Combustion Characteristics of Waste Cooking Oil–Butanol/Diesel/Gasoline Blends for Cleaner Emission
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
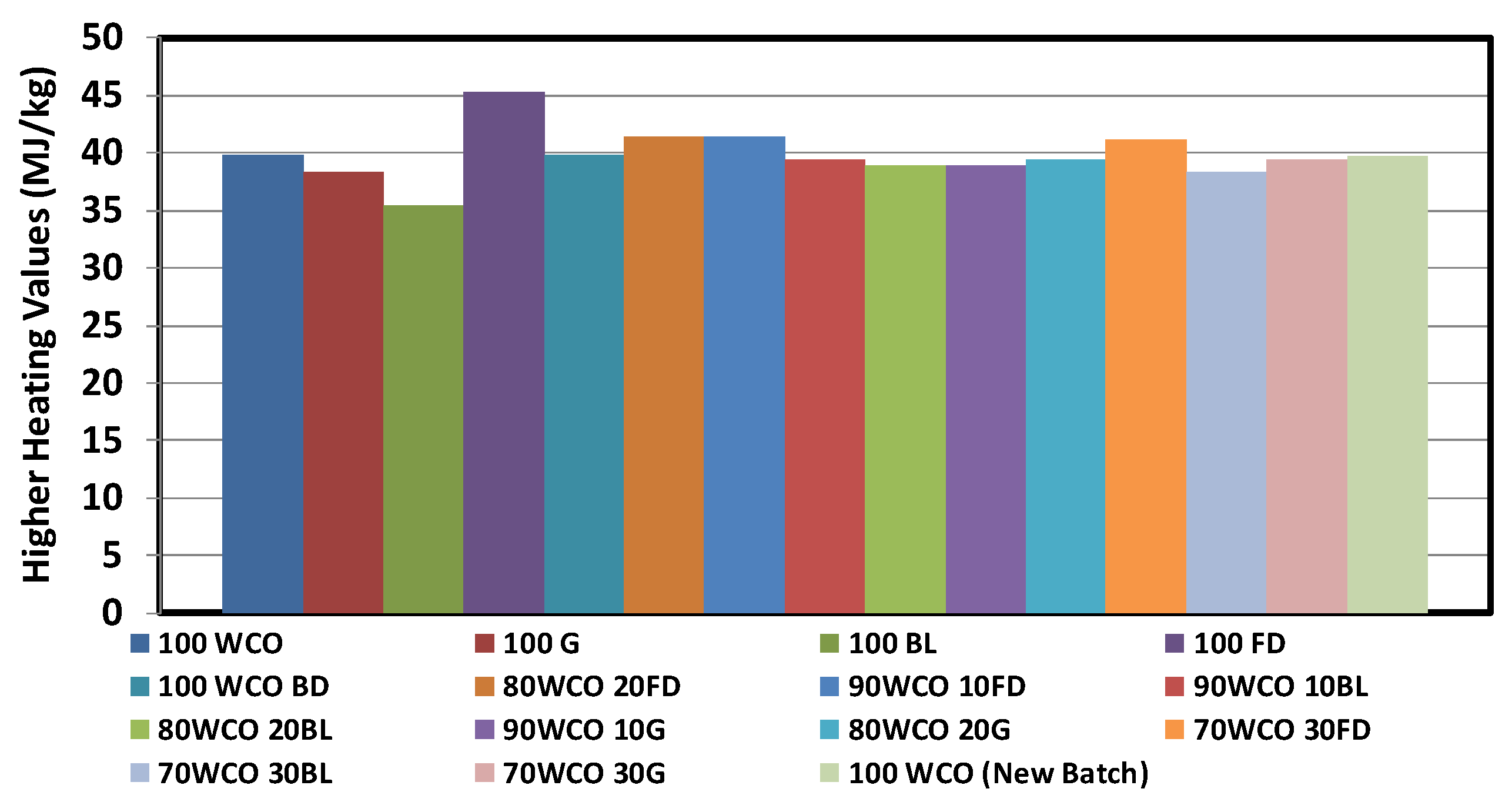
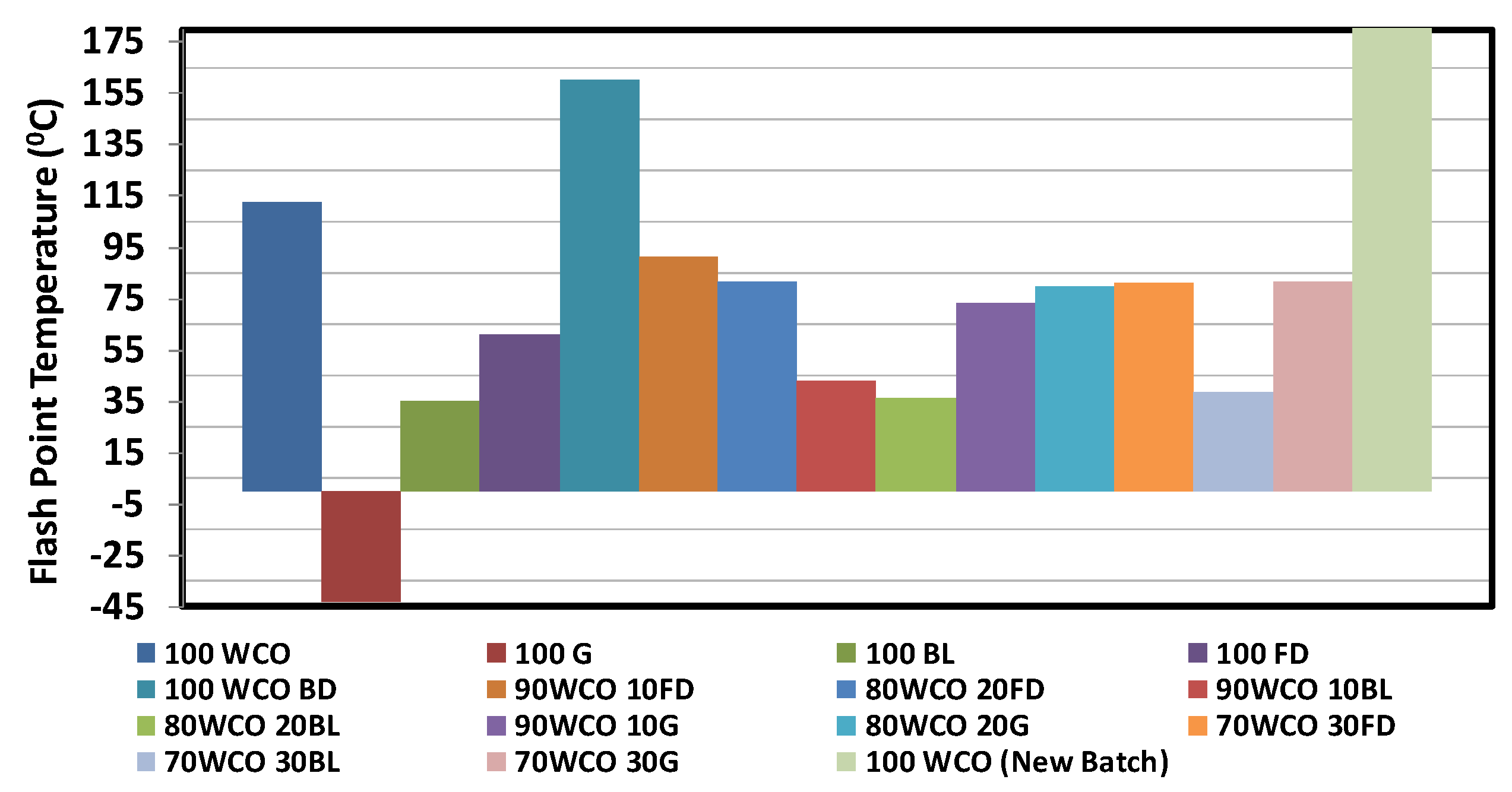
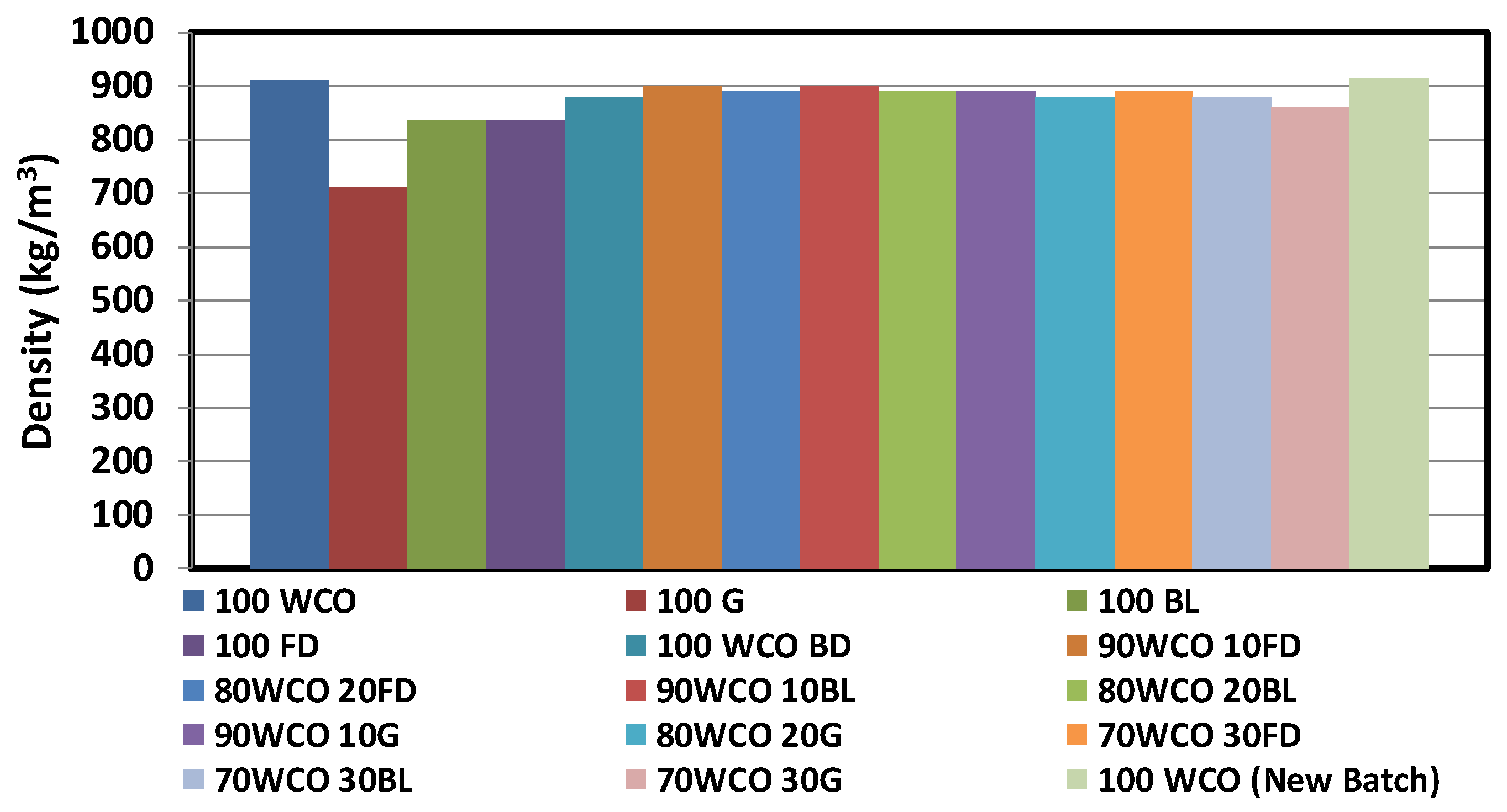
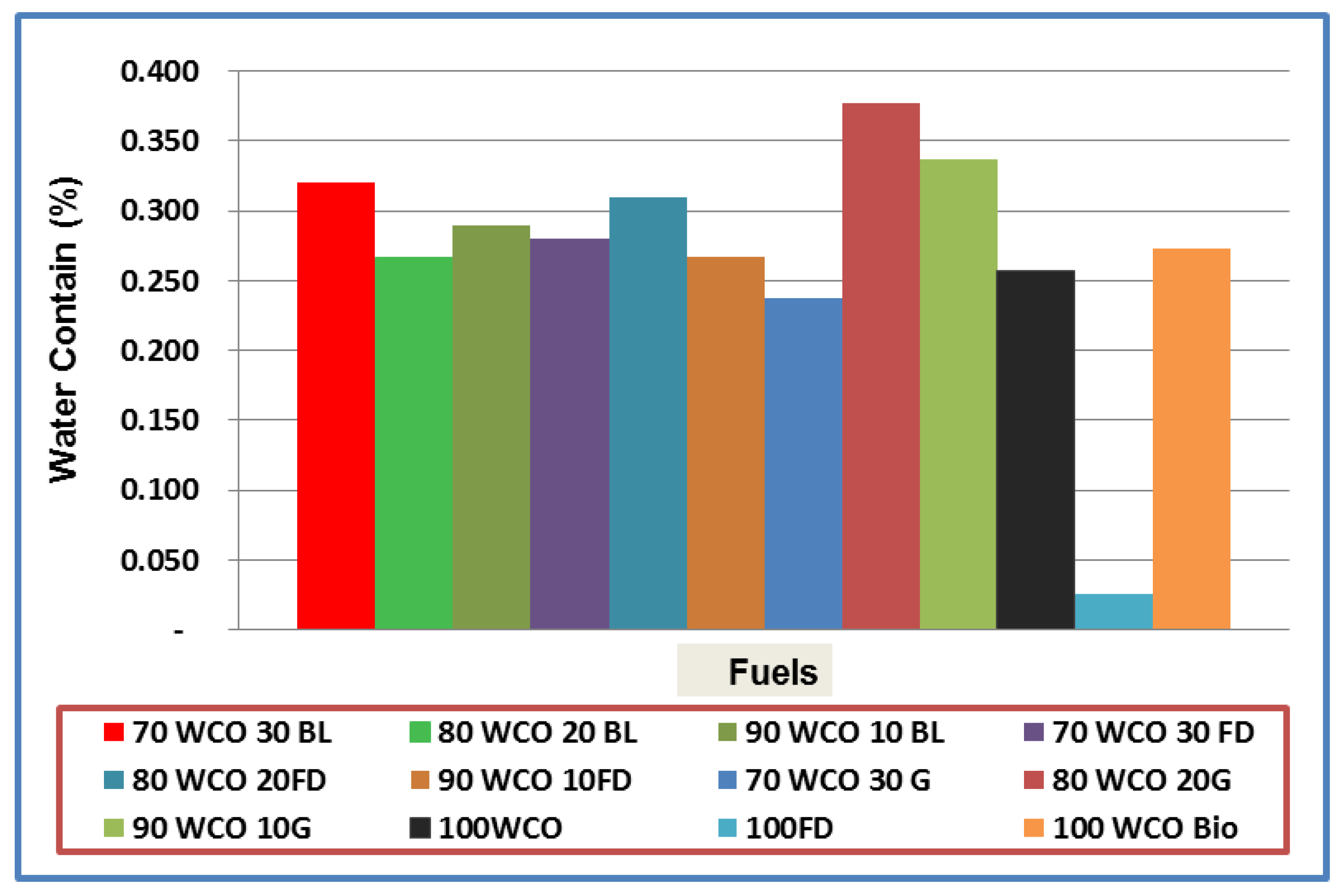
3.1. Characterisation of Neat Fuels and Blends
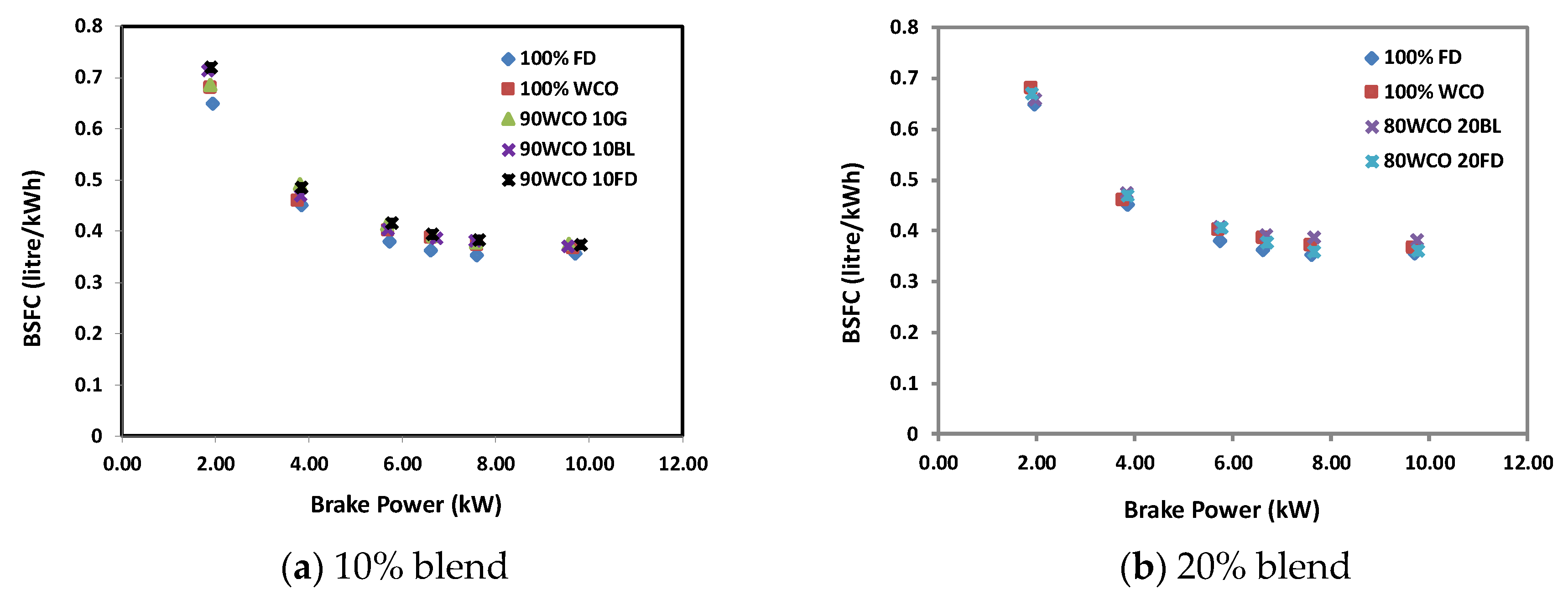
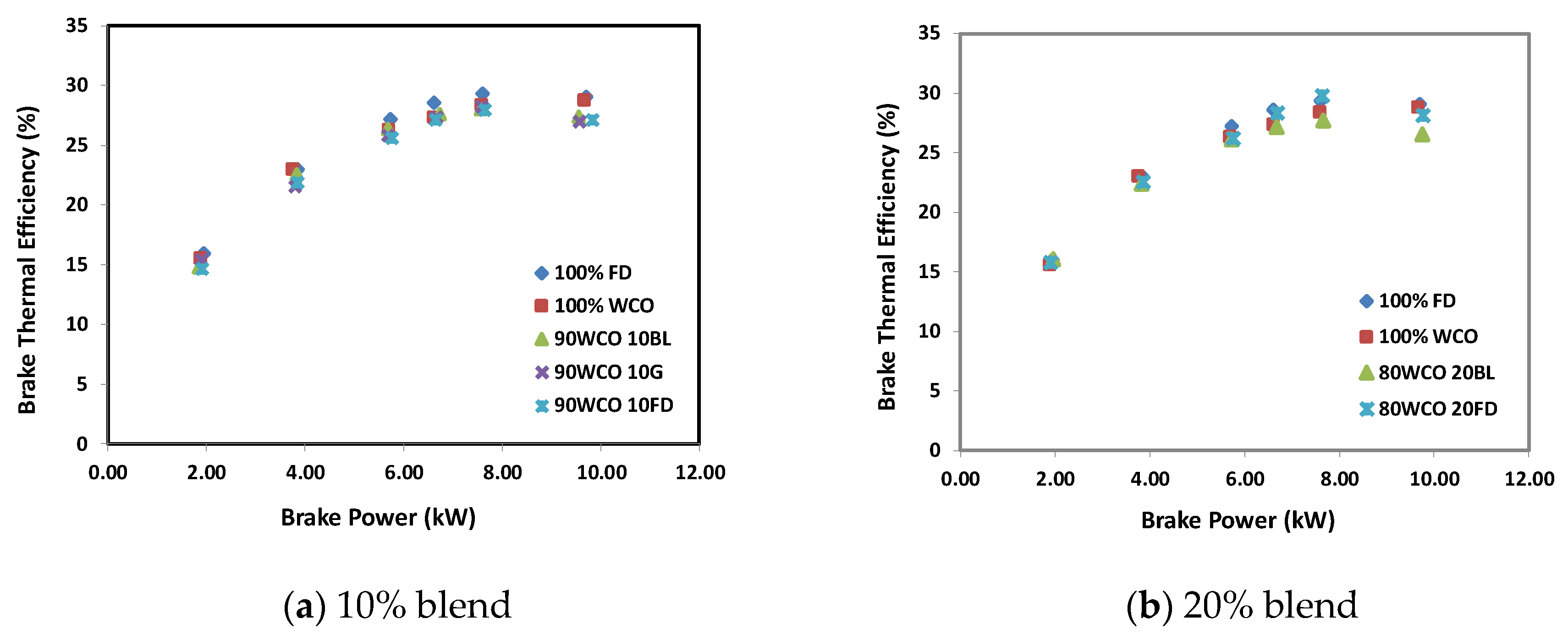
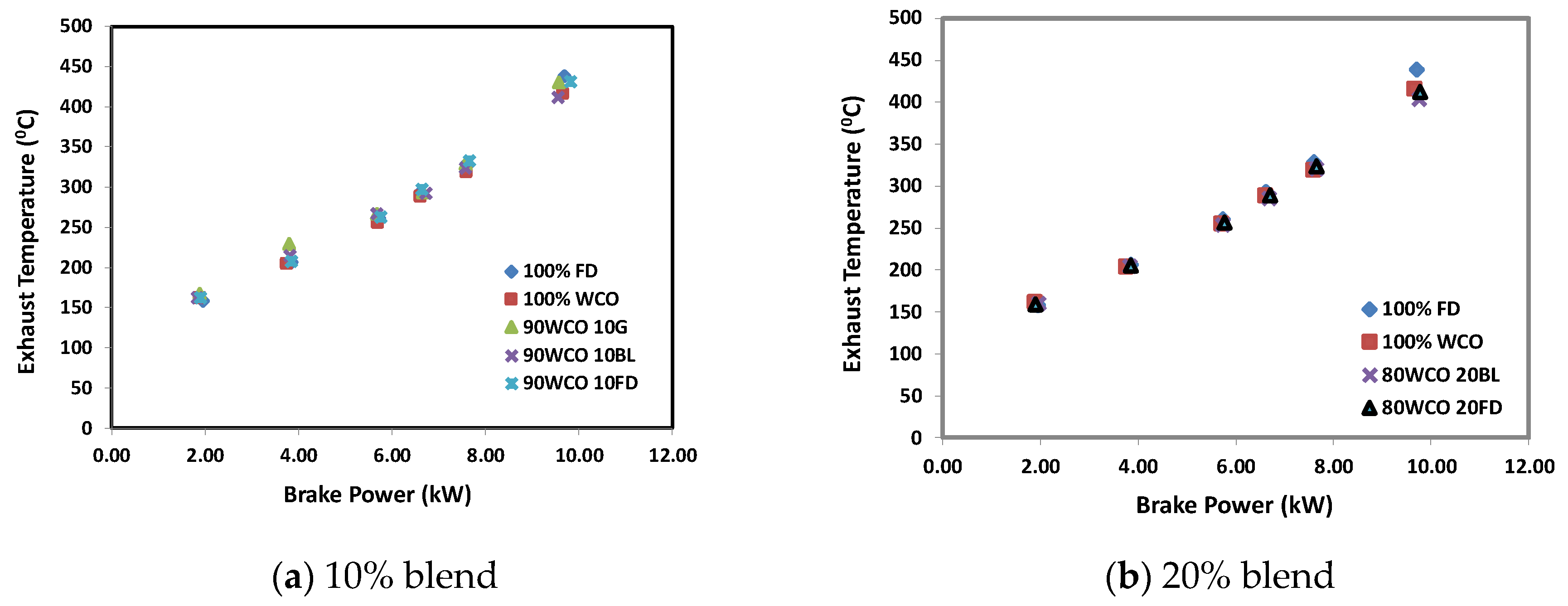
3.2. Engine Test Results
4. Error and Uncertainty Analysis
