Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site of Real Samples
2.2. Synthetic Samples
2.3. Membrane Crossflow System Setup
2.4. Commercial Membranes
2.5. Experimental Procedure
3. Results and Discussion
3.1. Effect of the Metals’ Feed Concentration on the Membrane Performance
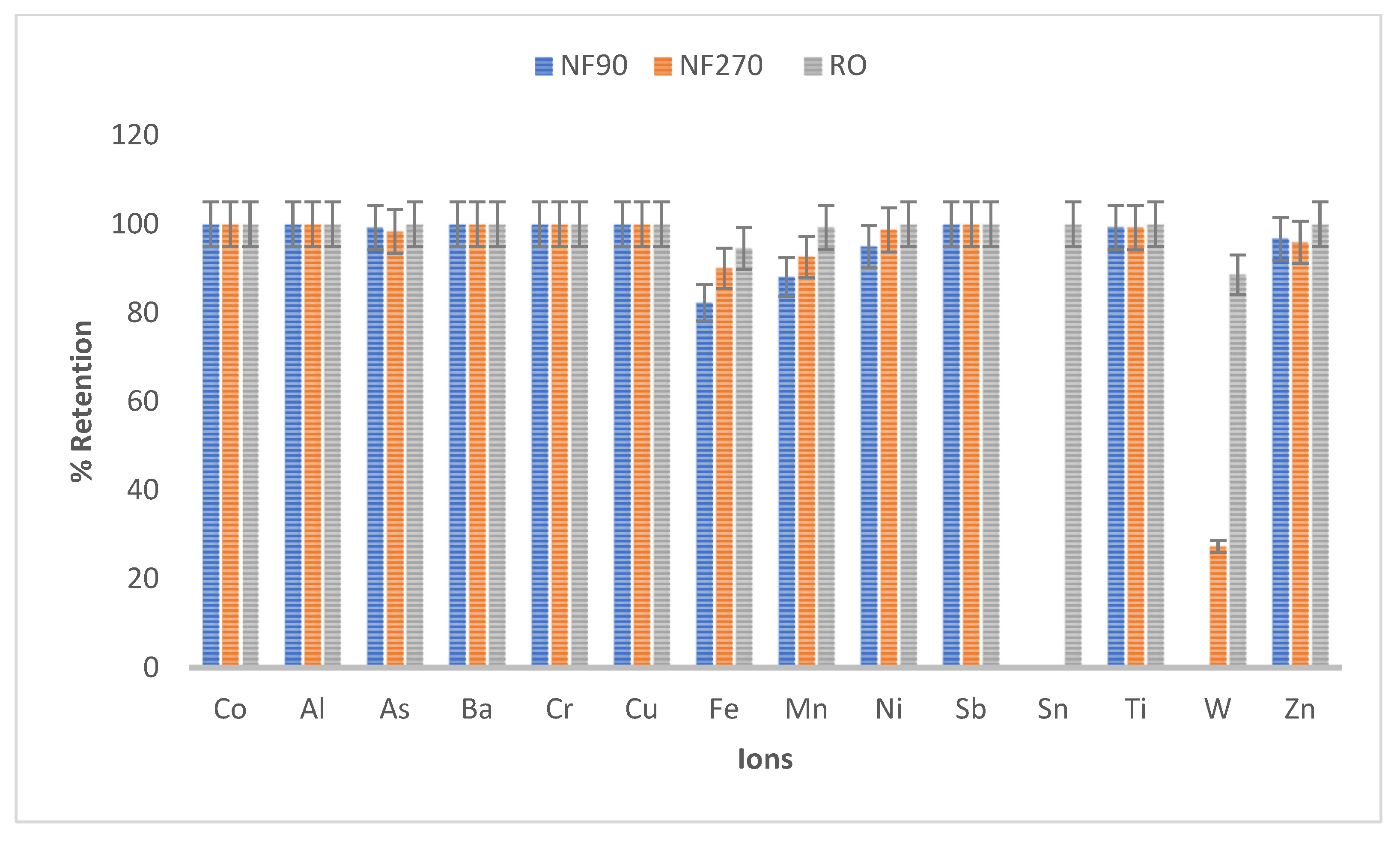
3.2. Ion Removal in Real Waters
3.3. Comparison of Real and Synthetic Water Retention
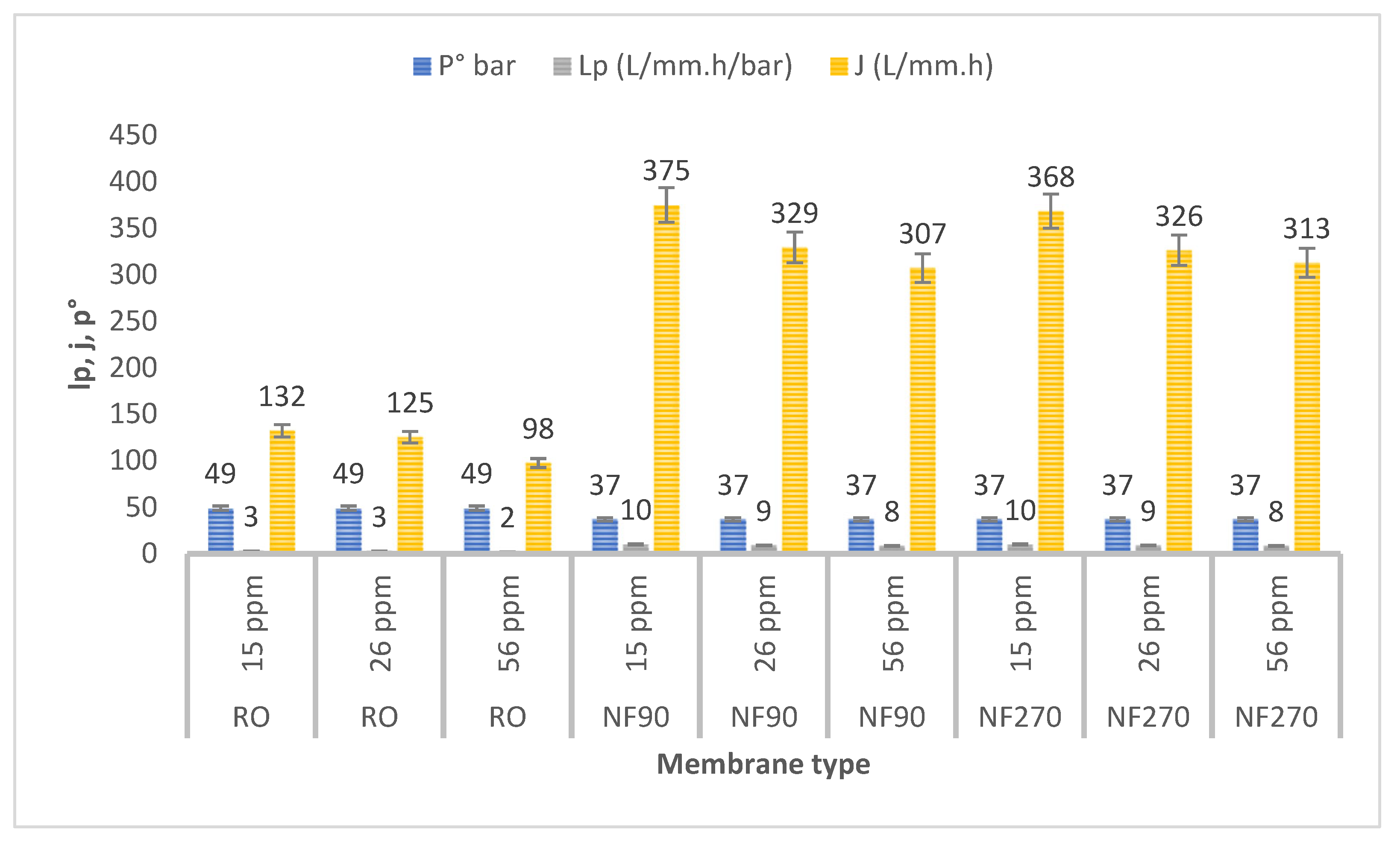
3.4. Transmembrane Flux
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Conc | Concentration |
| DRC | Democratic Republic of Congo |
| FO | Forward osmosis |
| ICP-MS | Inductively coupled plasma mass spectrometer |
| ICP-OES | inductively coupled plasma optical emission spectroscopy |
| Init | Initial |
| MF | Microfiltration |
| MMM | Mixed-matrix membranes |
| Na2EDTA | Disodium salt of ethylenediaminetetraacetic acid |
| NA | not available |
| NF | Nanofiltration |
| ND | No data |
| PA | Polyamide |
| RO | Reverse osmosis |
| SWRO | Seawater reverse osmosis |
| TFC | Thin-film composite |
| UF | Ultrafiltration |
| WHO | World Health Organization |
References
- Naccari, C.; Cicero, N.; Ferrantelli, V.; Giangrosso, G.; Vella, A.; Macaluso, A.; Naccari, F.; Dugo, G. Toxic Metals in Pelagic, Benthic and Demersal Fish Species from Mediterranean FAO Zone 37. Bull. Environ. Contam. Toxicol. 2015, 95, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Bua, G.D.; Licata, P.; Cicero, N.; Dugo, G. Trace elements in Thunnus thynnus from Mediterranean Sea and benefit–risk assessment for consumers. Food Addit. Contam. Part B Surveill. 2015, 8, 175–818. [Google Scholar] [CrossRef] [PubMed]
