On the Removal and Desorption of Sulfur Compounds from Model Fuels with Modified Clays
Abstract
:1. Introduction
2. Experiments
2.1. Conditioning of the Adsorbents
2.2. Synthetic Fuels and Solutions
2.3. Adsorption Experiments
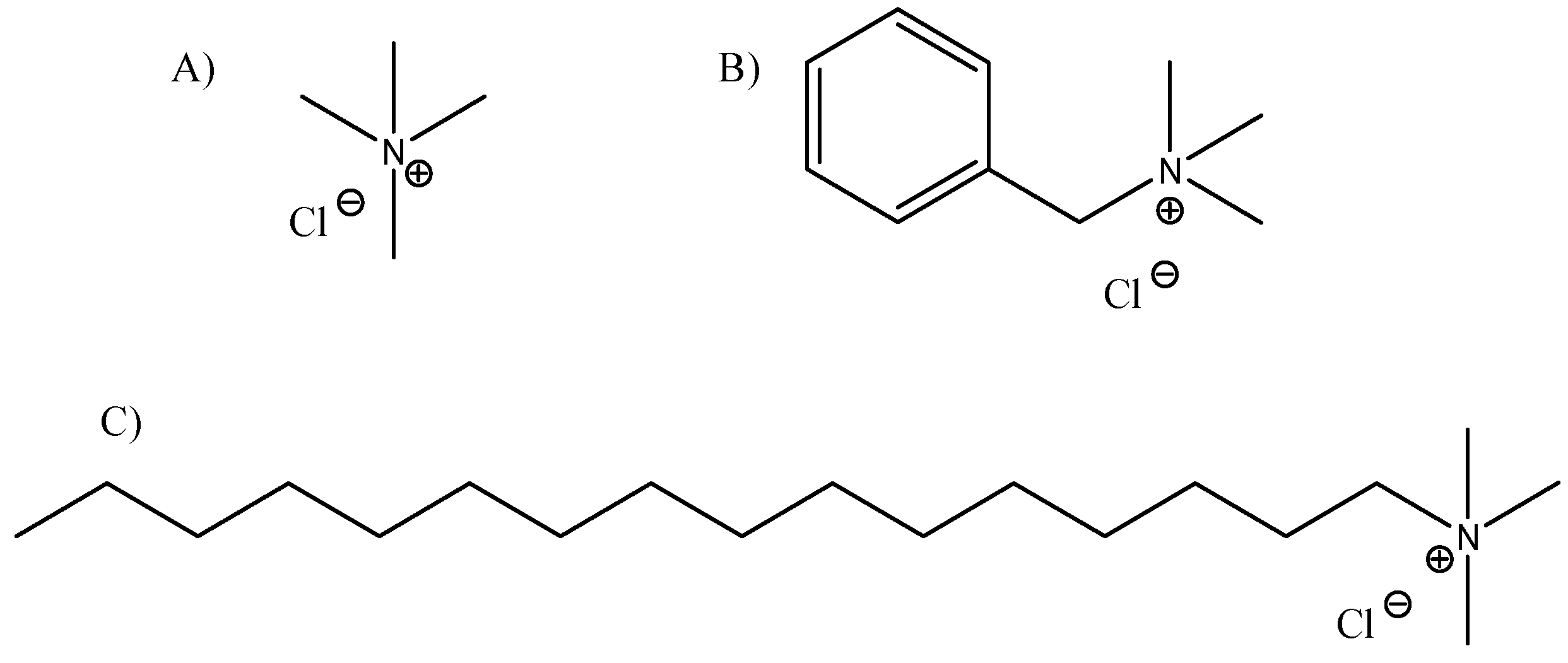
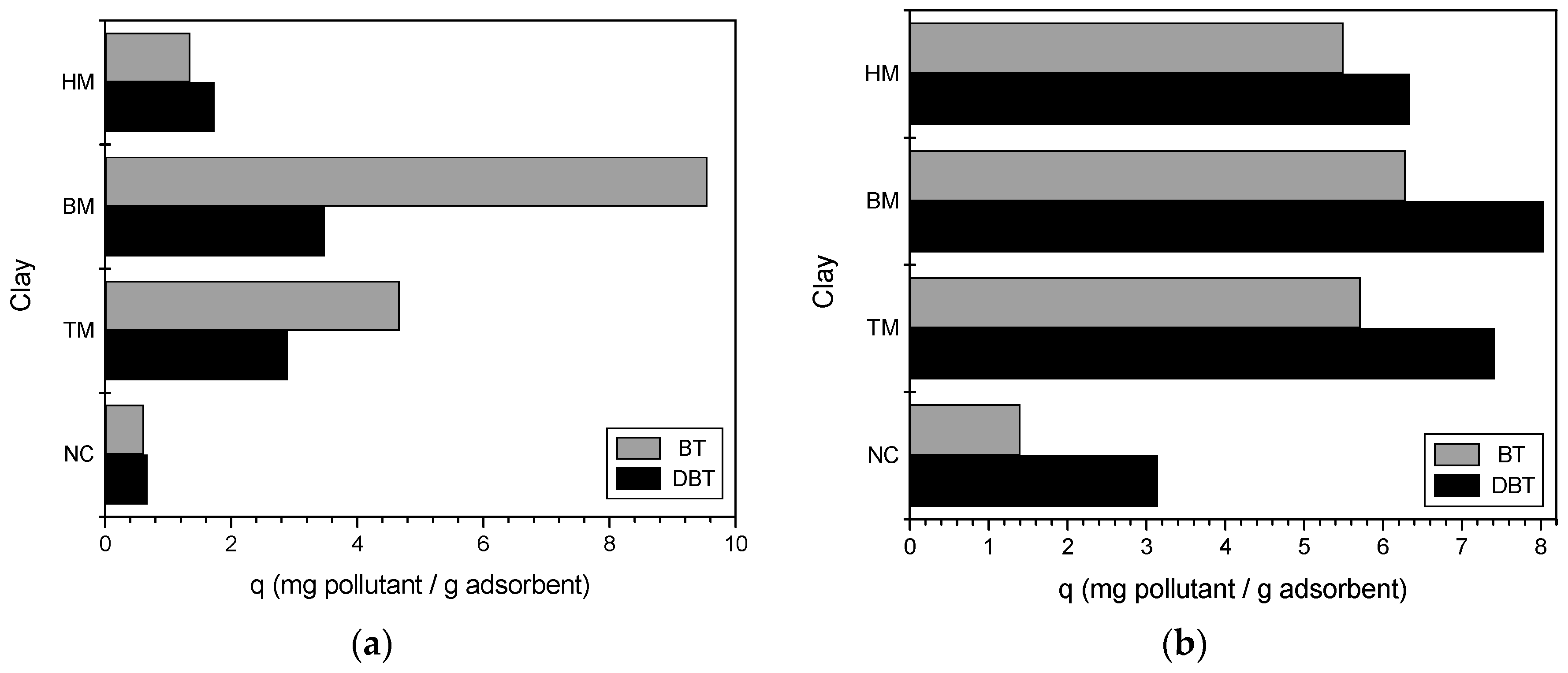
2.3.1. Best Modified Clay
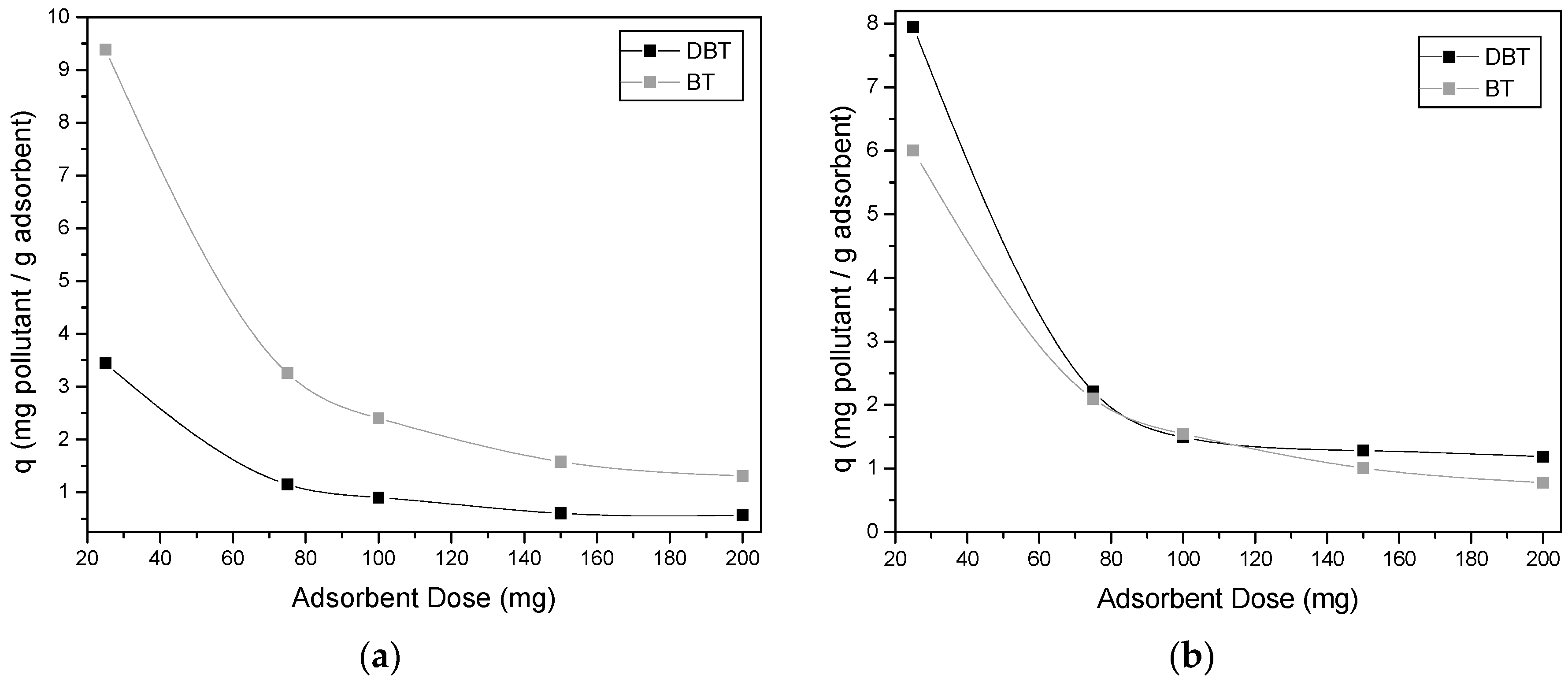
2.3.2. Adsorption Isotherms and Effect of Adsorbent Dosage
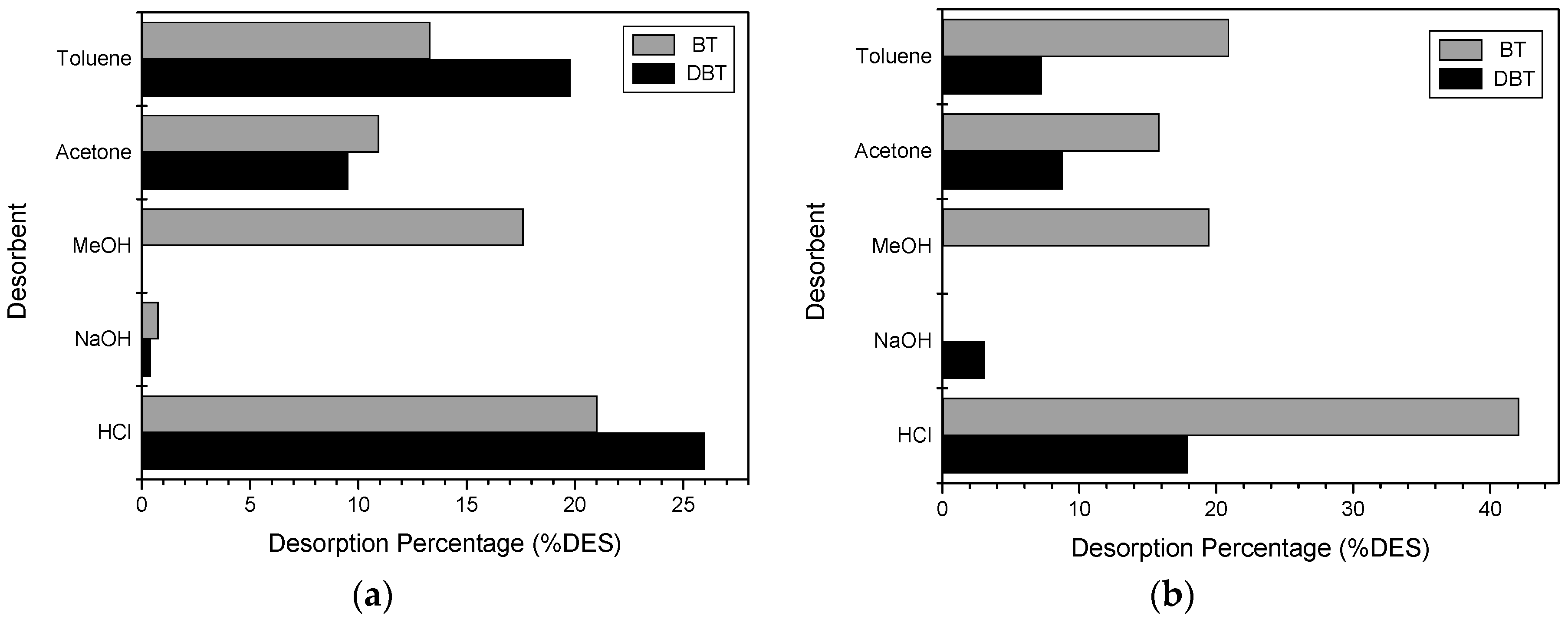
2.4. Desorption Experiments
2.5. Data Analysis
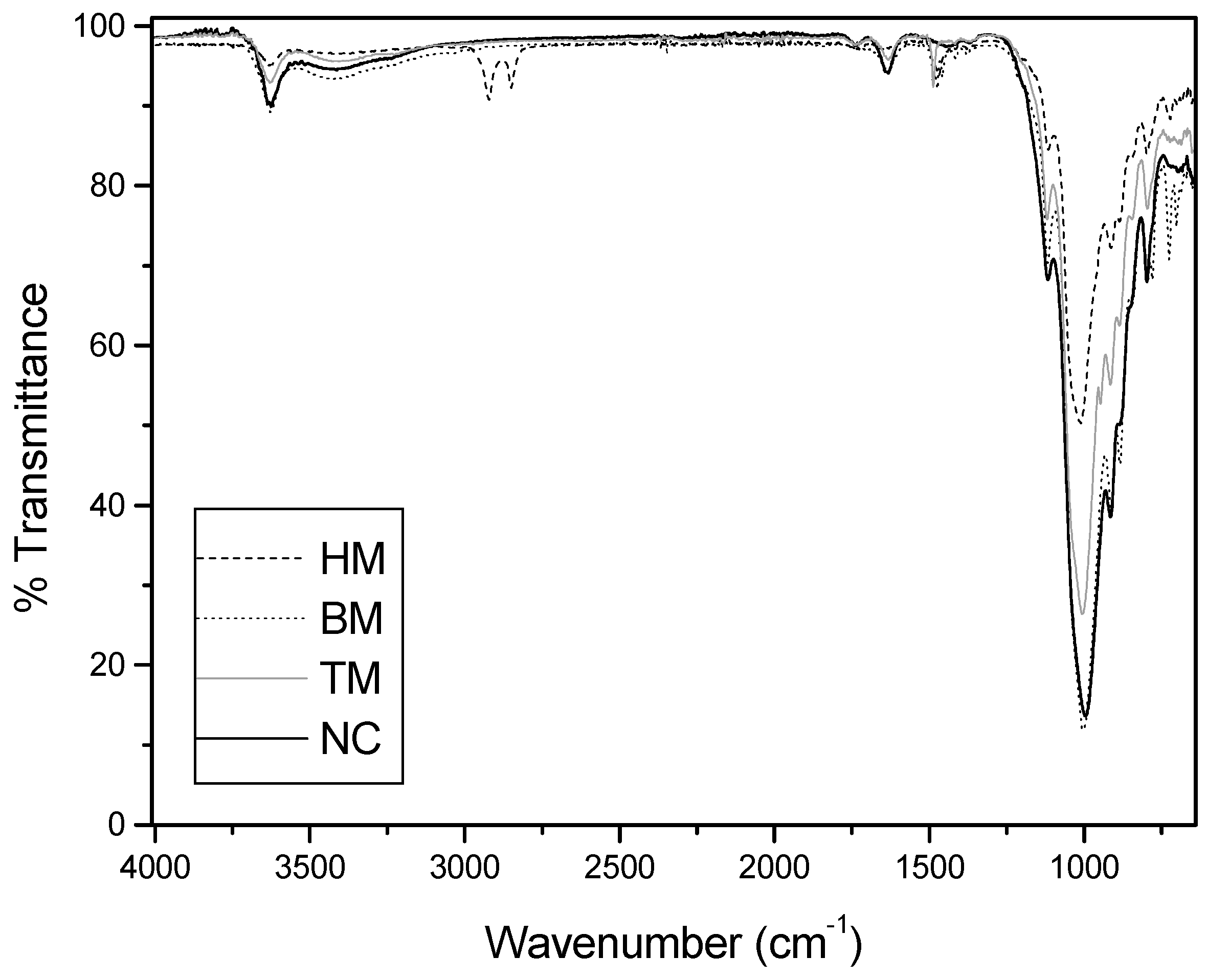
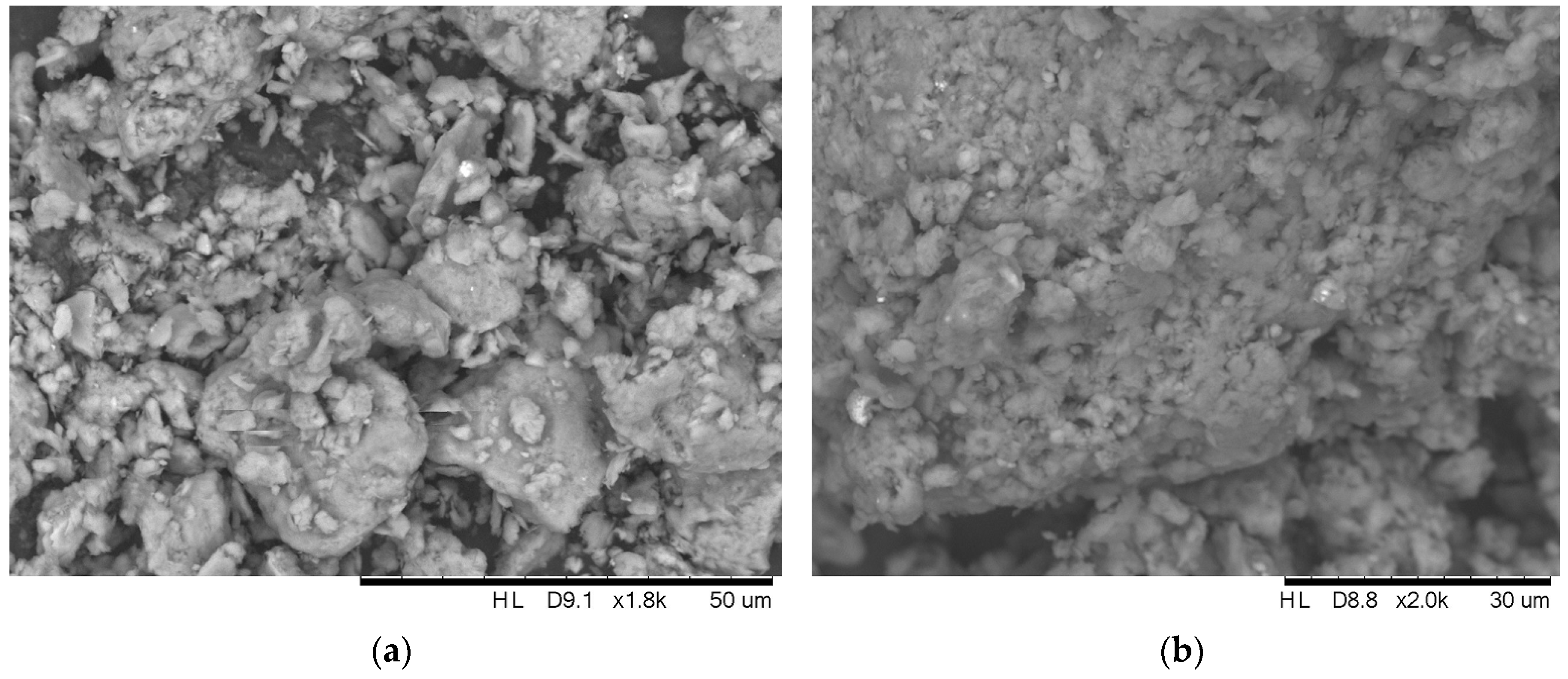
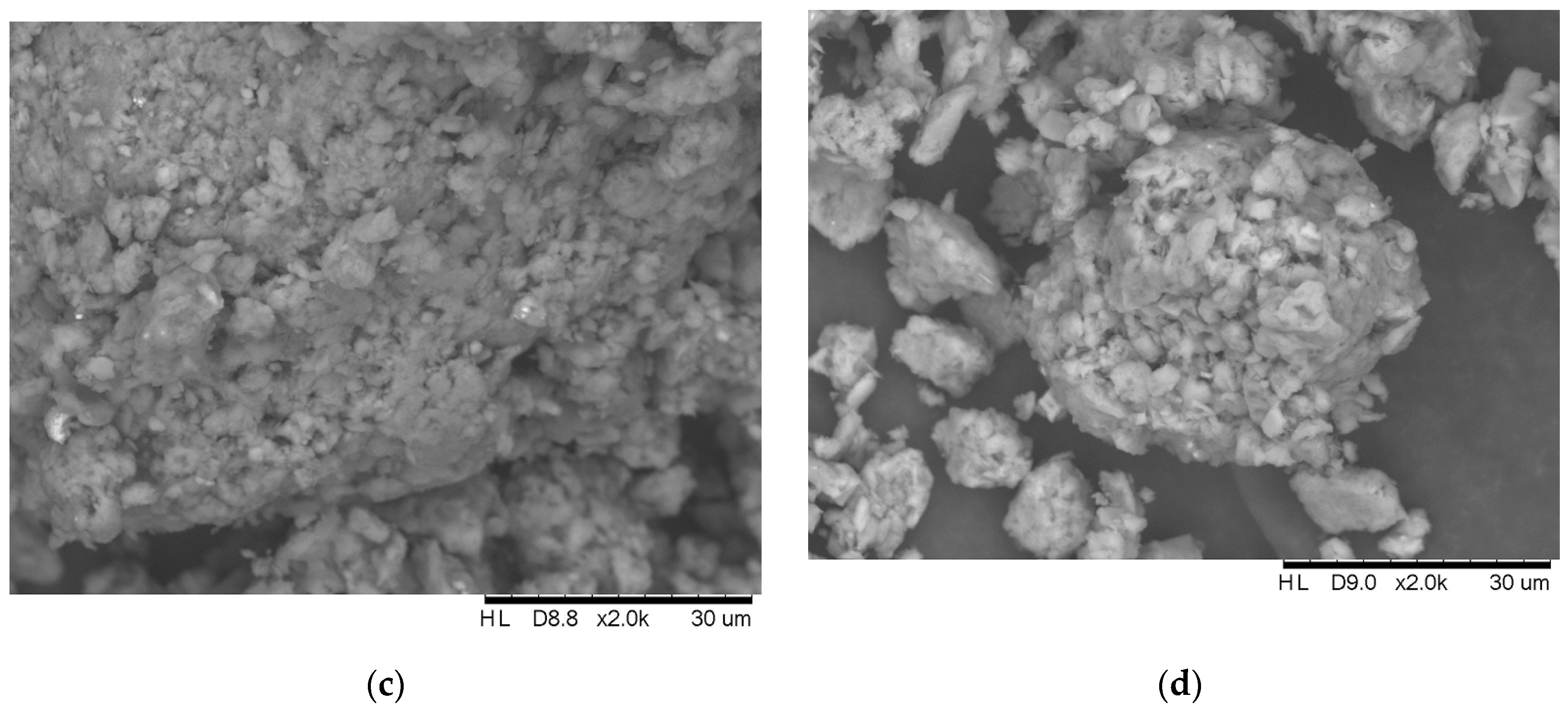
2.6. Physical and Chemical Characterization of the Adsorbents
3. Results
3.1. Best Modified Clay