5. Conclusions
- (i)
- The calorific value of the blends increased with the addition of fossil diesel additive. The flash point temperatures of the WCO blends reduced with the addition of additives. At room temperature, the density of the WCO blends with 20% diesel/butanol/gasoline contents dropped by 1%, 2.1% and 3.2%, respectively. The viscosities of WCO blends reduced with the addition of additives.
- (ii)
- At low loads, the BSFC of the WCO blends with 10% FD/BL/G additives were higher than FD by 11%, 10% and 5.5%, respectively; whereas, at high load, they were higher than the FD by 5%, 2% and 5.5%, respectively. For 20% additives blends, the BSFC for FD and butanol blends were higher than neat FD by 1.1% and 7.4%, respectively. The brake thermal efficiency decreased by about 0.3–8% when the additives were used.
- (iii)
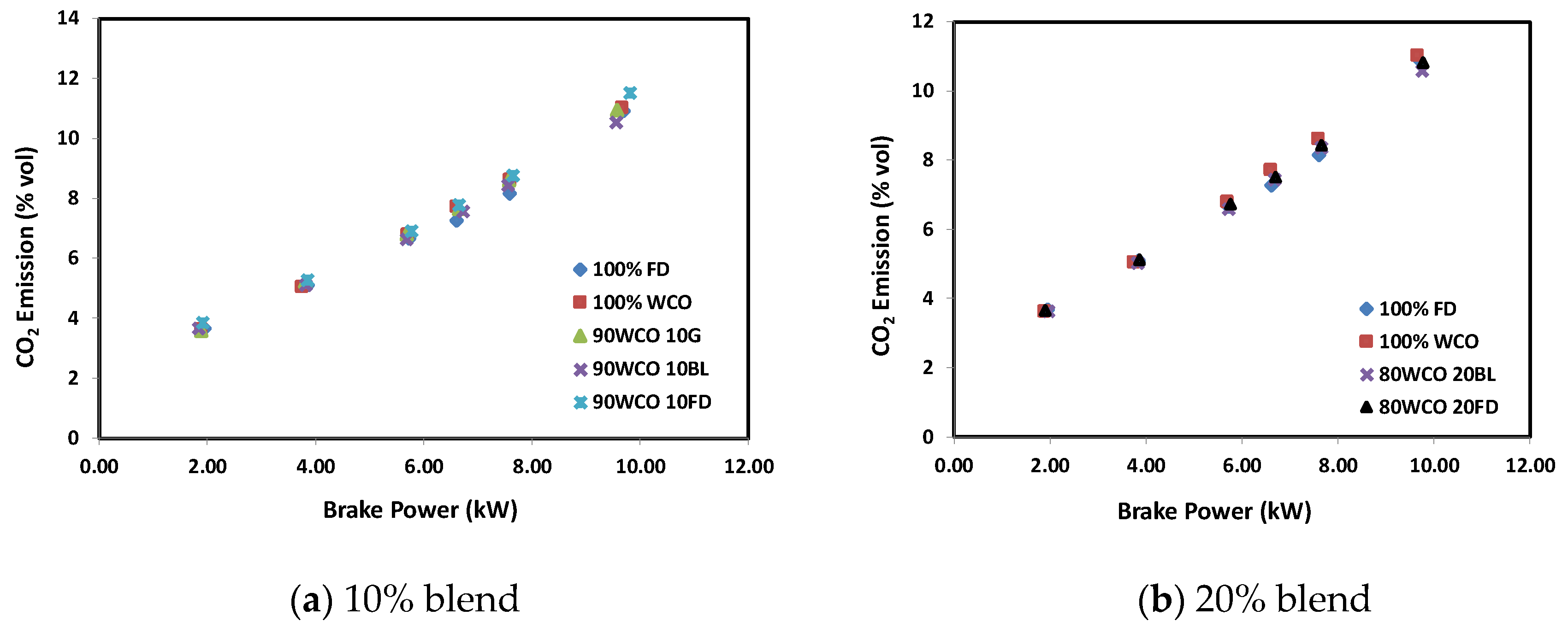
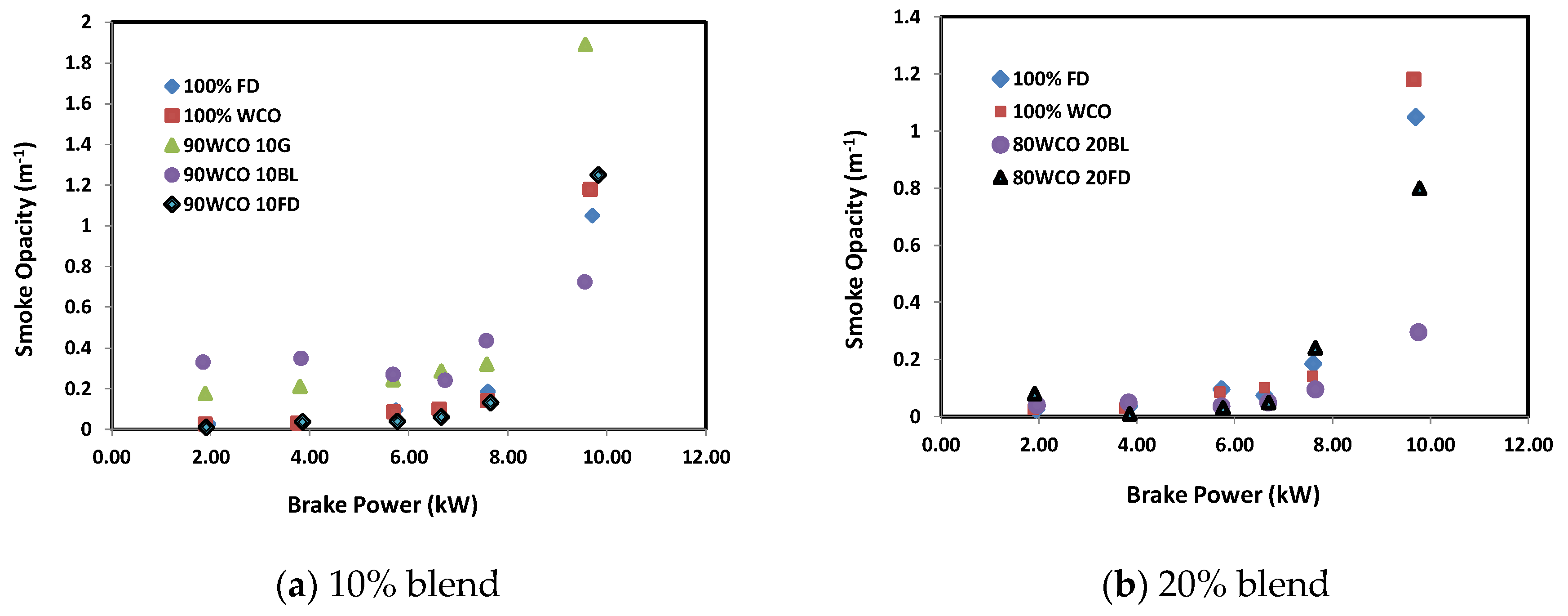
- At high loads, the CO2 emission decreased with the increase in additive content. At 100% load, 90WCO10BL fuel gave about 3.5% and 4.3% lower