- Salvo, A.; Potortì, A.G.; Cicero, N.; Bruno, M.; Turco, V.L.; Di Bella, G.; Dugo, G. Statistical characterisation of heavy metal contents in Paracentrotus lividus from Mediterranean Sea. Nat. Prod. Res. 2013, 28, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Turco, V.L.; Di Bella, G.; Furci, P.; Cicero, N.; Pollicino, G.; Dugo, G. Heavy metals content by ICP-OES in Sarda sarda, Sardinella aurita and Lepidopus caudatus from the Strait of Messina (Sicily, Italy). Nat. Prod. Res. 2013, 27, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ziccarelli, I.; Espro, C.; Galvagno, S.; Giofré, S.V.; Romeo, R.; Cicero, N.; Bua, G.D.; Lanza, G.; et al. Removal of heavy metal ions from wastewaters using dendrimer-functionalized multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2017, 24, 14735–14747. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Salvo, A.; Cicero, N.; Vadalà, R.; Mottese, A.F.; Bua, D.; Mallamace, D.; Giannetto, C.; Dugo, G. Toxic and essential metals determination in commercial seafood: Paracentrotus lividus by ICP-MS. Nat. Prod. Res. 2015, 30, 657–664. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Somasundaran, P. Encyclopedia of Surface and Colloid Science, 2004 Update Supplement, 1st ed.; CRC Press: London, UK, 2014. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Chaudhary, H.J. Microbial biotechnology as an emerging industrial wastewater treatment process for arsenic mitigation: A critical review. J. Clean. Prod. 2017, 151, 427–438. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Chen, W.; Mo, J.; Du, X.; Zhang, Z.; Zhang, W. Biomimetic dynamic membrane for aquatic dye removal. Water Res. 2018, 151, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Ali, I. Chapter 1. In Environmental Water; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.elsevier.com/books/environmental-water/gupta/978-0-444-59399-3 (accessed on 9 March 2021).