3.2. Effect of Adsorbent Dosage
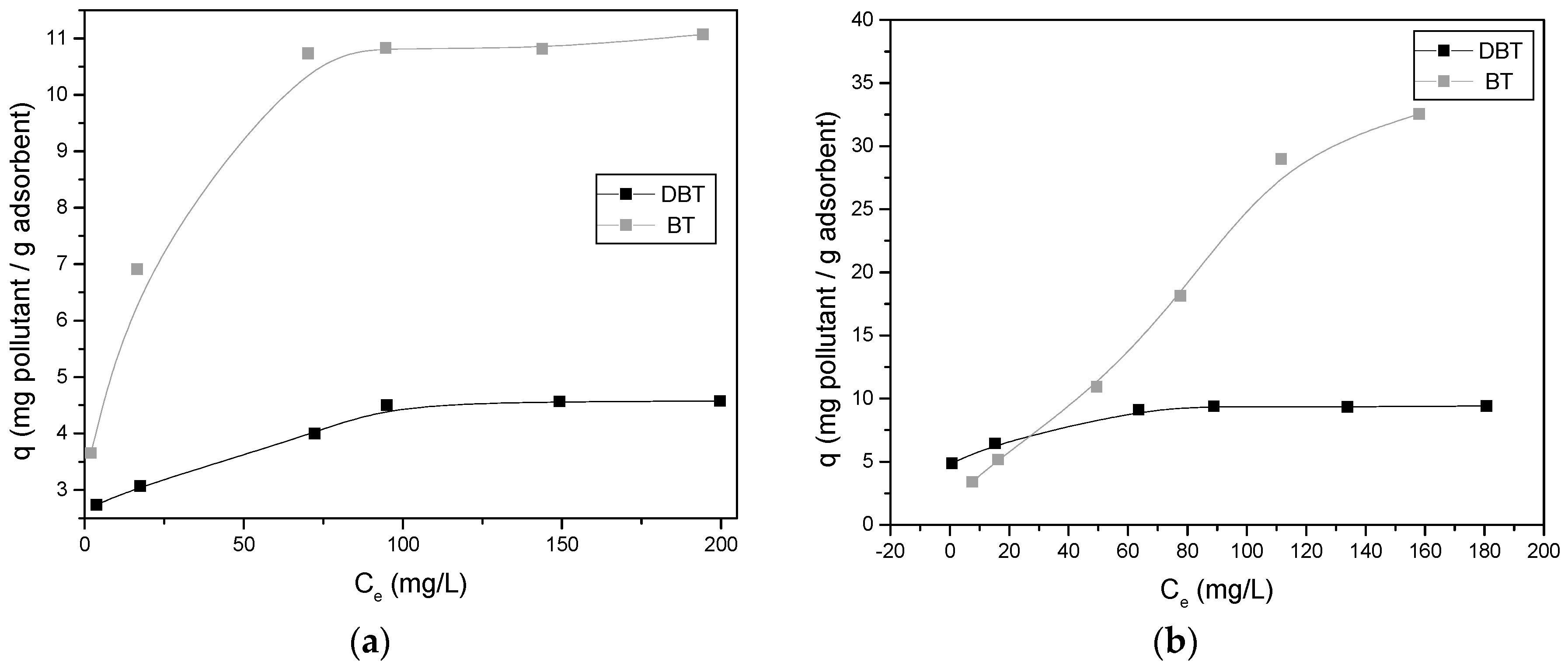
3.3. Effect of the Concentration of Pollutant—Adsorption Isotherms
3.4. Desorption Experiments
3.5. Physical and Chemical Characterization of the Adsorbents
4. Discussion
4.1. Best Modified Clay
4.2. Effect of Adsorbent Dosage
4.3. Adsorption Isotherms
4.4. Desorption Experiments
4.5. Insights into the Adsorption Mechanism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brock, W.A.; Taylor, M.S. Economic Growth and the Environment: A Review of Theory and Empirics; En NBER Working Paper 10854; National Bureau of Economic Research: Cambridge, MA, USA, 2004. [Google Scholar]
- Stern, D.I. Beyond the environmental Kuznets curve: Diffusion of emissions reducing technology. J. Environ. Dev. 2005, 14, 101–124. [Google Scholar] [CrossRef]
