Investigating Combustion Process of N-Butanol-Diesel Blends in a Diesel Engine with Variable Compression Ratio
Abstract
:1. Introduction
- n-butanol has a lower auto-ignition temperature than methanol and ethanol, making it easier to ignite in diesel engines;
- n-butanol has better evaporation properties, higher cetane number, and energy density than ethanol and methanol;
- n-butanol is able to clean the fuel system, including the injector, and has better mixing properties.
2. Experimental
2.1. Engine and Method
2.2. Investigated Fuel Blends
- Lower heating value, cetane number decrease, and kinematic viscosity increase. All these are negative factors regarding combustion efficiency.
- Density and boiling point drop, which is a good direction but increasing self-ignition temperature and heat of evaporation of butanol also takes the blend in a negative direction.
3. Results and Discussion
4. Conclusions
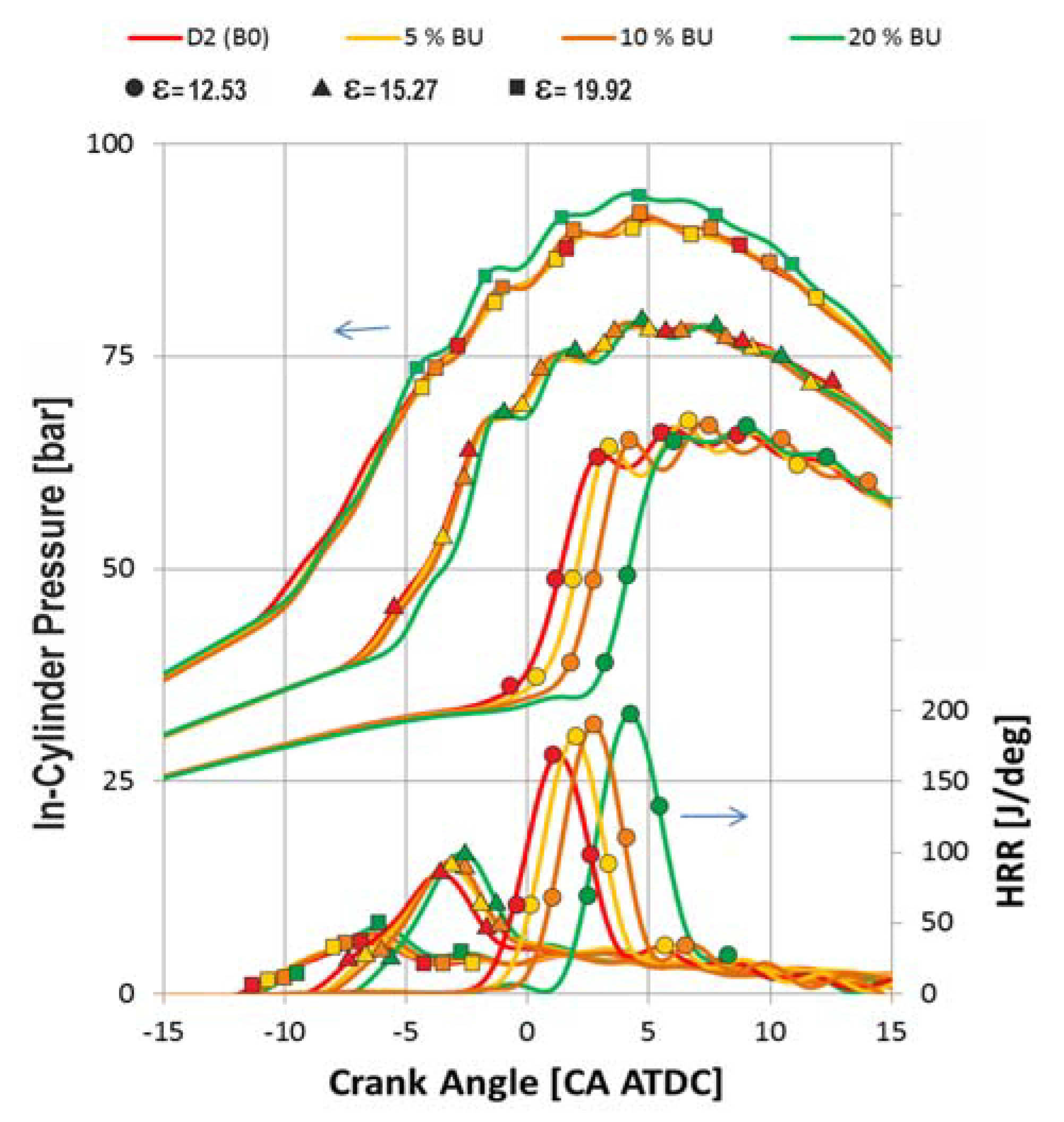
- In the case of the pre-chamber diesel engine when the fuel flow rate and pre-injection angle were constant, the ignition delay increased accordingly when the butanol ratio increased in the blend, but it was inversely proportional to the compression ratio. The maximal value of the heat release rate would be higher with increasing n-butanol in the fuel mixture, and it was also proportional to the increased compression ratio. Against the lowered LHV of the fuel, the blend contains n-butanol and the combustion peak pressure remained constant or a slightly increasing could be obtained.
