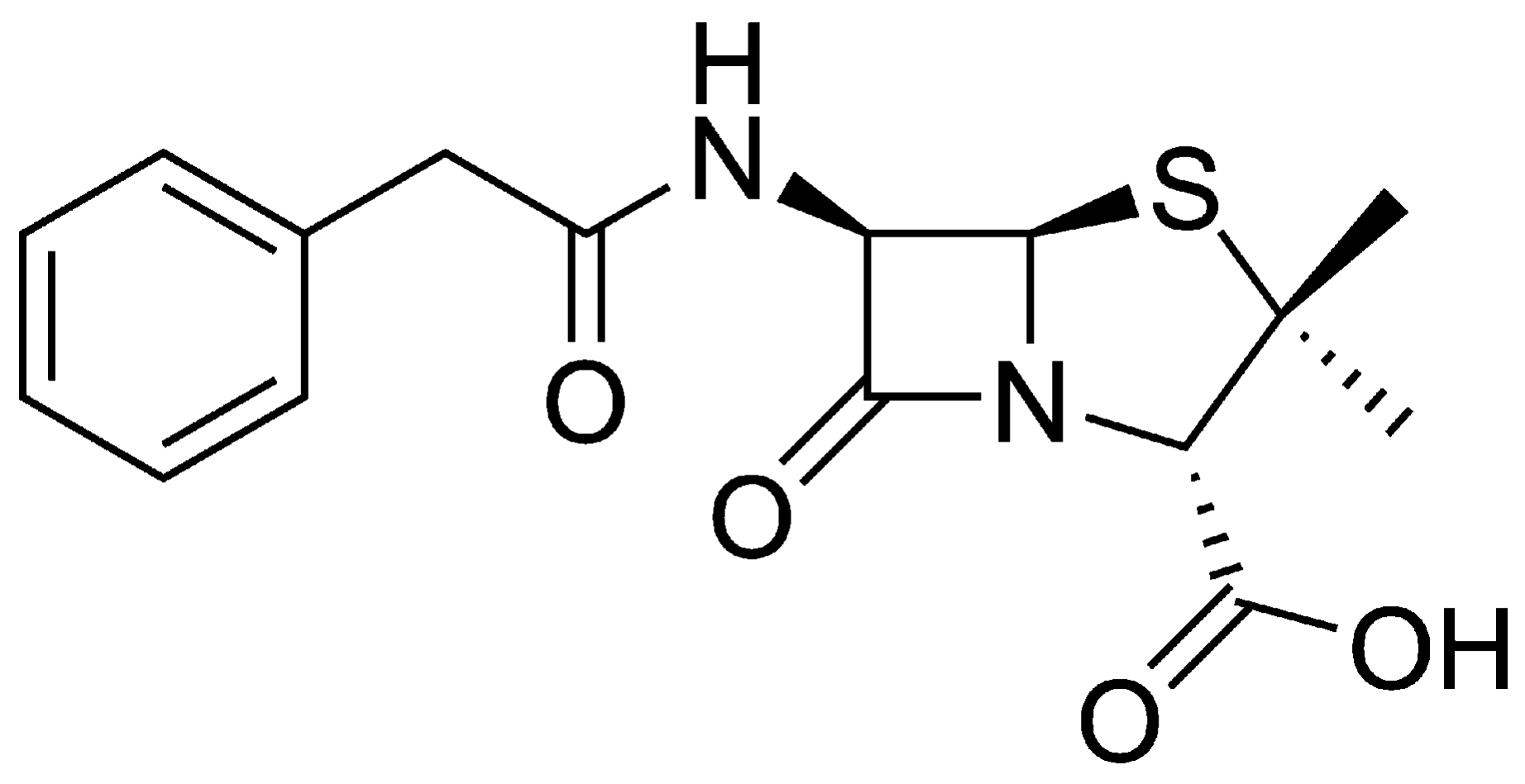
Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G
Abstract
:1. Introduction
2. Experiments
2.1. Conditioning of the Adsorbents
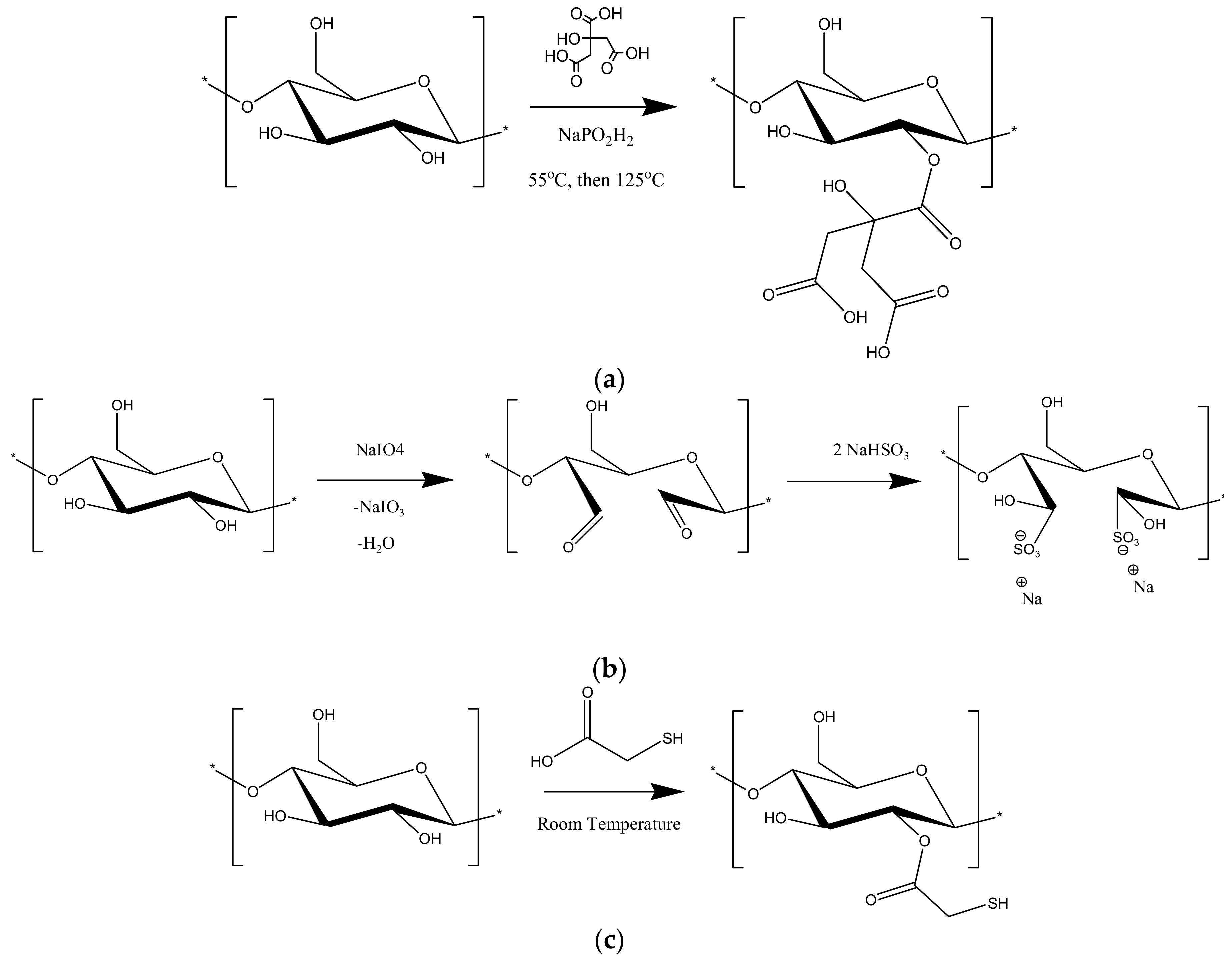
2.2. Chemical Modification of the Adsorbents
2.3. Preparation and Quantification of Penicillin G Solutions
2.4. Adsorption Experiments
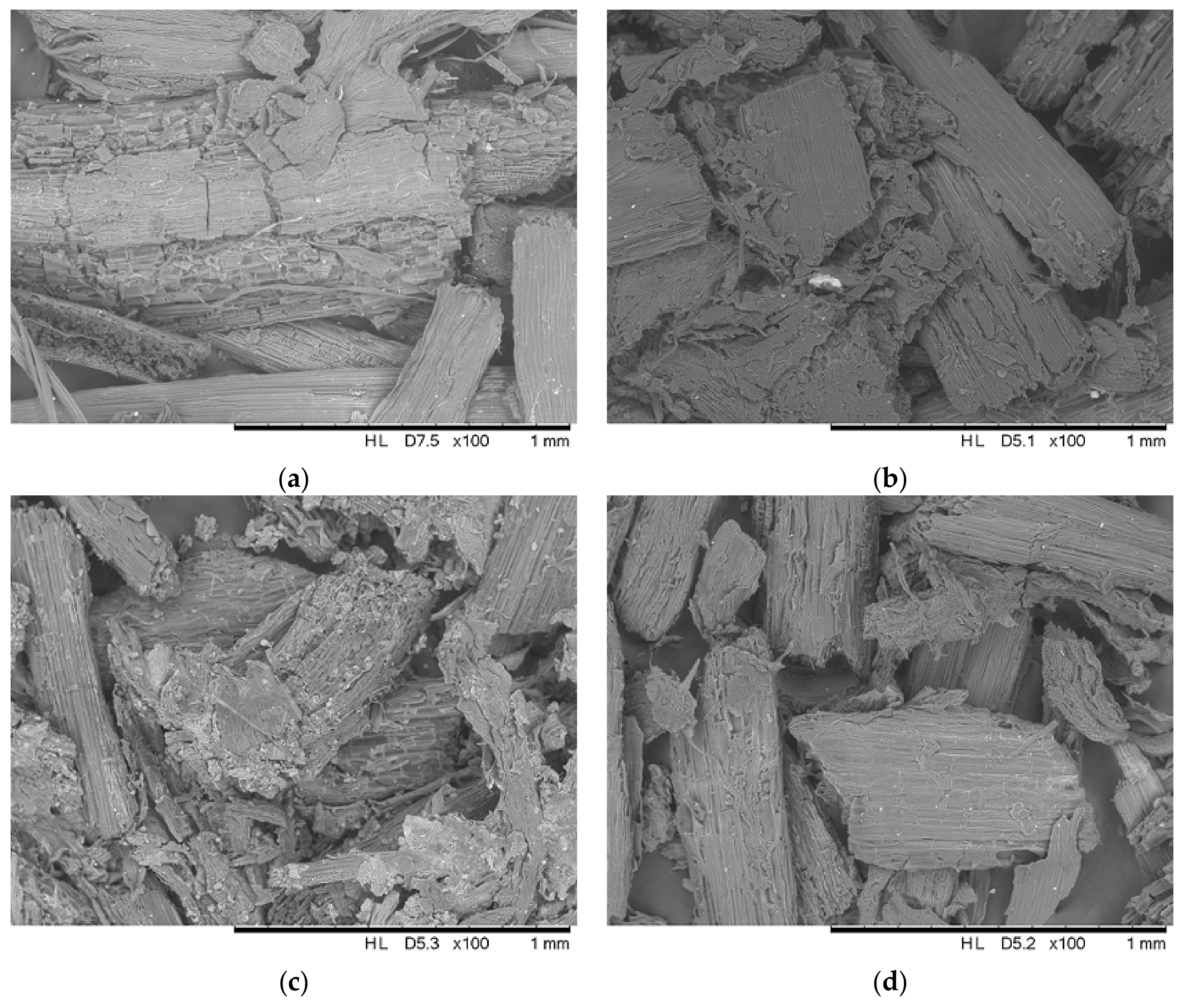
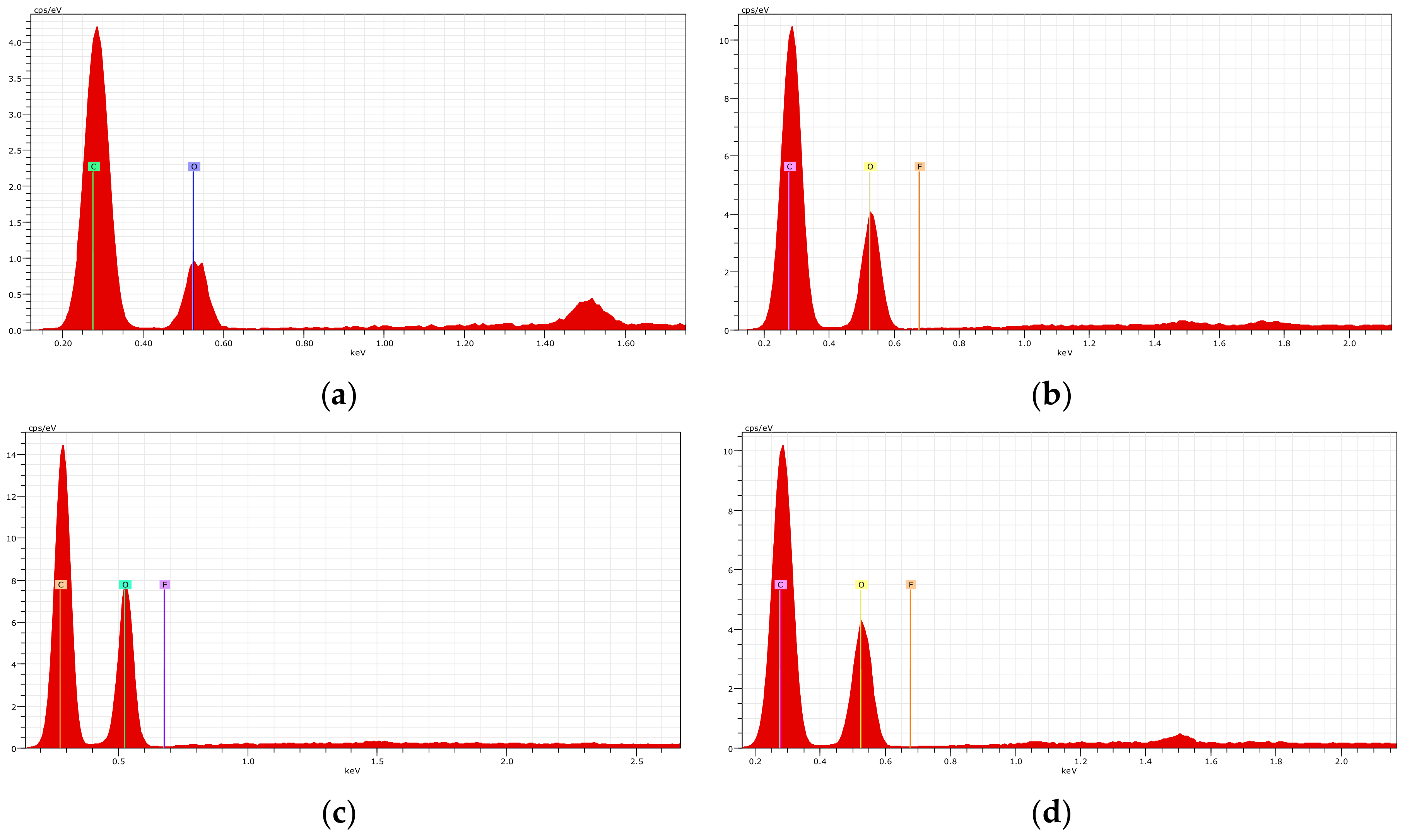
2.5. Characterization of the Adsorbents
2.6. Data Analysis of the Adsorption Experiments
3. Results and Discussion
3.1. Chemical Modification of the Adsorbents
3.2. Characterization of the Adsorbents
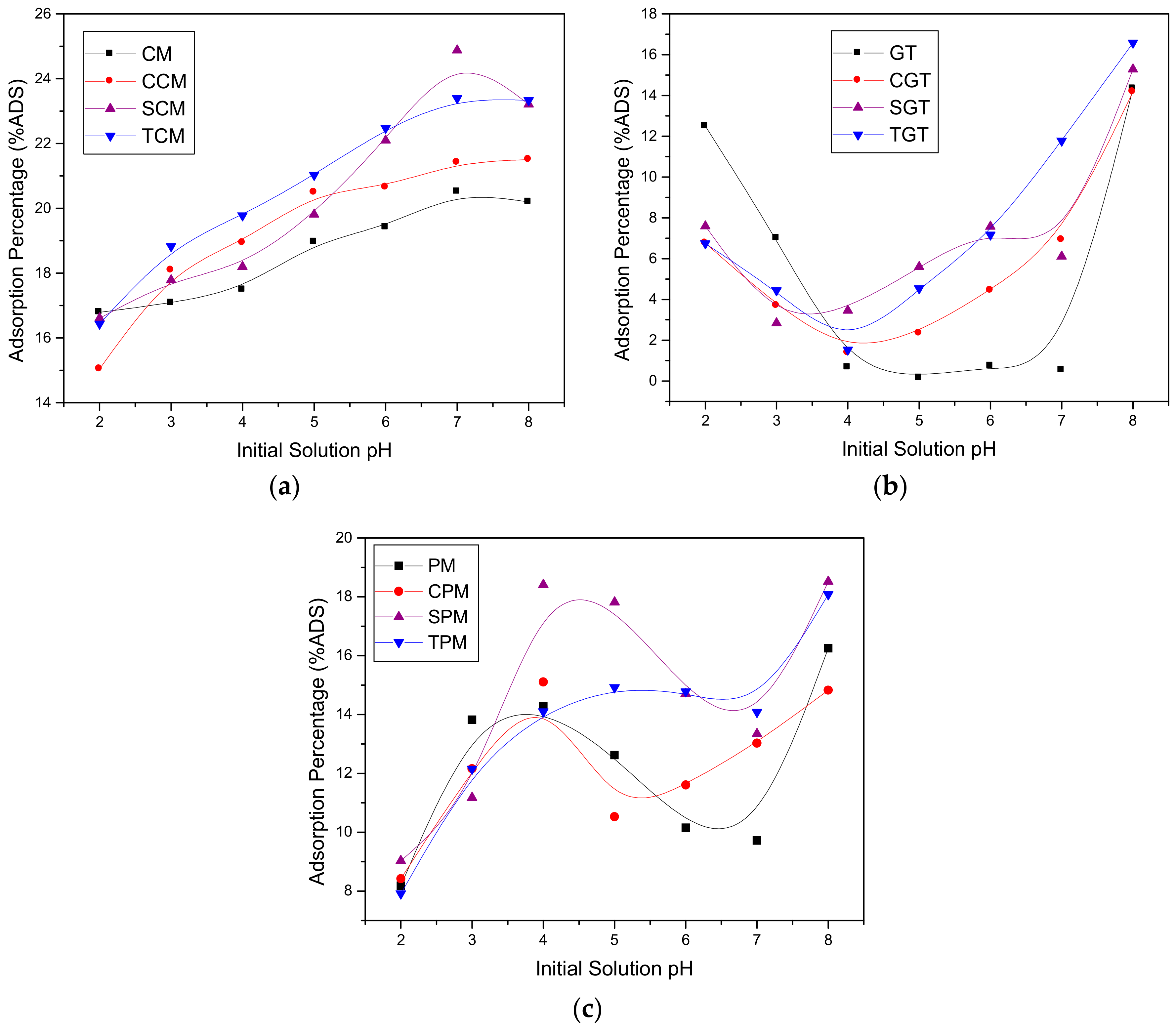
3.3. Effect of the Initial Solution pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunningham, V.; Buzby, M.; Hutchinson, T.; Mastrocco, F.; Parke, N.; Roden, N. Effects of Human Pharmaceuticals on Aquatic Life: Next Steps. Environ. Sci. Technol. 