- Dharaskar, S.; Sillanpaa, M.; Tadi, K.K. Sulfur extraction from liquid fuels using trihexyl(tetradecyl)phosphonium tetrafluoroborate: As a promising solvent. Environ. Sci. Pollut. Res. Int. 2018, 25, 17156–17167. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Al-Hammadi, S.A.; Tanimu, A.; Alooshani, K. Ultra-deep adsorptive desulfurization of fuels on cobalt and molybdenum nanoparticles loaded on activated carbon derived from waste rubber. J. Colloid Interface Sci. 2018, 513, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ahmad, I.; Ahmad, W. Adsorptive Desulphurization study of liquid fuels using Tin (Sn) impregnated charcoal. J. Hazard. Mater. 2016, 304, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.P.; Kasinathan, P.; Kim, J.; Lee, D.; Woo, H.C. Deep desulfurization of fuel gas by adsorption on Cu-impregnated activated carbons in practical conditions. Korean J. Chem. Eng. 2016, 33, 1908–1916. [Google Scholar] [CrossRef]
- Baltzopoulou, P.; Kallis, K.; Karagiannakis, G.; Konstandopoulos, A. Diesel fuel desulfurization via adsorption with the aid of activated carbon: Laboratory- and Pilot-scale studies. Energy Fuels 2015, 29, 5640–5648. [Google Scholar] [CrossRef]
- Navarro, A.E.; Cuizano, N.; Lazo, J.; Sun-Kou, M.; Llanos, B.P. Comparative study of the removal of phenolic compounds by biological and non-biological adsorbents. J. Hazard. Mater. 