) |
|---|---|
| -CH3 | 214 |
| -CH2- | 133 |
| -CH< | 28 |
| >C< | −93 |
| -OH | 83 |
| -O- | 70 |
| -H (variable) | 80–100 |
| >C=O | 275 |
| Polymer | Structure | δ(MPa1/2) Calculated | δ(MPa1/2) Available Data [29] |
|---|---|---|---|
| PET |  | 24.3 | 17.8–24.8 |
| PBS | 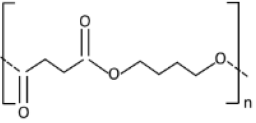 | 22.3 | 20.9 |
| Sample | Impact Strength (kJ/m2) | Hardness (Shore D) |
|---|---|---|
| PET | 19 ± 2 | 81 ± 1 |
| PET-2.5PBS | 12 ± 1 | 76 ± 1 |
| PET-5PBS | 10 ± 1 | 77 ± 1 |
| PET-7.5PBS | 2 ± 1 | 78 ± 1 |
| PET-10PBS | 2 ± 1 | 78 ± 1 |
| PET-15PBS | 2 ± 1 | 79 ± 1 |
| PBS | 11 ± 2 | 63 ± 1 |
| Sample | PBS Weight Content (%) | Tg (°C) ** | Tcc (°C) ** | Tm (°C) | ΔHm (J g −1) | ꭕc (%) |
|---|---|---|---|---|---|---|
| PET | 0 | 72.4 ± 0.2 | 125.7 ± 0.3 | 250.1 ± 0.2 | −36.6 ± 1.0 | 26.1 ± 1.0 |
| PET-2.5PBS | 2.5 | - | - | 250.6 ± 0.5 | −22.2 ± 1.3 | 16.3 ± 1.0 |
| PET-5PBS | 5 | - | - | 250.7 ± 0.4 | −22.8 ± 1.0 | 17.1 ± 1.0 |
| PET-7.5PBS | 7.5 | - | - | 247.1 ± 0.8 | −23.1 ± 1.6 | 17.8 ± 1.0 |
| PET-10PBS | 10 | - | - | 247.0 ± 1.0 | −20.9 ± 1.5 | 16.6 ± 1.0 |
| PET-15PBS | 15 | - | - | 245.4 ± 1.3 | −20.8 ± 1.2 | 17.5 ± 1.0 |
| PBS | 100 | −44 * | - | 112 ± 0.2 | −42.4 ± 1.0 | 30.3 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavon, C.; Aldas, M.; Bertomeu, D.; de la Rosa-Ramírez, H.; Samper, M.D.; López-Martínez, J. Influence of the Presence of Poly(butylene succinate) in the Poly(ethylene terephthalate) Recycling Process. Clean Technol. 2023, 5, 190-202. https://doi.org/10.3390/cleantechnol5010011
Pavon C, Aldas M, Bertomeu D, de la Rosa-Ramírez H, Samper MD, López-Martínez J. Influence of the Presence of Poly(butylene succinate) in the Poly(ethylene terephthalate) Recycling Process. Clean Technologies. 2023; 5(1):190-202. https://doi.org/10.3390/cleantechnol5010011
Chicago/Turabian StylePavon, Cristina, Miguel Aldas, David Bertomeu, Harrison de la Rosa-Ramírez, María Dolores Samper, and Juan López-Martínez. 2023. "Influence of the Presence of Poly(butylene succinate) in the Poly(ethylene terephthalate) Recycling Process" Clean Technologies 5, no. 1: 190-202. https://doi.org/10.3390/cleantechnol5010011
APA StylePavon, C., Aldas, M., Bertomeu, D., de la Rosa-Ramírez, H., Samper, M. D., & López-Martínez, J. (2023). Influence of the Presence of Poly(butylene succinate) in the Poly(ethylene terephthalate) Recycling Process. Clean Technologies, 5(1), 190-202. https://doi.org/10.3390/cleantechnol5010011