- Additionally, with this engine with a constant flow rate of fuel and pre-injection angle, the ignition delay of blend with butanol increased most significantly with the highest compression ratio compared to that of diesel. It changed almost equally at lower compression ratios. Maximal values of heat release rate increased when a higher butanol part in the blend was investigated. This is the most intensive at a high compression ratio, while for the lower level of compression ratio, it changed almost equally. Clarification for that is that in case of a high compression ratio the ignition delay is lower than 2–3 CA°, so in this case, after the injection, a high quantity of butanol had evaporated, while only a small fraction of the diesel had done so. So, the mixture contained more n-butanol damp at the start of the combustion.
- When the compression ratios were lower, the ignition delay times were longer during which the butanol and also the diesel evaporated continuously. So at the start of the combustion, it appeared that the ratio of diesel to n-butanol was higher, as it was the situation in case of shorter ignition delays. It is supported by the above findings, so in the case of the used adjustments, the injection period was 5–6 CA°, so it can be obtained that if the compression ratio was 12.53, the period of the diffuse combustion process was not significantly in contrast to the process when the compression ratio was set to 19.92.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BU | n-butanol |
| B20 | a fuel mixture that contains 20 V/V% of sunflower methyl-ester, and 80 V/V% of diesel |
| BU0S20D80 | a fuel mixture that contains 0 V/V% of butanol, 20 V/V% of soybean oil methyl ester, and 80 V/V% of diesel |
| BU10S10D80 | a fuel mixture that contains 10 V/V% of butanol, 10 V/V% of soy bean oil methyl ester, and 80 V/V% of diesel |
| BU20S80 | a fuel mixture that contains 20 V/V% of butanol and 80 V/V% of soy bean oil |
| BU5S15D80 | a fuel mixture that contains 5 V/V% of butanol, 15 V/V% of soybean oil methyl ester, and 80 V/V% of diesel |
| BMEP | brake mean effective pressure |
| BU20 | a fuel mixture that contains 20 V/V% of butanol and 80 V/V% of diesel |
| BTDC | before top dead center |
| CA° | degree of Crankshaft Angle |
| CFR F-5 | code of a special engine used for determining cetane number of a fuel |
| CO | chemical formula of the gas molecule carbon-monoxide |
| CO2 | chemical formula of the gas molecule carbon-monoxide |
| CR | common rail |
| D, D2 | diesel fuel |
| E20 | a fuel mixture that contains 20 V/V% of ethanol and 80 V/V% of diesel |
| E20S80 | a fuel mixture that contains 20 V/V% of ethanol and 80 V/V% of soybean oil methyl ester |
| FAME | fatty acid methyl ester |
| GHG | greenhouse gas |
| HRR | heat release rate |
| ICE | internal combustion engine |
| LHV | lower heating value |
| M20 | a fuel mixture that contains 20 V/V% of methanol and 80 V/V% of diesel |
| NOx | general chemical formula describing a molecule of oxides of nitrogen |
| PM | particulate matter as an emission component of an internal combustion engine |
| RPM | revolution per minute, which is a used measurement unit of engine speed |
| S | soybean oil methyl ester |
| THC | total hydrogen carbon as an emission component of an internal combustion engine |
| Acronyms | |
| dpin-cyl./dφ (max.) | a maximal value of the ratio between elemental change in in-cylinder pressure and elemental change in angular rotation of the engine’s crankshaft [bar/CA°] |
| ε | compression ratio of an engine [-] |
| σHRR | uncertainty value of the parameter heat release rate [%] |
| σPi | uncertainty value of the parameter in-cylinder pressure [%] |
References
- Liu, Y.; Cheng, W.L.; Huo, M.; Lee, C.F.; Li, J.; City, C.C.; Province, J.L.; Street, W.G. Effects of Micro-Explosion on Butanol-Biodiesel-Diesel Spray and Combustion. In Proceedings of the ILASS-Americas 22nd Annual Conference on Liquid Atomization and Spray Systems, Cincinnati, OH, USA, May 2010; pp. 16–19. Available online: https://www.researchgate.net/profile/Ming-Huo-6/publication/228511913_Effects_of_Micro-Explosion_on_Butanol-Biodiesel-Diesel_Spray_and_Combustion/links/555c743508ae8f66f3ae352e/Effects-of-Micro-Explosion-on-Butanol-Biodiesel-Diesel-Spray-and-Combustion.pdf (accessed on 2 February 2021).
