State of Charge and Capacity Tracking in Vanadium Redox Flow Battery Systems
Abstract
:1. Introduction
2. Materials and Methods
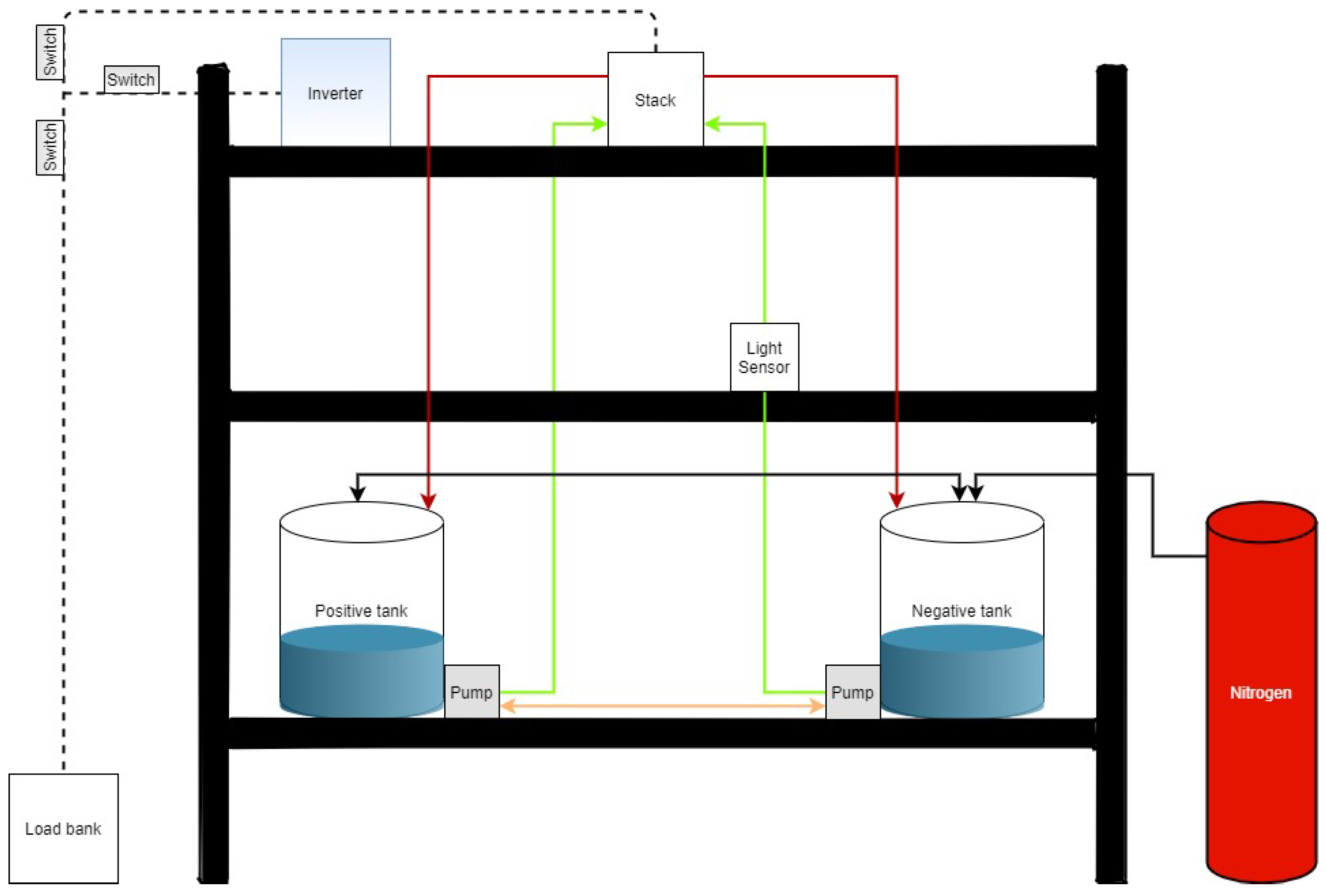
2.1. VRFB System
2.2. Absorbance-Based Monitoring System
3. Discussion
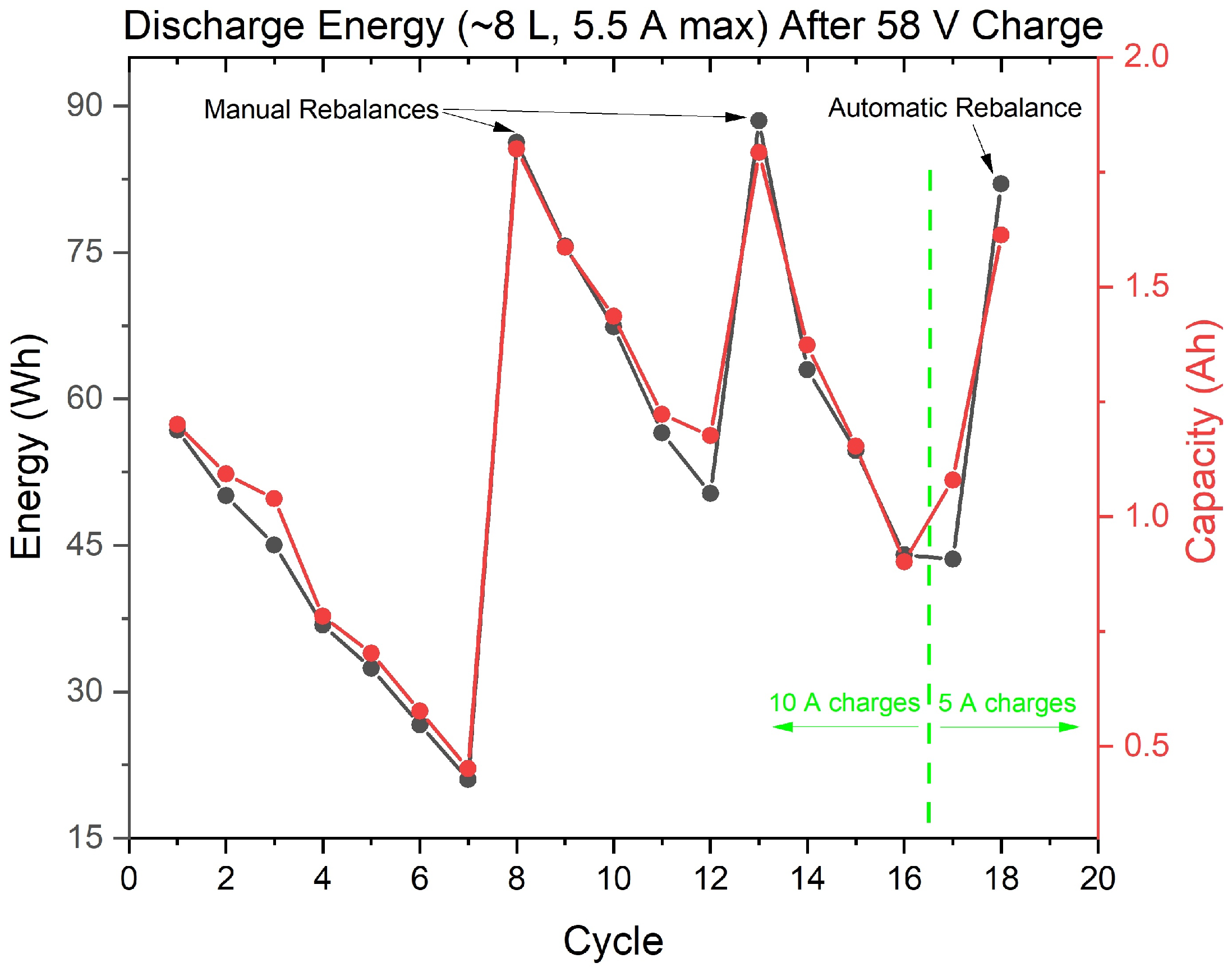
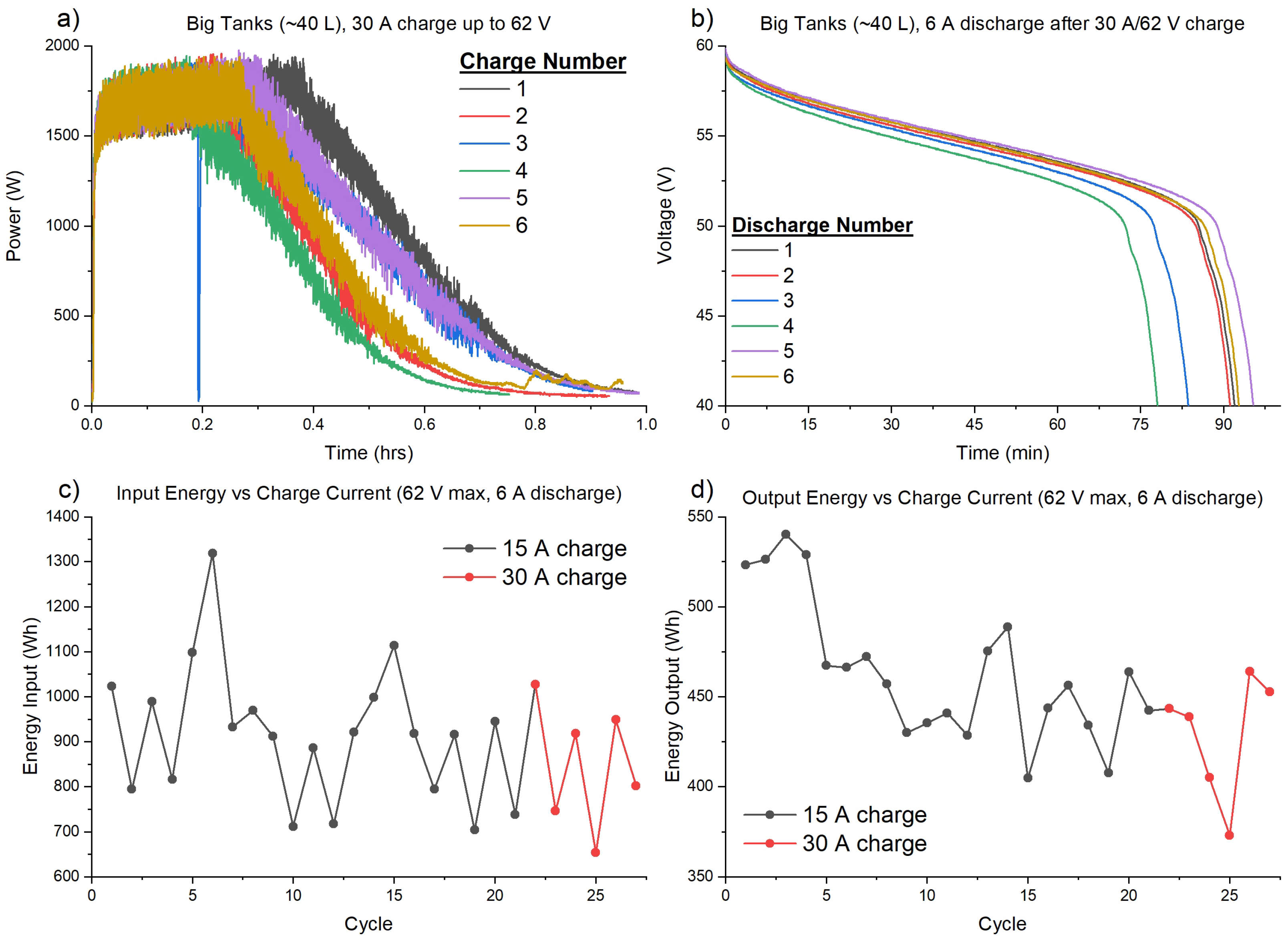
3.1. Capacity Loss Tracking and Mitigation
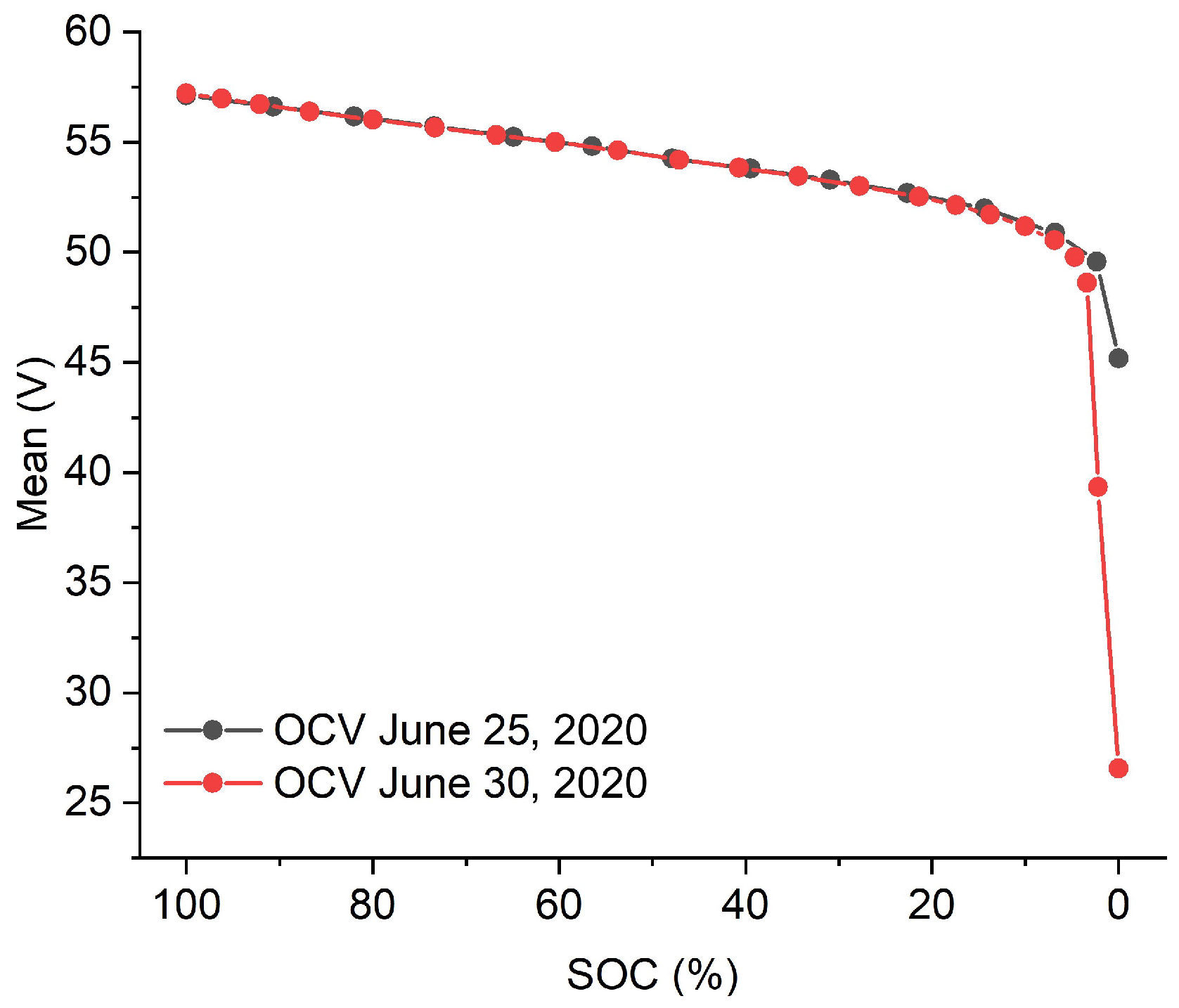
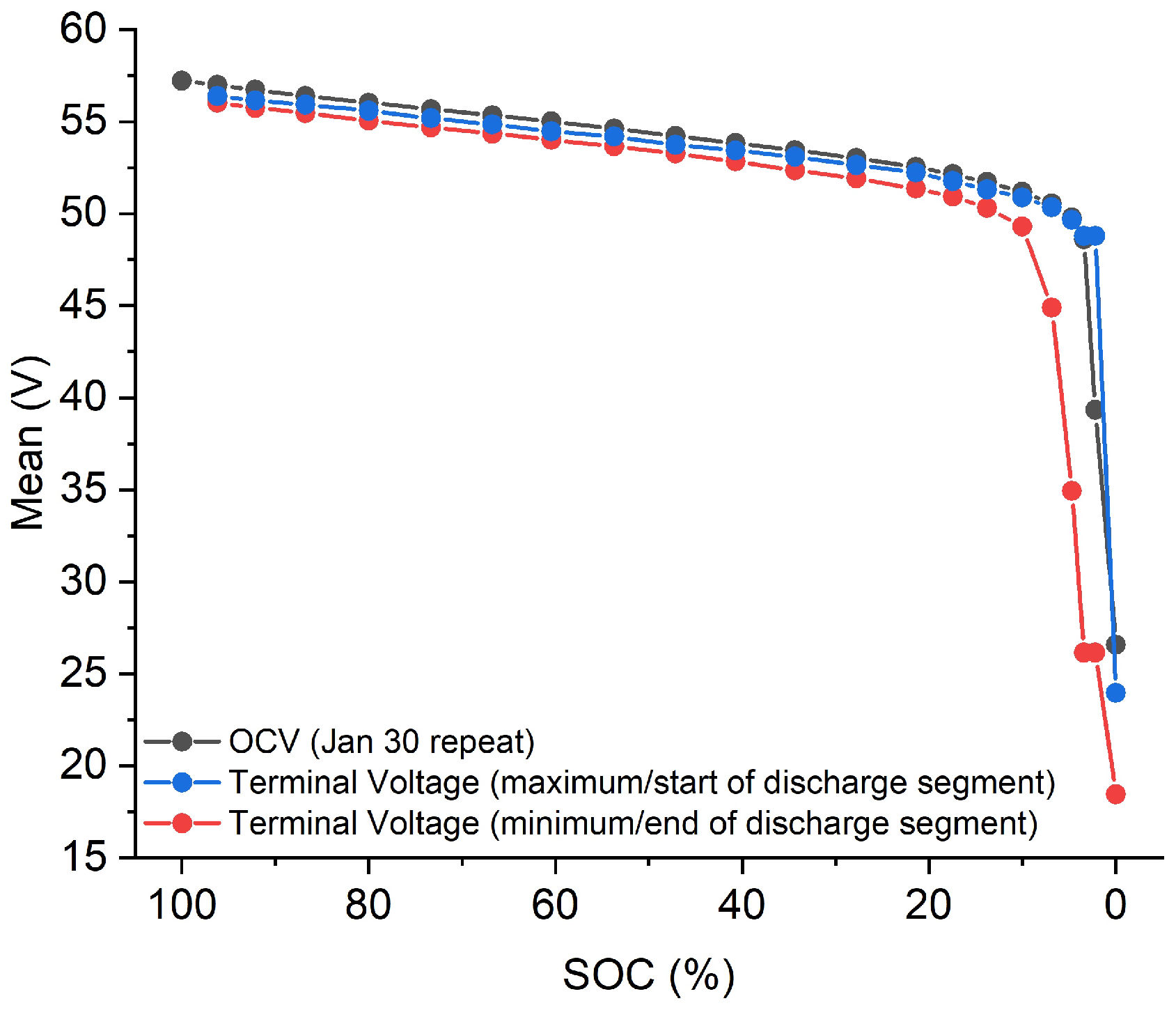
3.2. SOC Tracking via OCV
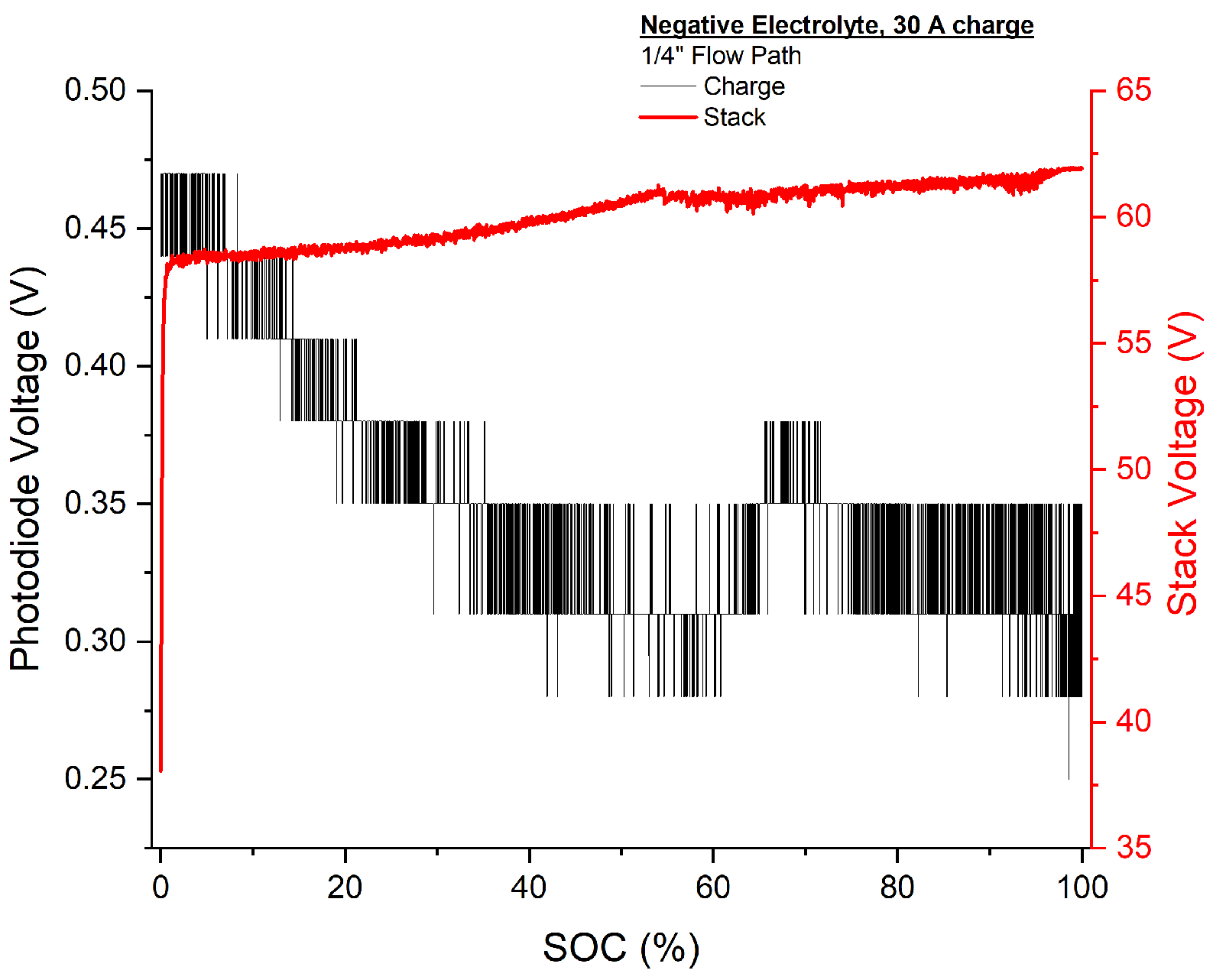
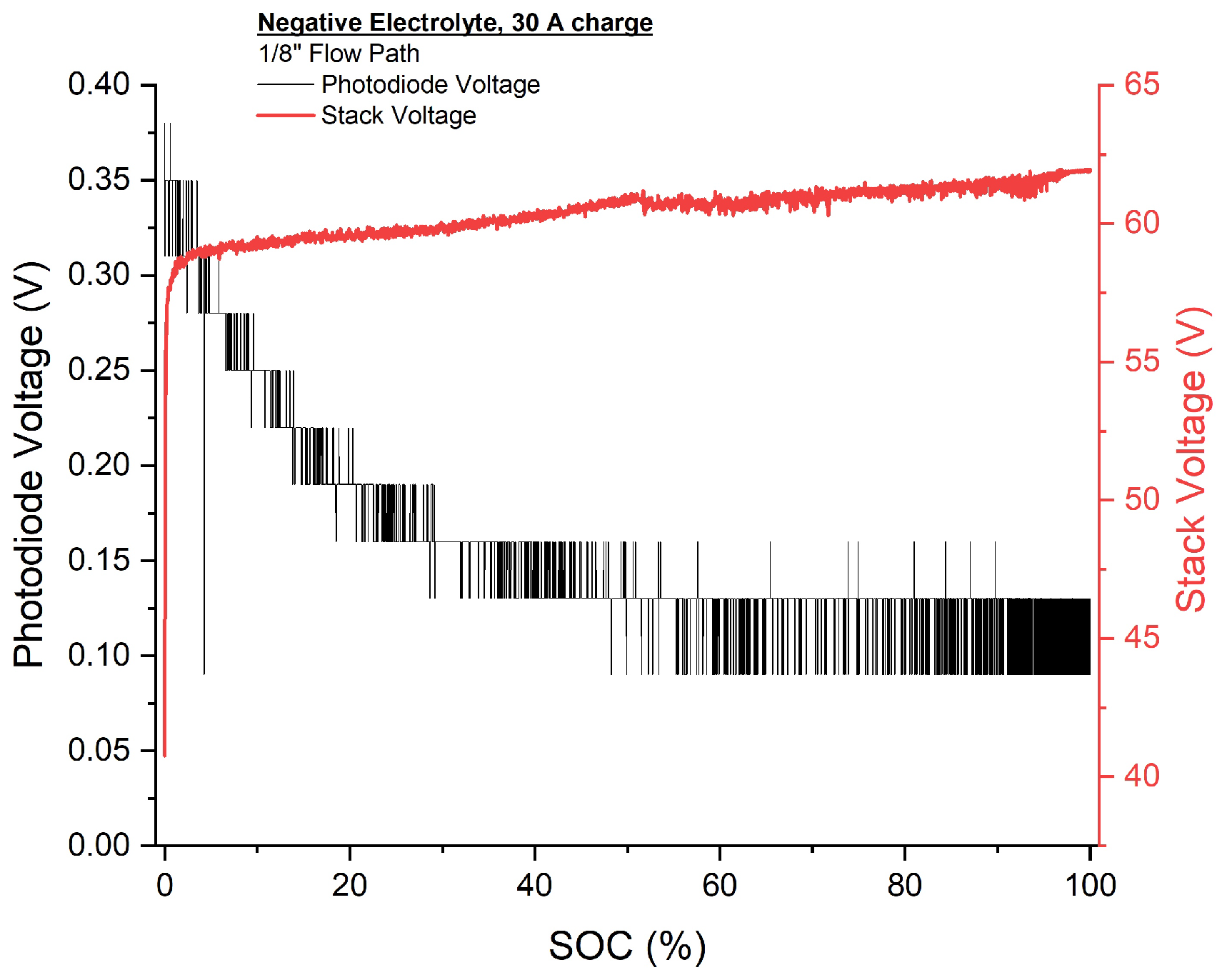
3.3. SOC Tracking via Absorbance
3.3.1. Positive Electrolyte
3.3.2. Negative Electrolyte
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OCV | Open circuit voltage |
| SOC | State of Charge |