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Lasat, M.M. Phytoextraction of Metals from Contaminated Soil: A Review of Plant/Soil/Metal Interaction and Assessment of Pertinent Agronomic Issues. J. Hazard. Subst. Res. 1999, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Estrella, L.R.; Guevara-Garcia, A.A. Heavy metal adaptation. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Mohod, C.V.; Dhote, J. Review of heavy metals in drinking water and their effect on human health. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2992–2996. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C. Efficient removal of heavy metal ions based on the optimized dissolution-diffusion-flow forward osmosis process. Chem. Eng. J. 2018, 334, 1128–1134. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Drochner, W.; Steingass, H.; Islam, K.M.S. Nutritive evaluation of some tree leaves from Bangladesh for feeding ruminant animals. Indian J. Anim. Sci. 2008, 78, 1273–1277. [Google Scholar]
- Noori, R.; Farahani, F.; Jun, C.; Aradpour, S.; Bateni, S.M.; Ghazban, F.; Hosseinzadeh, M.; Maghrebi, M.; Naseh, M.R.V.; Abolfathi, S. A non-threshold model to estimate carcinogenic risk of nitrate-nitrite in drinking water. J. Clean. Prod. 2022, 363, 132432. [Google Scholar] [CrossRef]
- Nsavyimana, G. Modélisation des Processus Physiques et Biologiques dans des Fosses Septiques et Voies de Valorisation des Boues de Vidange: Application à Bujumbura-Burundi. Ph.D. Thesis, Université de Liège, Liege, Belgium, 2015. Available online: https://orbi.uliege.be/bitstream/2268/174036/1/Th%C3%A8se%20Gaston%20NSAVYIMANA_PDF.pdf (accessed on 15 April 2017).