- Lapuerta, M.; Hernández, J.J.; Fernández-Rodríguez, D.; Cova-Bonillo, A. Autoignition of blends of n-butanol and ethanol with diesel or biodiesel fuels in a constant-volume combustion chamber. Energy 2017, 118, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lee, C.F.; Huo, M.; Yao, M. Comparison of ethanol and butanol as additives in soybean biodiesel using a constant volume combustion chamber. Energy Fuels 2011, 25, 1837–1846. [Google Scholar] [CrossRef]
- Merola, S.S.; Tornatore, C.; Iannuzzi, S.E.; Marchitto, L.; Valentino, G. Combustion process investigation in a high speed diesel engine fuelled with n-butanol diesel blend by conventional methods and optical diagnostics. Renew. Energy 2014, 64, 225–237. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Balasubramanian, R. Influence of butanol–diesel blends on particulate emissions of a non-road diesel engine. Fuel 2014, 118, 130–136. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X. Individual hydrocarbons and particulate matter emission from a turbocharged CRDI diesel engine fueled with n-butanol/diesel blends. Fuel 2015, 154, 188–195. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X.; Kim, Y.K.; Jung, G.; Lee, C.; Choi, I.; Song, C.S. Effect of diesel fuel blend with n-butanol on the emission of a turbocharged common rail direct injection diesel engine. Appl. Energy 2015, 146, 20–28. [Google Scholar] [CrossRef]
- Valentino, G.; Corcione, F.E.; Iannuzzi, S.E.; Serra, S. Experimental study on performance and emissions of a high speed diesel engine fuelled with n-butanol diesel blends under premixed low temperature combustion. Fuel 2012, 92, 295–307. [Google Scholar] [CrossRef]
- Valentino, G.; Corcione, F.E.; Iannuzzi, S.E. Effects of gasoline–diesel and n-butanol–diesel blends on performance and emissions of an automotive direct-injection diesel engine. Int. J. Engine Res. 2012, 13, 199–215. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Mazzei, A.; Valentino, G.; Corcione, F.E.; Merola, S.S. Effect of Butanol Blend on in-Cylinder Combustion Process. Part 2: Compression Ignition Engine. J. KONES 2011, 18, 473–483. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-article-BUJ5-0040-0059 (accessed on 3 February 2021).
- Chen, G.; Shen, Y.; Zhang, Q.; Yao, M.; Zheng, Z.; Liu, H. Experimental study on combustion and emission characteristics of a diesel engine fueled with 2, 5-dimethylfuran–diesel, n-butanol–diesel and gasoline–diesel blends. Energy 2013, 54, 333–342. [Google Scholar] [CrossRef]
- Lujaji, F.; Kristóf, L.; Bereczky, A.; Mbarawa, M. Experimental investigation of fuel properties, engine performance, combustion and emissions of blends containing croton oil, butanol, and diesel on a CI engine. Fuel 2011, 90, 505–510. [Google Scholar] [CrossRef]
- Dobai, A.; Bereczky, Á. Investigation of Diesel–n-Butanol Fuel Blend in the Function of Pre-injection Angle. In Vehicle and Automotive Engineering; Jármai, K., Bolló, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–13. [Google Scholar]
- Economics, B.E. BP Energy Outlook 2018 Edition. 2018. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2018.pdf (accessed on 2 May 2020).
- Exxon Mobil. Outlook for Energy: A View to 2040. 2018. Available online: Cdn.exxonmobil.com/~/media/global/files/outlook-for-energy/2018/2018-outlook-for-energy.pdf (accessed on 2 May 2020).
- Shell. Shell Energy Transition Report. 2017. Available online: https://www.shell.com/energy-and-innovation/the-energy-future/shell-energy-transition-report/_jcr_content/par/toptasks.stream/1524757699226/f51e17dbe7de5b0eddac2ce19275dc946db0e407ae60451e74acc7c4c0acdbf1/web-shell-energy-transition-report.pdf (accessed on 2 May 2020).
- Shell International BV. Shell Energy Scenarios to 2050. 2016. Available online: https://rjohnwilliams.files.wordpress.com/2016/02/shell-energy-scenarios2050.pdf (accessed on 2 May 2020).