CO2 emissions than 100FD and 100WCO fuels. Compared to FD, the CO emission of the WCO fuel decreased by about 30%. A decrease in CO emission up to about 75% was observed when additives were added to WCO. The 90WCO10BL fuel gave 25% more NOx reduction than FD fuel. The smoke level was reduced considerably. The minimum smoke opacity was recorded for 80WCO20BL fuel, lower than FD by 71%.
- (iv)
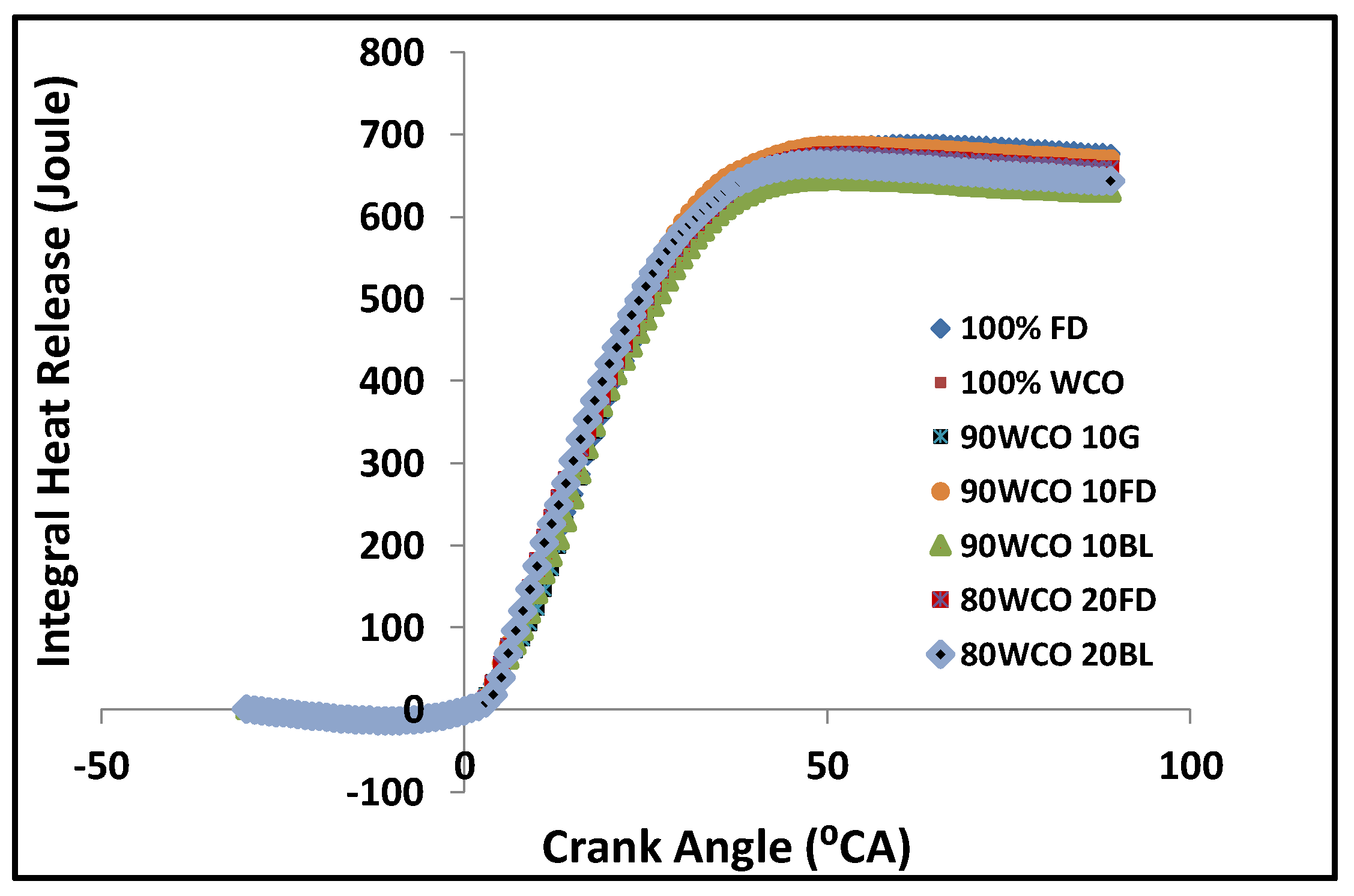
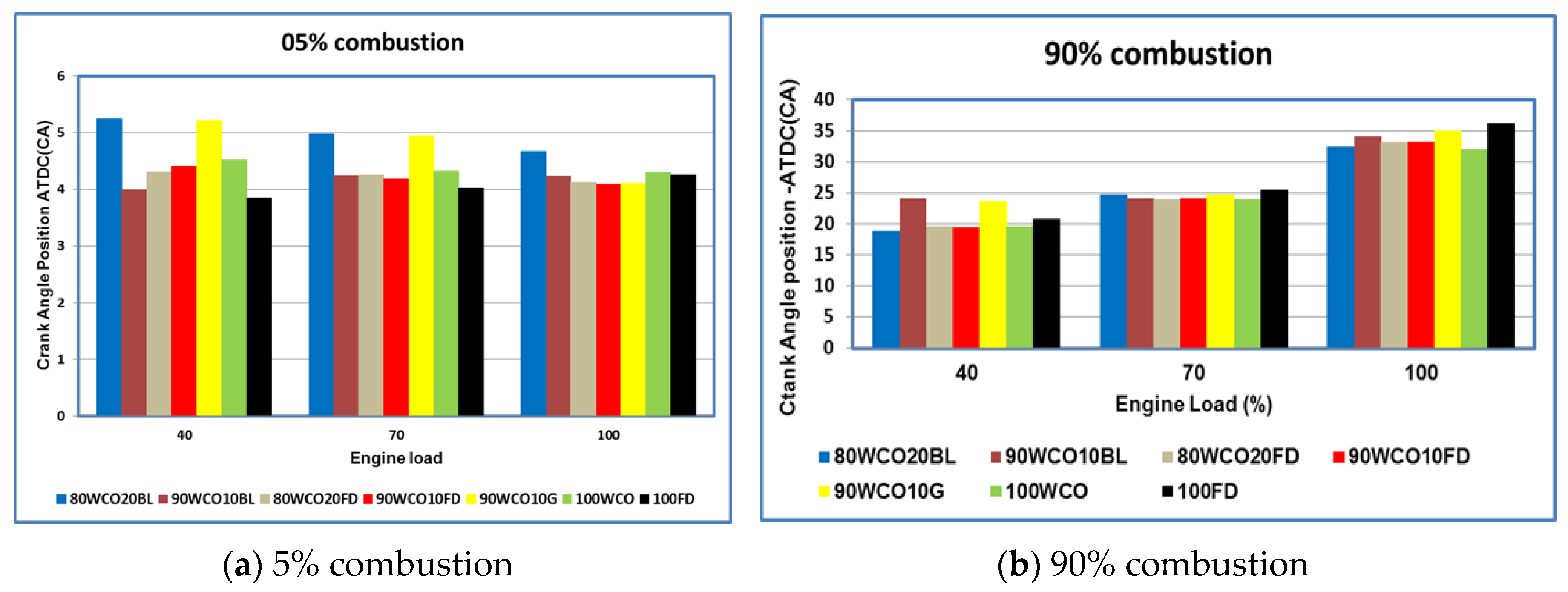
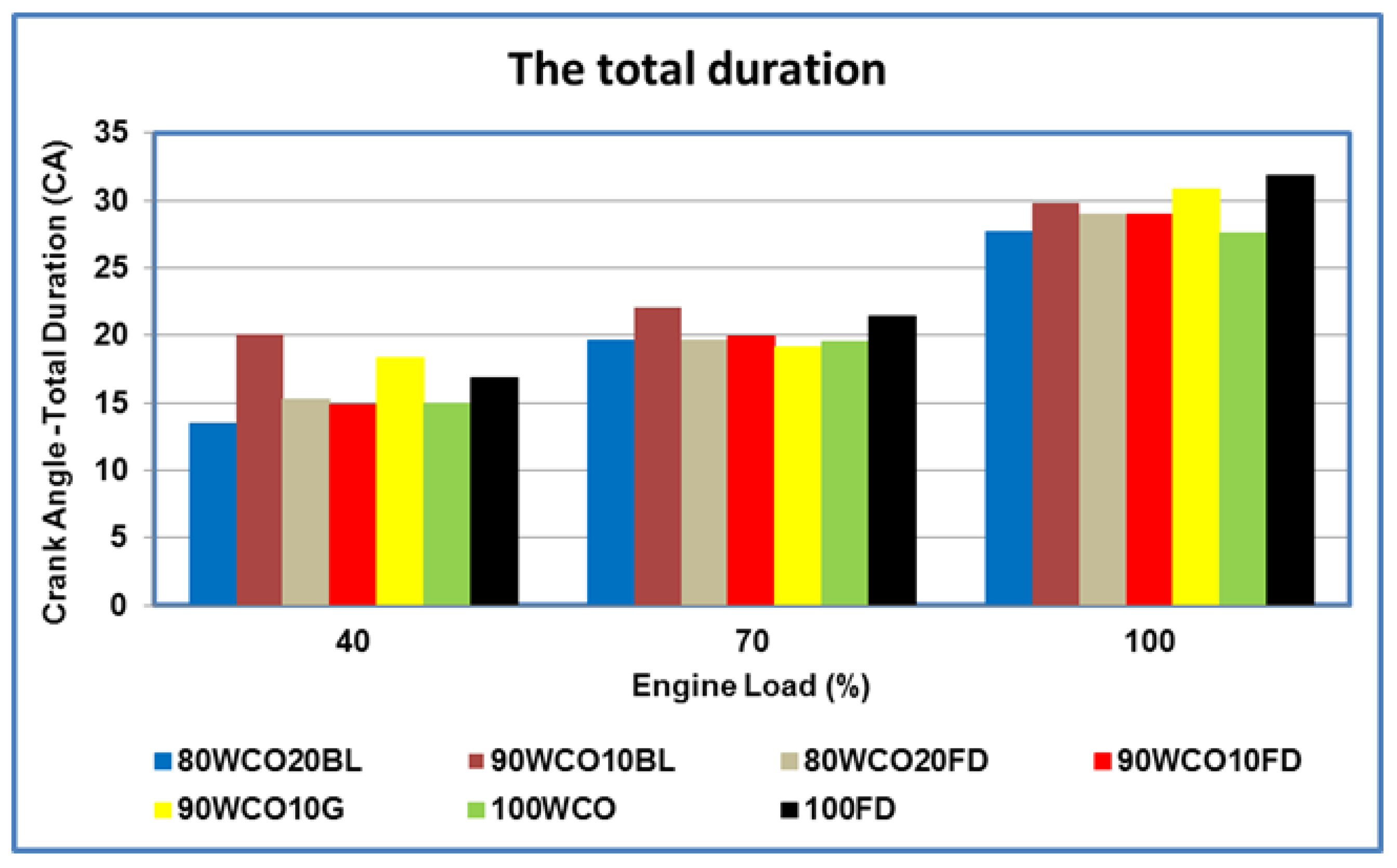
- At high loads, the peak in-cylinder pressures of 80WCO10-20FD fuels were approximately 4.4% higher than FD. At 70% load, in-cylinder pressures of the 80WCO20BL, WCO fuels and FD fuels were almost identical; the lowest peak in-cylinder pressure value was recorded for 90WCO10G fuel, lower than FD by 10%. At 100% load, the peak in-cylinder pressures for 80WCO20BL were 3.6% and 3.1% higher than 90WCO10BL and 100 FD. The 90WCO10G fuel gave a 5.4% lower in-cylinder pressure than neat FD operation in the full-load condition. The maximum heat release was recorded for FD. At 100% load, minimum heat release was observed for 90WCO10BL fuel, lower than FD by approximately 6.5%. The 80WCO20BL fuel gave a 2% higher heat release than the 90WCO10BL fuel; however, the peak heat release value of 80WCO20BL was about 5% lower than FD. Compared to FD, and in the full-load condition, the total combustion duration of the 80WCO20BL and WCO fuels decreased by approximately 13%. At 70% load, the combustion duration of the 80WCO20BL, 80WCO20FD and WCO fuels were lower than FD by approximately 8.4%.