| VRFB | Vanadium Redox Flow Battery |
Appendix A

Appendix B

References
- Schavan, A. Germany’s Energy Research Plan. Science 2010, 330, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anvari, M.; Lohmann, G.; Wächter, M.; Milan, P.; Lorenz, E.; Heinemann, D.; Tabar, M.R.R.; Peinke, J. Short term fluctuations of wind and solar power systems. New J. Phys. 2016, 18, 063027. [Google Scholar] [CrossRef]
- Álvaro Cunha; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, M. State of charge monitoring methods for vanadium redox flow battery control. J. Power Sources 2011, 196, 8822–8827. [Google Scholar] [CrossRef]
- Tang, Z.; Aaron, D.; Papandrew, A.; Zawodzinski, T. Monitoring the state of charge of operating vanadium redox flow batteries. Ecs Trans. 2012, 41, 1. [Google Scholar] [CrossRef]
- Haisch, T.; Ji, H.; Holtz, L.; Struckmann, T.; Weidlich, C. Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount. Membranes 2021, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Struckmann, T.; Kuhn, P.; Ressel, S. A combined in situ monitoring approach for half cell state of charge and state of health of vanadium redox flow batteries. Electrochim. Acta 2020, 362, 137174. [Google Scholar] [CrossRef]
- Haisch, T.; Ji, H.; Weidlich, C. Monitoring the state of charge of all-vanadium redox flow batteries to identify crossover of electrolyte. Electrochim. Acta 2020, 336, 135573. [Google Scholar] [CrossRef]