2006, 40, 3456–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jjemba, P. Excretion and Ecotoxicity of Pharmaceutical and Personal Care Products in the Environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances: The Micropollutant Challenge for Urban Water Management; IWA Publishing: London, UK, 2006; Volume 21, p. 53. [Google Scholar]
- Liu, Y.; Wang, J. Fundamentals and Applications of Biosorption Isotherms, Kinetics and Thermodynamics; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Kotrba, P.; Machova, M.; Macek, T. Microbial Biosorption of Metals; Springer Science: New York, NY, USA, 2011. [Google Scholar]
- Volesky, B. Sorption and Biosorption; BV SORBEX Inc.: Quebec, QC, Canada, 2004. [Google Scholar]
- Choi, Y.; Isaac, P.; Irkakhujaev, S.; Masud, M.E.; Navarro, A.E. Use of spent tea wastes-chitosan capsules for the removal of divalent copper ions. J. Environ. Sci. 2015, 1, 003. [Google Scholar]
- Park, R.; Kim, G.; Shen, L.; Hong, M.; Navarro, A.E. Batch adsorption of heavy metals onto chai tea residues for the bioremediation of contaminated solutions. Curr. Top. Biotechnol. 2014, 8, 51–62. [Google Scholar]
- Navarro, A.E.; Musaev, H.; Serrano, K.; Masud, M.E. Adsorption kinetics of cobalt (II) ions onto alginate beads from aqueous solutions. J. Earth Sci. Clim. Chang. 2014, 5, 223. [Google Scholar] [CrossRef]
- Zahir, H.; Naidoo, M.; Kostadinova, R.M.; Ortiz, K.A.; Sun-Kou, R.; Navarro, A.E. Decolorization of hair dye by lignocellulosic waste materials from contaminated waters. Front. Environ. Toxicol. 2014, 2, 28. [Google Scholar] [CrossRef]
- Zarzar, A.; Hong, M.; Llanos, B.; Navarro, A.E. Insights into the eco-friendly adsorption of caffeine from contaminated solutions by using hydrogel beads. J. Environ. Anal. Chem. 2015, 2, 4. [Google Scholar]
- Japhe, T.; Zhdanova, K.; Rodenburg, L.; Roberson, L.; Navarro, A.E. Factors affecting the Biosorption of 2-Chlorophenol using spent tea leaf wastes as adsorbents. J. Environ. Sci. 2015, 1, 010. [Google Scholar]