- OMS. Eau, Assainissement et Hygiène Directives de Qualité de l’eau de Boisson: Quatrième Édition Intégrant le Premier Additif (par Chapitre). Available online: https://apps.who.int/iris/bitstream/handle/10665/258887/9789242549959-fre.pdf;jsessionid=CF724E272D2B8A73F855C7F9DF87216B?sequence=1 (accessed on 20 November 2019).
- Barbooti, M.M.; Altameemi, A.; Najah, M. Removal of Heavy Metals Using Chemicals Precipitation. Eng. Tech. J. 2011, 29, 595–612. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, M.; Walters, T.J.; Ainscough, P.M.; Williams, A.W.; Mohammad, N. Hilal Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Ejraei, A.; Aroon, M.A.; Saravani, A.Z. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Hamid, M.; Abdullah, N.; Yusof, N.; Ismail, N.; Ismail, A.; Salleh, W.; Jaafar, J.; Aziz, F.; Lau, W. Effects of surface charge of thin-film composite membrane on copper (II) ion removal by using nanofiltration and forward osmosis process. J. Water Process Eng. 2020, 33, 101032. [Google Scholar] [CrossRef]
- Boussouga, Y.-A.; Frey, H.; Schäfer, A.I. Removal of arsenic(V) by nanofiltration: Impact of water salinity, pH and organic matter. J. Membr. Sci. 2020, 618, 118631. [Google Scholar] [CrossRef]
- Kang, M.; Kawasaki, M.; Tamada, S.; Kamei, T.; Magara, Y. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 2000, 131, 293–298. [Google Scholar] [CrossRef]
- Abu Qdais, H.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Ipek, U. Removal of Ni(II) and Zn(II) from an aqueous solutionby reverse osmosis. Desalination 2005, 174, 161–169. [Google Scholar] [CrossRef]
- Jekel, M.; Amy, G.L. Interface Science in Drinking Water Treatment; Newcombe, G., Dixon, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Maher, A.; Sadeghi, M.; Moheb, A. Heavy metal elimination from drinking water using nanofiltration membrane technology and process optimization using response surface methodology. Desalination 2014, 352, 166–173. [Google Scholar] [CrossRef]
- Basaran, G.; Kavak, D.; Dizge, N.; Aşçı, Y.; Şölener, M.; Ozbey, B.; Asci, Y. Comparative study of the removal of nickel(II) and chromium(VI) heavy metals from metal plating wastewater by two nanofiltration membranes. Desalination Water Treat. 2015, 57, 21870–21880. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Mikulášek, P. Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 2014, 343, 67–74. [Google Scholar] [CrossRef]
- Algureiri, A.H.; Abdulmajeed, Y.R. Removal of Heavy Metals from Industrial Wastewater by Using RO Membrane. Iraqi J. Chem. Pet. Eng. 2016, 17, 125–136. [Google Scholar]
- ISO 17294-1; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines. ISO: Geneva, Switzerland, 2004.
- ISO 17294-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2004.