- Ressel, S.; Bill, F.; Holtz, L.; Janshen, N.; Chica, A.; Flower, T.; Weidlich, C.; Struckmann, T. State of charge monitoring of vanadium redox flow batteries using half cell potentials and electrolyte density. J. Power Sources 2018, 378, 776–783. [Google Scholar] [CrossRef]
- Jirabovornwisut, T.; Arpornwichanop, A. A review on the electrolyte imbalance in vanadium redox flow batteries. Int. J. Hydrogen Energy 2019, 44, 24485–24509. [Google Scholar] [CrossRef]
- Bhattarai, A.; Ghimire, P.C.; Whitehead, A.; Schweiss, R.; Scherer, G.G.; Wai, N.; Hng, H.H. Novel Approaches for Solving the Capacity Fade Problem during Operation of a Vanadium Redox Flow Battery. Batteries 2018, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Stolze, C.; Hager, M.; Schubert, U. State-of-charge monitoring for redox flow batteries: A symmetric open-circuit cell approach. J. Power Sources 2019, 423, 60–67. [Google Scholar] [CrossRef]
- Ngamsai, K.; Arpornwichanop, A. Measuring the state of charge of the electrolyte solution in a vanadium redox flow battery using a four-pole cell device. J. Power Sources 2015, 298, 150–157. [Google Scholar] [CrossRef]
- Shin, K.; Jin, C.; So, J.; Park, S.; Kim, D.; Yeon, S. Real-time monitoring of the state of charge (SOC) in vanadium redox-flow batteries using UV–Vis spectroscopy in operando mode. J. Energy Storage 2020, 27, 101066. [Google Scholar] [CrossRef]
- Roznyatovskaya, N.; Noack, J.; Mild, H.; Fühl, M.; Fischer, P.; Pinkwart, K.; Tübke, J.; Skyllas-Kazacos, M. Vanadium Electrolyte for All-Vanadium