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATDC | After Top Dead Centre |
| BL | Butanol |
| BSFC | Brake Specific Fuel Consumption |
| BTE | Brake Thermal Efficiency |
| CA | Crank Angle |
| CI | Compression Ignition |
| FD | Fossil Diesel |
| G | Gasoline |
| HHV | Higher Heating Values |
| UBHC | Unburnt Hydrocarbon |
| WCO | Waste Cooking Oil |
| 100WCO | 100% Waste Cooking Oil |
| 100 FD | 100% Fossil diesel |
| 100WCO–Bio | 100% Waste Cooking Oil Biodiesel |
| 90WCO 10BL | 90% Waste Cooking Oil 10%Butanol |
| 80WCO 20BL | 80% Waste Cooking Oil 20%Butanol |
| 70WCO 30BL | 70% Waste Cooking Oil 30%Butanol |
| 90WCO 10FD | 90% Waste Cooking Oil 10% Fossil Diesel |
| 80WCO 20FD | 80% Waste Cooking Oil 20% Fossil Diesel |
| 70WCO 30FD | 70% Waste Cooking Oil 30% Fossil Diesel |
| 90WCO 10G | 90% Waste Cooking Oil 10% Gasoline |
| 80WCO 20G | 80% Waste Cooking Oil 20% Gasoline |
| 70WCO 30G | 70% Waste Cooking Oil 30% Gasoline |
References
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Karavalakis, G.; Hilari, D.; Givalou, L.; Karonis, D.; Stournas, S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 2011, 36, 369–374. [Google Scholar] [CrossRef]
- Department for Transport. UK Bioenergy Strategy; Department of Energy and Climate Change: London, UK, 2012.
- E4tech. A Harmonised Auto-Fuel Biofuel Roadmap for the EU to 2030; E4tech: London, UK, 2013. [Google Scholar]
- Hossain, A.K.; Davies, P.A. Plant oils as fuels for compression ignition engines: A technical review and life-cycle analysis. Renew Energy 2010, 35, 1–13. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Rao, G.L.N.; Sampath, S.; Rajagopal, K. Experimental Studies on the Combustion and Emission Characteristics of a Diesel Engine Fuelled with Used Cooking Oil Methyl Esterand its Diesel Blends. World Acad. Sci. Eng. Technol. Int. J. Mech. Mechatron. Eng. 2008, 2, 1–25. [Google Scholar]
- Abuhabaya, A.A.; Fieldhouse, J.D.; Robert, D.R. Evaluation of Properties and USE of waste Vegetable Oil (WVO), Pure Vegetable Oils and Standard Diesel as Used in a Compression Ignition Engine. In Proceedings of the Future Technologies in Computing and Engineering Annual Researchers’ Conference (CEARC’10); The University of Huddersfield: Huddersfield, UK, 2010; pp. 71–76. [Google Scholar]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Performance, emission and combustion characteristics of an indirect injection (IDI) multi-cylinder compression ignition (CI) engine operating on neat jatropha and karanj oils preheated by jacket water. Biomass Bioenergy 2012, 46, 332–342. [Google Scholar] [CrossRef]
- Department for Transport. Renewable Transport Fuel Obligation Statistics: Obligation Period 6, 2013/14 Report 4; Department for Transport: London, UK, 2014.
- Algasim, H.M.; Fieldhouse, J. Performance and Emissions of Compression Ignition Engines Using Waste Cooked Oil as Fuel. In Proceedings of the Future Technologies in Computing and Engineering Annual Reserachers’s Conference (CEARC’10); The University of Huddersfield: Huddersfield, UK, 2010; pp. 65–70. ISBN 9781862180932. [Google Scholar]
- Agarwal, D.; Agarwal, A.K. Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl. Therm. Eng. 2007, 27, 2314–2323. [Google Scholar] [CrossRef]
- EPA. Guidance for Biodiesel Producers and Biodiesel Blenders/Users; EPA: Washington, DC, USA, 2007.
- Mahfouz, A.; Gad, M.S.; Faith, A.E.; Emara, A. Comparative study of combustion characteristics and exhaust emissions of waste cooking-diesel oil blends. Ain Shams Eng. J. 2018, 9, 3123–3134. [Google Scholar] [CrossRef]
- Delalibera, H.C.; Johann, A.L.; de Figueiredo, P.R.; Toledo, A.D.; Weirich Neto, P.H.; Ralisch, R. Performance of diesel engine fuelled with four vegetable oils, preheated and at engine operating temperature. Eng. Agric. 2017, 37, 302–314. [Google Scholar]
- Kumar, A.; Atul, D. Performance, Emission and Combustion Characteristics of Preheated and Blended Jatropha Oil. Chall. New Energy Crop 2012, 491–508. [Google Scholar]
- Pramanik, K. Properties and use of jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew. Energy 2003, 28, 239–248. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Antonopoulos, K.A.; Rakopoulos, D.C.; Hountalas, D.T.; Giakoumis, E.G. Comparative performance and emissions study of a direct injection diesel engine using blends of diesel fuel with vegetable oils or bio-diesels of various origins. Energy Convers. Manag. 2006, 47, 3272–3287. [Google Scholar] [CrossRef]
- Gopal, K.N.; Pal, A.; Sharma, S.; Samanchi, C.; Sathyanarayanan, K.; Elango, T. Investigation of emissions and combustion characteristics of a CI engine fueled with waste cooking oil methyl ester and diesel blends. Alex. Eng. J. 2014, 53, 281–287. [Google Scholar] [CrossRef]