- Luis, P. Chapter 1-Introduction. In Fundamental Modelling of Membrane Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Bruggen, B. Chapter 2-Microfiltration, Ultrafiltration, Nanofiltration, Reverse Osmosis, and Forward Osmosis. In Fundamental Modelling of Membrane Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–70. ISBN 9780128134832. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Pauer, V.; Mizsey, P. Recovery of copper from process waters by nanofiltration and reverse osmosis. Desalination 2009, 240, 132–142. [Google Scholar] [CrossRef]
- Chan, B.; Dudeney, A. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates. Miner. Eng. 2008, 21, 272–278. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Chaudhari, L.B. Separation of binary heavy metals from aqueous solutions by nanofiltration and cha-racterization of the membrane using SpieglereKedem model. Chem. Eng. J. 2009, 150, 181–187. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2002, 122, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Brandhuber, P.; Amy, G. Alternative methods for membrane filtration of arsenic from drinking water. Desalination 1998, 117, 1–10. [Google Scholar] [CrossRef]
- Seidel, A.; Waypa, J.J.; Elimelech, M. Role of Charge (Donnan) Exclusion in Removal of Arsenic from Water by a Negatively Charged Porous Nanofiltration Membrane. Environ. Eng. Sci. 2001, 18, 105–113. [Google Scholar] [CrossRef]
- Oh, J.-I.; Lee, S.-H.; Yamamoto, K. Relationship between molar volume and rejection of arsenic species in groundwater by low-pressure nanofiltration process. J. Membr. Sci. 2004, 234, 167–175. [Google Scholar] [CrossRef]
- Worou, C.N.; Chen, Z.-L.; Bacharou, T. Arsenic removal from water by nanofiltration membrane: Potentials and limitations. Water Pract. Technol. 2021, 16, 291–319. [Google Scholar] [CrossRef]
- Sato, Y.; Kang, M.; Kamei, T.; Magara, Y. Performance of nanofiltration for arsenic removal. Water Res. 2002, 36, 3371–3377. [Google Scholar] [CrossRef]
- Sen, M.; Manna, A.; Pal, P. Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J. Membr. Sci. 2010, 354, 108–113. [Google Scholar] [CrossRef]
- Abolfathi, S.; Cook, S.; Yeganeh-Bakhtiary, A.; Borzooei, S.; Pearson, J. Microplastics transport and mixing mechanisms in the nearshore region. Coast. Eng. Proc. 2020, 63. [Google Scholar] [CrossRef]
- Shigidi, I.; Anqi, A.E.; Elkhaleefa, A.; Mohamed, A.; Ali, I.H.; Brima, E.I. Temperature Impact on Reverse Osmosis Permeate Flux in the Remediation of Hexavalent Chromium. Water 2021, 14, 44. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Marques, L.S.; Martins, S.S.; Reis, B.G.; Amaral, M.C. Assessment of the chemical stability of nanofiltration and reverse osmosis membranes employed in treatment of acid gold mining effluent. Sep. Purif. Technol. 2017, 174, 301–311. [Google Scholar] [CrossRef]
- Thaçi, B.S.; Gashi, S.T. Reverse Osmosis Removal of Heavy Metals from Wastewater Effluents Using Biowaste Materials Pretreatment. Pol. J. Environ. Stud. 2018, 28, 337–341. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]




| Element | Concentration Normally Found in Surface Water [28] | WHO Guidelines |
|---|---|---|
| Sb | <4 μg/L | 0.02 mg/L |
| As | - | 0.01 mg/L |
| Cd | <1 µg/L | 0.003 mg/L |
| Mn | - | 0.1–0.2 mg/L |
| Cr3+, Cr6+ | <2 µg/L | Chrome total: 0.05 mg/L |
| Cu+2 | - | 2 mg/L |
| Pb | - | 0.01 mg/L |
| Hg | <0.5 µg/L | 0.01 mg/L |