Redox-Flow Batteries: The Effect of the Counter Ion. Batteries 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xi, J.; Wu, Z.; Zhang, W.; Zhou, H.; Li, W.; Qiu, X. State of charge monitoring for vanadium redox flow batteries by the transmission spectra of V(IV)/V(V) electrolytes. J. Appl. Electrochem. 2012, 42, 1025–1031. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Xi, J.; Zhou, H.; Wu, Z.; Qiu, X. Rapid detection of the positive side reactions in vanadium flow batteries. Appl. Energy 2017, 8, 452–462. [Google Scholar] [CrossRef]
- Buckley, D.N.; Gao, X.; Lynch, R.P.; Quill, N.; Leahy, M.J. Towards Optical Monitoring of Vanadium Redox Flow Batteries (VRFBs): An Investigation of the Underlying Spectroscopy. J. Electrochem. Soc. 2014, 161, A524–A534. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Liu, L. An on-line spectroscopic monitoring system for the electrolytes in vanadium redox flow batteries. RSC Adv. 2015, 5, 100235–100243. [Google Scholar] [CrossRef]
- Spectrometer SEC2000. 2020. Available online: https://www.als-japan.com/1352.html (accessed on 17 June 2022).
- Si Photodiode FD11A. 2012. Available online: https://www.thorlabs.com/thorproduct.cfm?partnumber=FD11A (accessed on 17 June 2022).
- Schofield, K. State of Charge and Capacity Tracking in VRFB Systems. Unpublished Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2022. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schofield, K.; Musilek, P. State of Charge and Capacity Tracking in Vanadium Redox Flow Battery Systems. Clean Technol. 2022, 4, 607-618. https://doi.org/10.3390/cleantechnol4030037
Schofield K, Musilek P. State of Charge and Capacity Tracking in Vanadium Redox Flow Battery Systems. Clean Technologies. 2022; 4(3):607-618. https://doi.org/10.3390/cleantechnol4030037
Chicago/Turabian StyleSchofield, Kalvin, and Petr Musilek. 2022. "State of Charge and Capacity Tracking in Vanadium Redox Flow Battery Systems" Clean Technologies 4, no. 3: 607-618. https://doi.org/10.3390/cleantechnol4030037
APA StyleSchofield, K., & Musilek, P. (2022). State of Charge and Capacity Tracking in Vanadium Redox Flow Battery Systems. Clean Technologies, 4(3), 607-618. https://doi.org/10.3390/cleantechnol4030037