2009, 164, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.E.; Lazo, J.; Cuizano, N.; Sun-Kou, M.; Llanos, B.P. Insights into the removal of phenol from aqueous solutions by low cost adsorbents: Clays versus algae. Sep. Sci. Technol. 2009, 44, 2491–2509. [Google Scholar] [CrossRef]
- Lazo, J.C.; Navarro, A.E.; Sun-Kou, M.R.; Llanos, B.P. Synthesis and characterization of organophilic clays and their use as adsorbents for phenol. Rev. Soc. Quím. Perú 2008, 74, 3–19. [Google Scholar]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Sastre de Vicente, M. Biosorption of phenolic compounds by the Brown algae Sargassum muticum. J. Chem. Technol. Biotechnol. 2006, 81, 1093–1099. [Google Scholar] [CrossRef]
- Navarro, A.E.; Portales, R.; Sun-Kou, R.; Llanos, B.P. Effect of pH on phenol biosorption by marine seaweeds. J. Hazard. Mater. 2008, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J. Fundamentals and Applications of Biosorption Isotherms and Kinetics and Thermodynamics; Nova Science Publishers, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Volesky, B. Sorption and Biosorption; BV Sorbex, Inc.: Quebec, QC, Canada, 2003. [Google Scholar]
- Chojnacka, K. Biosorption and Bioaccumulation in Practice; Nova Science Publishers, Inc.: New York, NY, USA, 2009. [Google Scholar]
| Isotherm Model and Parameters | Gasoline | Diesel | ||
|---|---|---|---|---|
| DBT | BT | DBT | BT | |
| Langmuir Model | ||||
| qmax (mg/g) | 4.74 | 11.311 | 9.404 | 31.32 |
| b (L/mg) | 0.103 | 0.245 | 1.407 | 0.015 |
| R2 | 0.979 | 0.999 | 0.998 | 0.964 |
| Freundlich Model | ||||