| Ni | <0.02 µg/L | 0.07 mg/L |
| Zn | - | 3 mg/L |
| Elements | Concentration before Filtration (ppm) * | Concentration after Filtration (ppb) * |
|---|---|---|
| Co | <0.05 | 28.13 |
| Al | 0.155 | <1 |
| As | <0.05 | 6.36 |
| Ba | 0.085 | 16.96 |
| Cd | <0.05 | <0.05 |
| Cr | 0.06 | <0.05 |
| Cu | <0.05 | 5.66 |
| Fe | 0.815 | 23 |
| Mn | 0.47 | 337.27 |
| Ni | 0.03 | 6.25 |
| Pb | <0.05 | <0.05 |
| Sb | <0.05 | 1.57 |
| Sn | <0.05 | <0.05 |
| Ti | <0.05 | 26.33 |
| W | <0.05 | 5.27 |
| Zn | <0.05 | 184.26 |
| Ca | 40 | 47,390 |
| K | 66.5 | 53,920 |
| Mg | 15 | 16,900 |
| Na | 78 | 930 |
| Si | 16 | 18,700 |
| Characteristics | RO X-20TM Membrane | NF90 Membrane | NF270 Membrane |
|---|---|---|---|
| Allowable operating pH range | 4–11 Continuous | NA | NA |
| Maximum operating temperature, °C | NA | 45 | 45 |
| Maximum feed turbidity, NTU | 2 | NA | NA |
| Maximum feed SDI, 15 min | 5 | NA | NA |
| Average salt discharge (%) | 99.5 | 97 | |
| Minimum salt discharge (%) | 98.5 | NA | NA |
| Filtration pressure (bar) | NA | 41 | 41 |
| Parameter | Formula | Equation | Ref |
|---|---|---|---|
| Retention (%) where Cp and Cr are the permeate and retentate Mass balance calculation | (C.V)f = (C.V)r + (C.V)p | (1) | Luis et al. (2018) [49] |
| Permeation flux flux (J) (L. m−2.h−1) | where Qp is the permeate flow rate (L h−1), S (0.00664 m2) is the membrane area | (2) | Luis et al. (2018) [49] |
| Driving pressure DP Δπ is the osmotic pressure gradient (bar) C is the total concentration of ions (mol L−1) R = 0.082 L atm K−1 mol−1 T (K) | DP = ∆P − ∆π with n as the total number of moles of ions | (3) | Van der Bruggen et al. (2018) [50] |
| Membrane Permeability Lp is the solvent (water) permeability (L m−2 h−1 bar−1) | (4) | Luis et al. (2018) [49] |
| Ions Conc (ppb) | |||
|---|---|---|---|
| Elements | NF90 | NF270 | RO |
| Co | 0 | 0 | 0 |
| Al | 0 | 0 | 0.00 |
| As | 0.30 | 0.36 | 0.00 |
| Ba | 0 | 0 | 0 |
| Cd | <0.05 | <0.05 | <0.05 |
| Cr | 0 | 0 | 0 |
| Cu | 0 | 0 | 0 |
| Fe | 64.66 | 17.96 | 6.54 |
| Mn | 78.10 | 90.70 | 0 |
| Ni | 1.30 | 0.91 | 0 |
| Pb | <0.05 | <0.05 | 0 |
| Sb | 0 | 0 | 0 |
| Sn | <0.05 | <0.05 | 0 |
| Ti | 0.33 | 0.41 | 0 |
| W | 5.06 | 3.80 | 1.51 |
| Zn | 12.90 | 17.85 | 0.00 |
| Type | Membrane | Element | Initial Conc | Type of Water | Removal Efficiency (%) | Conditions | Conc Permeate | Ref |
|---|---|---|---|---|---|---|---|---|
| NF | NF270 | As (V) | 250 µg/L | Synthetic | 82–88 | Feed pressure of 20 bars | Youssef et al., 2021 [37] | |
| NF90 | 93–98 | |||||||
| RO | ES-10 | As III | 50 µg/L | Drinking water | 75 | 0.75 MPa | ND | Kang et al., 2000 [38] |
| Sb III | 60 | |||||||
| As V | 95 | |||||||
| Sb V | 95 | |||||||
| NTR-729Hf | As III | 20–43 | ||||||
| Sb III | 45.7–60.2 | |||||||
| As V | 80–95 | |||||||
| Sb V | 95 | |||||||
| RO | Polyamide | Cd2+ | 250 ppm | Synthetic | 98.5 | ND | 3.2 ppm | Qdais et al., 2004 [39] |
| NF | 82–97 | 13.16 ppm | ||||||
| RO | Ni2+ | 500 mg/l | 99.5 | Operation pressure 5 atm | 2.01 ppm | Mohsen-Nia et al., 2007 [40] | ||
| Cu II | ||||||||
| As III | <500 µg/L | 20–55 | 3 atm | ND | Chan et al., 2008 [52] | |||
| As V | 91–99 | |||||||
| Ni2+ | 44–169 mg/L | 99.3 | Operational pressure 1100 kPa | Ipek et al., 2005 [41] | ||||
| As III | ND | 99 | ND | ND | Jekel et al., 2006 [42] | |||
| As V | 99 | |||||||
| NF | NF70 | As III | <30 | |||||