- Fischer, G.; Schrattenholzer, L. Global bioenergy potentials through 2050. Biomass Bioenergy 2001, 20, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Zöldy, M. Potential future renewable fuel challenges for internal combustion engine. Jármûvek és Mobilgépek II. évf 2009, 397–403. [Google Scholar]
- Ricardo. Europe’s Clean Mobility Outlook: Scenarios for the EU Light-Duty Vehicle Fleet, Associated Energy Needs and Emissions, 2020–2050. 2018. Available online: https://www.epure.org/media/1751/ed51122_epure_cmp_modelling_final-report_issue3-1.pdf (accessed on 2 May 2020).
- Hancsók, J.; Krár, M.; Baladincz, J.; Vuk, T. Dízelgázolajok bioeredetű komponensei (Zsírsav-metilészterek) (Bio-origin components of diesel (Fatty acid methyl esters)). Magy. Kémikusok Lapja 2006, 61, 228–235. (In Hungarian) [Google Scholar]
- Hancsók, J.; Magyar, S.; Szoboszlai, Z. Motorbenzinek bioeredetű komponensei I. (Bioalkoholok) (Bio-origin components of gasoline I. (Bioalcohols)). Magy. Kémikusok Lapja 2006, 61, 153–159. (In Hungarian) [Google Scholar]
- European Parliament and Council. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; European Parliament and Council: Strasbourg, France, 2009. [Google Scholar]
- Mączyńska, J.; Krzywonos, M.; Kupczyk, A.; Tucki, K.; Sikora, M.; Pińkowska, H.; Wielewska, I. Production and use of biofuels for transport in Poland and Brazil–The case of bioethanol. Fuel 2019, 241, 989–996. [Google Scholar] [CrossRef]
- Mollenhauer, K.; Tschoke, H. Handbook of Diesel Engines; Springer: Berlin, Germany, 2010; Volume 1. [Google Scholar]
- Hartmann Braun Meß und Regeltechik Verbrennungstechnik; Hartmann Braun AG: Frankfurt, Germany, 1973.
- Herbinet, O.; Pitz, W.J.; Westbrook, C.K. Detailed chemical kinetic mechanism for the oxidation of biodiesel fuels blend surrogate. Combust. Flame 2010, 157, 893–908. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, C.K.; Naik, C.V.; Herbinet, O.; Pitz, W.J.; Mehl, M.; Sarathy, S.M.; Curran, H.J. Detailed chemical kinetic reaction mechanisms for soy and rapeseed biodiesel fuels. Combust. Flame 2011, 158, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Heywood, J.B. Combustion Engine Fundamentals, 1st ed.; McGraw-Hill, Inc.: New York, NY, USA, 1988. [Google Scholar]
- European Committee for Standardization. European Standard EN 590 Automotive Fuels. Diesel. Requirements and Test Methods; CEN-European Committee for Standardization: Brussels, Belgium, 2005. [Google Scholar]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Dimaratos, A.M.; Kyritsis, D.C. Effects of butanol–diesel fuel blends on the performance and emissions of a high-speed DI diesel engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Horváth, I.T.; Cséfalvay, E.; Mika, L.T.; Debreczeni, M. Sustainability metrics for biomass-based carbon chemicals. ACS Sustain. Chem. Eng. 2017, 5, 2734–2740. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. n-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, V.; Murthy, M. Butanol and pentanol: The promising biofuels for CI engines–A review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar]
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Davis, S.G.; Law, C.K. Determination of and fuel structure effects on laminar flame speeds of C1 to C8 hydrocarbons. Combust. Sci. Technol. 1998, 140, 427–449. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabados, G.; Lukács, K.; Bereczky, Á. Investigating Combustion Process of N-Butanol-Diesel Blends in a Diesel Engine with Variable Compression Ratio. Clean Technol. 2021, 3, 618-628. https://doi.org/10.3390/cleantechnol3030037
Szabados G, Lukács K, Bereczky Á. Investigating Combustion Process of N-Butanol-Diesel Blends in a Diesel Engine with Variable Compression Ratio. Clean Technologies. 2021; 3(3):618-628. https://doi.org/10.3390/cleantechnol3030037
Chicago/Turabian StyleSzabados, György, Kristóf Lukács, and Ákos Bereczky. 2021. "Investigating Combustion Process of N-Butanol-Diesel Blends in a Diesel Engine with Variable Compression Ratio" Clean Technologies 3, no. 3: 618-628. https://doi.org/10.3390/cleantechnol3030037
APA StyleSzabados, G., Lukács, K., & Bereczky, Á. (2021). Investigating Combustion Process of N-Butanol-Diesel Blends in a Diesel Engine with Variable Compression Ratio. Clean Technologies, 3(3), 618-628. https://doi.org/10.3390/cleantechnol3030037