- Imtenan, S.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Arbab, M.I.; Sajjad, H.; Rahman, S.M.A. Impact of oxygenated additives to palm and jatropha biodiesel blends in the context of performance and emissions characteristics of a light-duty diesel engine. Energy Convers. Manag. 2014, 83, 149–158. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Benalil, K.; Davis, S.M.; Calva, A. Effect of biodiesel-butanol fuel blends on emissions and performance characteristics of a diesel engine. Fuel 2014, 135, 46–50. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Dimaratos, A.M.; Kyritsis, D.C. Effects of butanol-diesel fuel blends on the performance and emissions of a high-speed di diesel engine. Energy Convers. Manag. 2010, 51, 1889–1997. [Google Scholar] [CrossRef]
- Rakopoulos, D.C. Combustion and emissions of cottonseed oil and its bio-diesel in blends with either n-butanol or diethyl ether in HSDI diesel engine. Fuel 2013, 105, 603–613. [Google Scholar] [CrossRef]
- Lujaji, F.; Hameer, S.; John, G.; Bereczky, A.; Mbarawa, M. Experimental investigation of NOx emission on crotonoil – 1-butanol – diesel in compression ignition (CI) engine. J. Mech. Eng. Res. 2013, 5, 104–111. [Google Scholar] [CrossRef][Green Version]
- Armas, O.; García-Contreras, R.; Ramos, Á. Pollutant emissions from engine starting with ethanol and butanol diesel blends. Fuel Process Technol. 2012, 100, 63–73. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S. Comparative performance of direct injection diesel engine operating on ethanol, petrol and rapeseed oil blends. Energy Convers. Manag. 2009, 50, 792–801. [Google Scholar] [CrossRef]
- Altun, E. Performance and exhaust emissions of a DI diesel engine fueled with waste cooking oil and inedible animal tallow methyl esters. Turk. J. Eng. Environ. Sci. 2011, 35, 107–114. [Google Scholar] [CrossRef]
- Subramaniam, D.; Murugesan, A. A comparative estimation of C.I. engine fuelled with methyl esters of punnai, neem and waste cooking oil. Int. J. Energy Environ. 2013, 4, 869–870. [Google Scholar]
- Deva Kumar, M.L.S.; Drakshayani, S.; Reddy, K.V.K. Effect of Fuel Injection Pressure on Performance of Single Cylinder Diesel Engine at Different Intake Manifold Inclinations. Int. J. Eng. Innov. Technol. 2012, 2, 20–25. [Google Scholar]
- Radheshyam; Santhosh, K.; Kumar, G.N. Effect of 1-pentanol addition and EGR on the combustion, performance and emission characteristic of a CRDI diesel engine, Renew. Energy 2020, 145, 925–936. [Google Scholar] [CrossRef]
- Gainy, B.; Longtin, J.P.; Lawler, B. A Guide to Uncertainty Quantification for Experimental Engine Research and Heat Release Analysis. SAE Int. J. Engines 2019, 12, 509–523. [Google Scholar] [CrossRef]
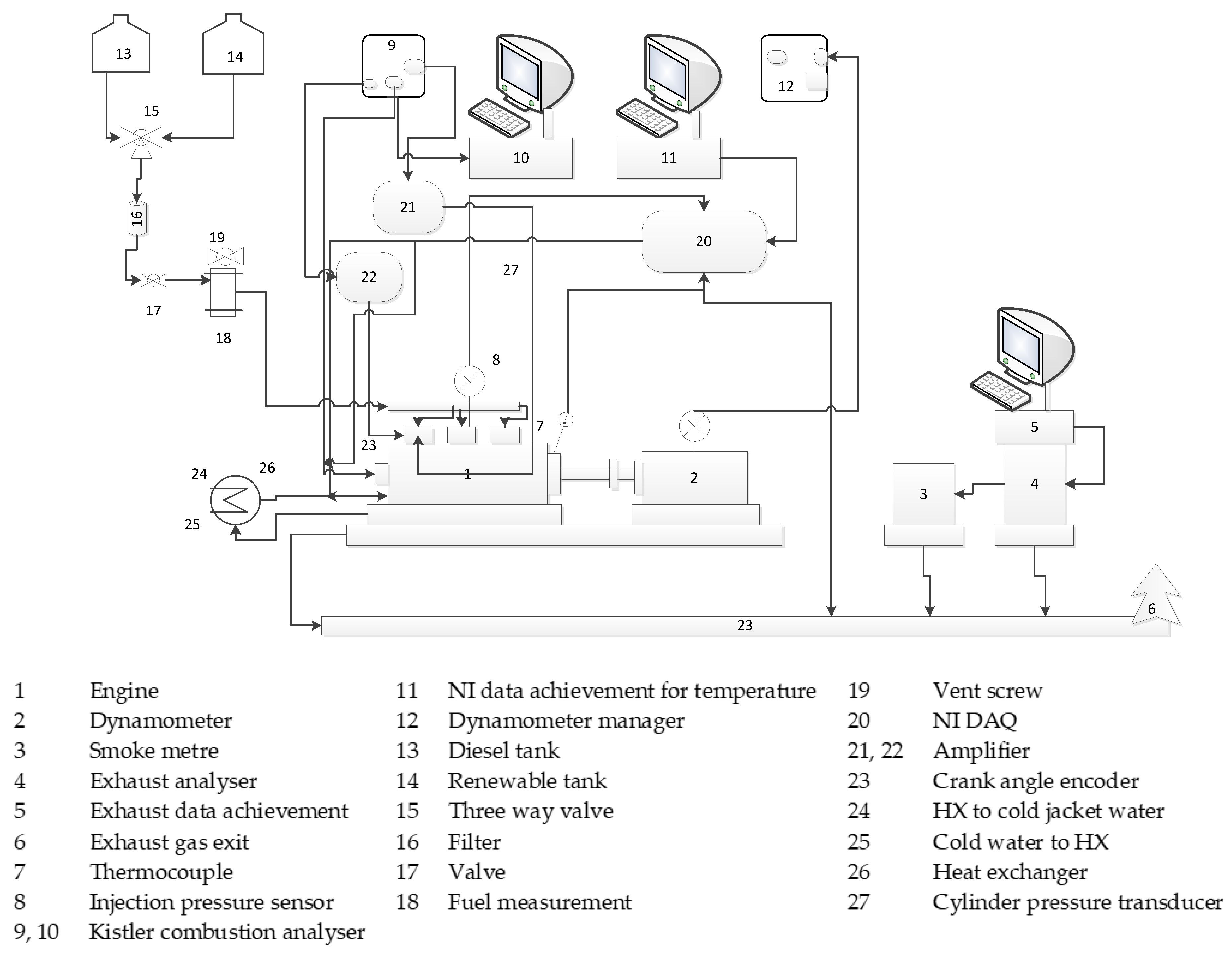




| Engine Manufacturer | Lister Petter (UK) |
|---|---|
| Model | LPWS Bio3 water cooled |
| Number of cylinders | 3 |
| Exhaust gas recirculation | None |
| Rated speed | 1500 rpm |
| Continuous power at rated speed | 9.9 kW |
| Fuel injection type | Indirect injection. Self-vent fuel system |
| with individual fuel injection pumps | |
| Fuel pump injection timing | 20° BTDC |
| Cylinder capacity | 1.395 litre |
| Compression ratio | 1:22 |
| Continuous power fuel consumption at 1500 rpm | 3.19 L/hr (fossil diesel) |
| Parameter | Uncertainties (%) | Parameter | Uncertainties (%) |
|---|---|---|---|
| Load (Torque) | 0.4 | Thermal efficiency | 0.8 |
| Speed | 0.6 | BSFC | 0.8 |
| Time | 0.5 | In-cylinder pressure | 0.6 |
| CO2 | 0.2 | Combustion duration | 0.8 |
| NOx | 0.1 | Heat release | 0.8 |
| CO | 0.1 | Crank angle | 0.2 |
| Smoke opacity | 0.8 | Time | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.K. Combustion Characteristics of Waste Cooking Oil–Butanol/Diesel/Gasoline Blends for Cleaner Emission. Clean Technol. 2020, 2, 447-461. https://doi.org/10.3390/cleantechnol2040028
Hossain AK. Combustion Characteristics of Waste Cooking Oil–Butanol/Diesel/Gasoline Blends for Cleaner Emission. Clean Technologies. 2020; 2(4):447-461. https://doi.org/10.3390/cleantechnol2040028
Chicago/Turabian StyleHossain, Abul K. 2020. "Combustion Characteristics of Waste Cooking Oil–Butanol/Diesel/Gasoline Blends for Cleaner Emission" Clean Technologies 2, no. 4: 447-461. https://doi.org/10.3390/cleantechnol2040028
APA StyleHossain, A. K. (2020). Combustion Characteristics of Waste Cooking Oil–Butanol/Diesel/Gasoline Blends for Cleaner Emission. Clean Technologies, 2(4), 447-461. https://doi.org/10.3390/cleantechnol2040028