| As V | 60–90 | |||||||
| NF | Polyamide | Pb II | 1 ppm | Drinking water | 86 | 0.75 MPa | ND | Maher et al., 2014 [43] |
| Ni II | 93 | |||||||
| Cu II | 98.72 | |||||||
| Pb II | 99.61 | |||||||
| Mn II | 99.31 | |||||||
| Ni II | 99.11 | |||||||
| Zn II | 99.51 | |||||||
| NF | NF90 | Ni II | Wastewater | 99.2 | 10, 20, and 30 bars | ND | Basaran et al., 2015 [44] | |
| Cr VI | 96.5 | |||||||
| NF270 | Ni II | 98.7 | ||||||
| Cr VI | 95.7 | |||||||
| NF | AFC 80 | Pb II | 5–250 mg/L | Synthetic | >98 | 25 bars | <0.05 ppm | Gherasim et al., 2014 [45] |
| Pb II | 15 mg/L | Wastewater | >99 | |||||
| Cd | 5 mg/L | >99 | ||||||
| RO | Ni II | Synthetic | 98.5 | 1 and 4 bars | ND | Algureiri et al., 2016 [46] | ||
| Cu II | 97.5 | |||||||
| Pb II | 96 | |||||||
| NF | Ni II | 50 ppm | 85 | 1, 2, 3 and 4 bars | ||||
| Cu II | 100 ppm | 66 | ||||||
| Pb II | 150–200 ppm | 78 | ||||||
| RO NF | Cu II | 2 g/L | Synthetic | >95 | Operating pressures 35 bar | ND | Cséfalvay et al., 2009 [51] | |
| RO | TFC PA | Co II | 39.4 | Synthetic | 98.6 | 41 bars | ND | Ricci et al., 2017 [63] |
| Ni II | 214.9 | 98.1 | ||||||
| Mg II | 242.9 | 98.6 | ||||||
| RO | Pb II | 0.034 | Real water | <1 ppb | 1.76 MPa | <1 ppb | Thaçi, et al., 2019 [64] | |
| Zn II | 0.153 | <0.002 ppb | <0.002 | |||||
| Cd II | 0.025 | <0.1 ppb | <0.1 ppb | |||||
| Co II | 0.018 | <0.2 ppb | <0.2 ppb | |||||
| Mn II | 1.146 | 99.48 | 0.006 ppb | |||||
| Ni II | 0.004 | <0.5 ppb | <0.5 ppb | |||||
| RO | BW30XFR | Cr VI | 5 | Synthetic | 99.8 | 10, 30, and 45 bars | ND | Shigidi et al., 2022 [62] |
| 30 | 94.3 | |||||||
| 100 | 77.2 | |||||||
| RO | X-20TM | Cr III | 15, 26, 56 ppm | Synthetic | 99 | 50 bars | 0.15–1.02 | This study |
| Ni II | 98–99 | |||||||
| Cd II | 99 | |||||||
| Pb II | 99 | |||||||
| Sb III | 98–99 | |||||||
| As III | 98–99 | |||||||
| NF | NF90 | Cr III | 15, 26, 56 ppm | Synthetic | 95–98 | 38 bars | 0.33–22 | |
| Ni II | 97–99 | |||||||
| Cd II | 89–92 | |||||||
| Pb II | 73–76 | |||||||
| Sb III | 61–64 | |||||||
| As III | 51–54 | |||||||
| NF270 | Cr III | 15, 26, 56 ppm | Synthetic | 98–99 | 38 bars | 0.11–22 | ||
| Ni II | 98 | |||||||
| Cd II | 92–95 | |||||||
| Pb II | 77–82 | |||||||
| Sb III | 61–64 | |||||||
| As III | 48–53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.-D.; Van Der Bruggen, B.; Luis, P. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technol. 2022, 4, 1300-1316. https://doi.org/10.3390/cleantechnol4040080
Lumami Kapepula V, García Alvarez M, Sang Sefidi V, Buleng Njoyim Tamungang E, Ndikumana T, Musibono D-D, Van Der Bruggen B, Luis P. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technologies. 2022; 4(4):1300-1316. https://doi.org/10.3390/cleantechnol4040080
Chicago/Turabian StyleLumami Kapepula, Vercus, Mar García Alvarez, Vida Sang Sefidi, Estella Buleng Njoyim Tamungang, Théophile Ndikumana, Dieu-Donné Musibono, Bart Van Der Bruggen, and Patricia Luis. 2022. "Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo" Clean Technologies 4, no. 4: 1300-1316. https://doi.org/10.3390/cleantechnol4040080
APA StyleLumami Kapepula, V., García Alvarez, M., Sang Sefidi, V., Buleng Njoyim Tamungang, E., Ndikumana, T., Musibono, D.-D., Van Der Bruggen, B., & Luis, P. (2022). Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technologies, 4(4), 1300-1316. https://doi.org/10.3390/cleantechnol4040080