| kF (L/g) | 2.222 | 3.242 | 4.928 | 0.631 |
| n | 7.465 | 4.041 | 7.562 | 1.286 |
| R2 | 0.966 | 0.979 | 0.954 | 0.982 |
| D-R Model | ||||
| qmax (mg/g) | 4.582 | 10.867 | 9.317 | 34.834 |
| K | 2.18 × 10−5 | 1.07 × 10−6 | 1.56 × 10−7 | 5.08 × 10−4 |
| E (J/mole) | 151.41 | 684.02 | 1789.68 | 31.362 |
| R2 | 0.999 | 0.999 | 0.998 | 0.955 |
| Temkin Model | ||||
| at | 44.9 | 4.237 | 220.145 | 0.072 |
| bt | 4857.62 | 1433.57 | 2715.39 | 195.736 |
| R2 | 0.955 | 0.985 | 0.936 | 0.891 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.W.; Japhe, T.; Demeke, T.; Moreno, B.; Navarro, A.E. On the Removal and Desorption of Sulfur Compounds from Model Fuels with Modified Clays. Clean Technol. 2019, 1, 58-69. https://doi.org/10.3390/cleantechnol1010005
Ha JW, Japhe T, Demeke T, Moreno B, Navarro AE. On the Removal and Desorption of Sulfur Compounds from Model Fuels with Modified Clays. Clean Technologies. 2019; 1(1):58-69. https://doi.org/10.3390/cleantechnol1010005
Chicago/Turabian StyleHa, Jeong W., Tenzing Japhe, Tesfamichael Demeke, Bertin Moreno, and Abel E. Navarro. 2019. "On the Removal and Desorption of Sulfur Compounds from Model Fuels with Modified Clays" Clean Technologies 1, no. 1: 58-69. https://doi.org/10.3390/cleantechnol1010005
APA StyleHa, J. W., Japhe, T., Demeke, T., Moreno, B., & Navarro, A. E. (2019). On the Removal and Desorption of Sulfur Compounds from Model Fuels with Modified Clays. Clean Technologies, 1(1), 58-69. https://doi.org/10.3390/cleantechnol1010005