- Kim, G.; Garcia, H.; Japhe, T.; Llanos, B.; Navarro, A.E. On the desalination of saline waters via batch adsorption with spent tea leaves. J. Pet. Environ. Biotechnol. 2017, 8, 3. [Google Scholar]
- Richardson, M.; Bowron, J. The Fate of Pharmaceutical Chemicals in the Aquatic Environment. J. Pharmacy Pharmacol. 1985, 37, 1–12. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Antimicrobial Agents. Curr. Opin. Chem. Biol. 1997, 1, 169–175. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Kong, W.; Ren, J.; Liu, C.; Wang, K.; Sun, R.; She, D. Preparation, characterization of carboxylated bamboo fibers and their adsorption for lead(II) ions in aqueous solution. Cellulose 2013, 20, 2091–2100. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirvio, J.; Hormi, O.; Niinimaki, J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose 2013, 20, 741–749. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Z.; Ma, W. Adsorption of Pb(II) from glucose solution on thiol-functionalized cellulosic biomass. Bioresour. Technol. 2012, 104, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Japhe, T.; Paulsingh, R.; Ko, K.; Hong, J.; Navarro, A.E. Bioremoval of antibiotics by using biodegradable hydrogel beads from aqueous solutions. J. Environ. Sci. 2015, 1, 002. [Google Scholar]
- Navarro, A.E.; Portales, R.; Sun-Kou, R.; Llanos, B. Effect of pH on phenol biosorption by seaweeds. J. Hazard. Mater. 2008, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Minton, B. Is Your Tea Loaded with Toxic Fluoride? Available online: https://naturalsociety.com/is-your-tea-loaded-with-toxic-fluoride/ (accessed on 10 May 2017).
| Adsorbent | CCOOH (mmol/g) | Adsorbent | CCOOH (mmol/g) | Adsorbent | CCOOH (mmol/g) |
|---|---|---|---|---|---|
| CM | 1.36 | GT | 1.72 | PM | 1.4 |
| CCM | 1.48 | CGT | 1.88 | CPM | 2 |
| SCM | 1.76 | SGT | 2.02 | SPM | 1.58 |
| TCM | 1.98 | TGT | 2.08 | TPM | 1.66 |
| Adsorbent | % Carbon | % Oxygen | % Sulfur |
|---|---|---|---|
| CM | 72.07 | 27.93 | 0 |
| CCM | 65.12 | 34.89 | 0 |
| SCM | 58.92 | 39 | 2.08 |
| TCM | 62.69 | 35.11 | 2.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Morante, L.; Demeke, T.; Baah-Twum, J.; Navarro, A.E. Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G. Clean Technol. 2019, 1, 114-124. https://doi.org/10.3390/cleantechnol1010008
Silva J, Morante L, Demeke T, Baah-Twum J, Navarro AE. Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G. Clean Technologies. 2019; 1(1):114-124. https://doi.org/10.3390/cleantechnol1010008
Chicago/Turabian StyleSilva, Jesie, Lizebel Morante, Tesfamichael Demeke, Jacqueline Baah-Twum, and Abel E. Navarro. 2019. "Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G" Clean Technologies 1, no. 1: 114-124. https://doi.org/10.3390/cleantechnol1010008
APA StyleSilva, J., Morante, L., Demeke, T., Baah-Twum, J., & Navarro, A. E. (2019). Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G. Clean Technologies, 1(1), 114-124. https://doi.org/10.3390/cleantechnol1010008





