Washing Procedure with Several Reagents for Ecological Rehabilitation of Soil Polluted with Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Analyzing
2.2. Preparation of Extraction Solutions
2.3. Metal Extraction
3. Results
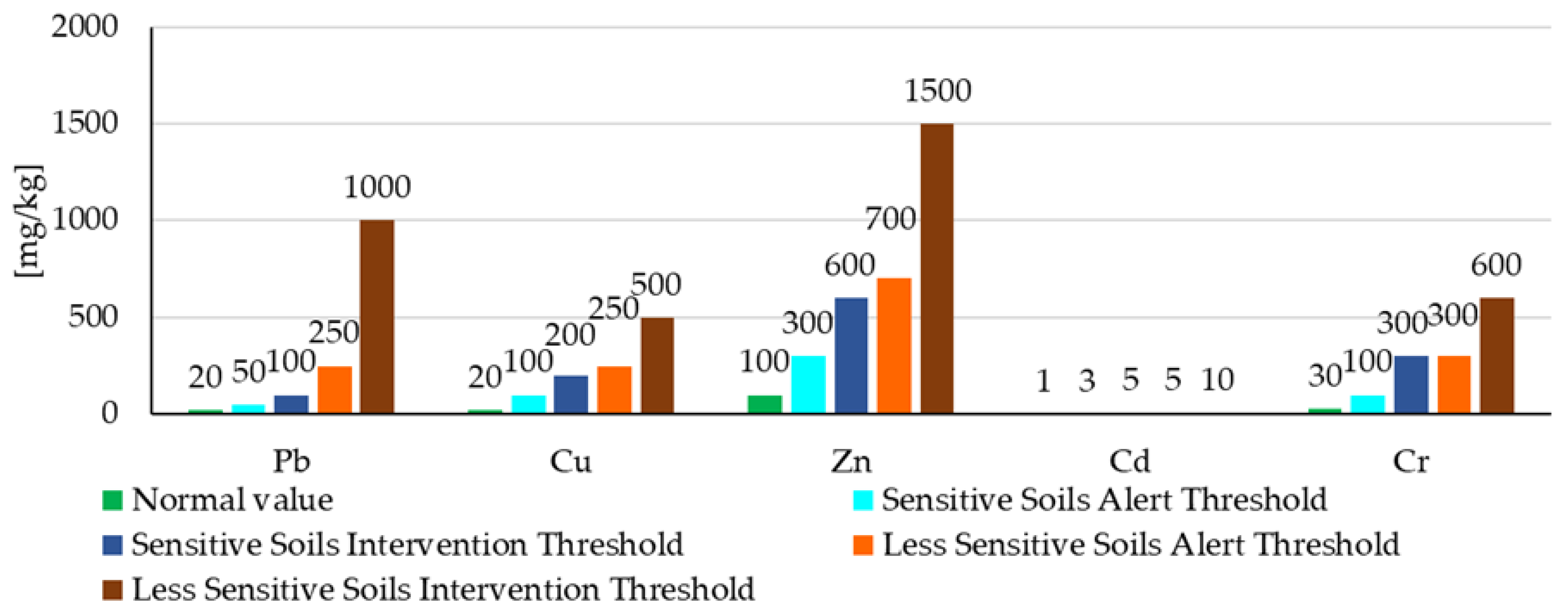
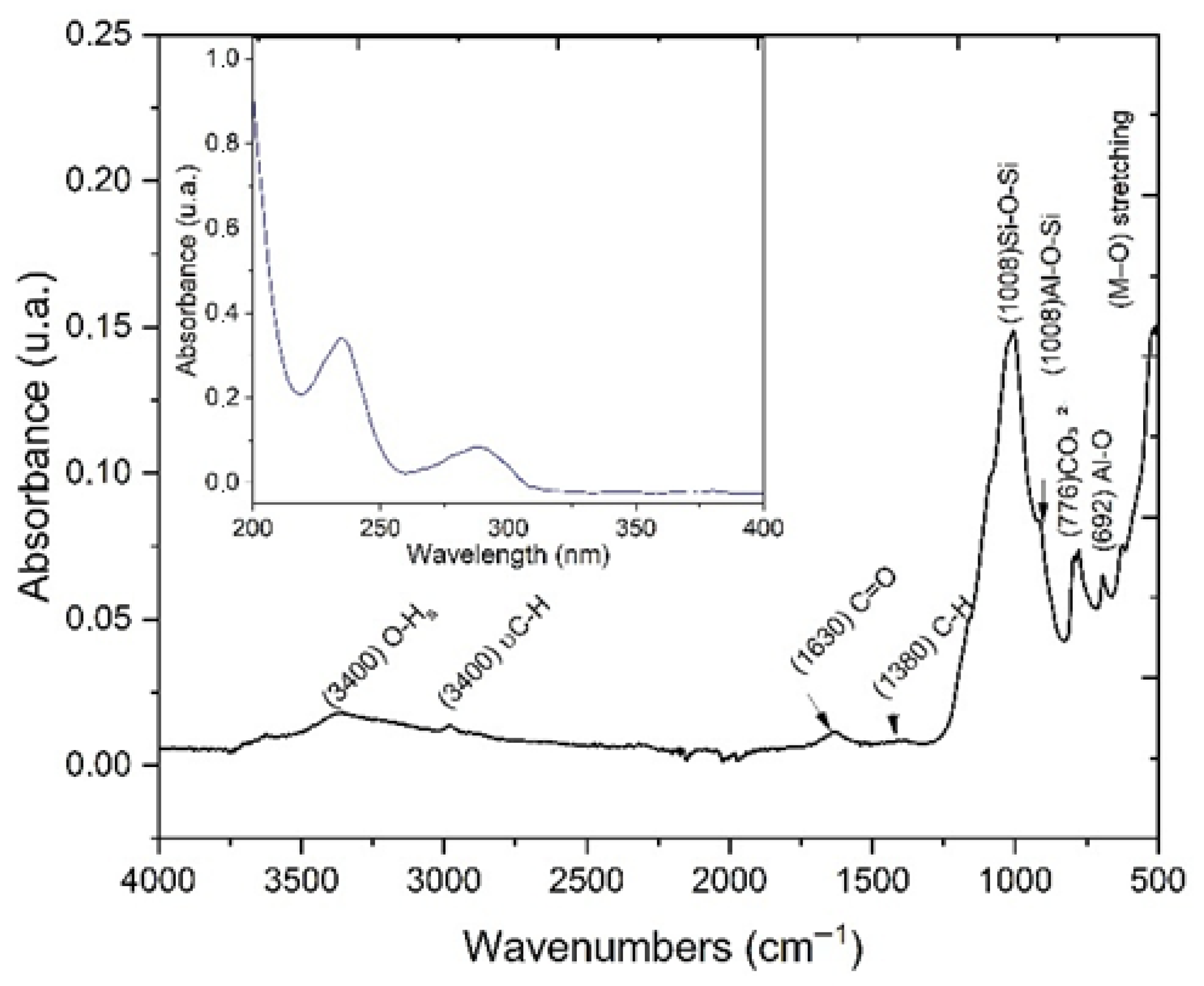
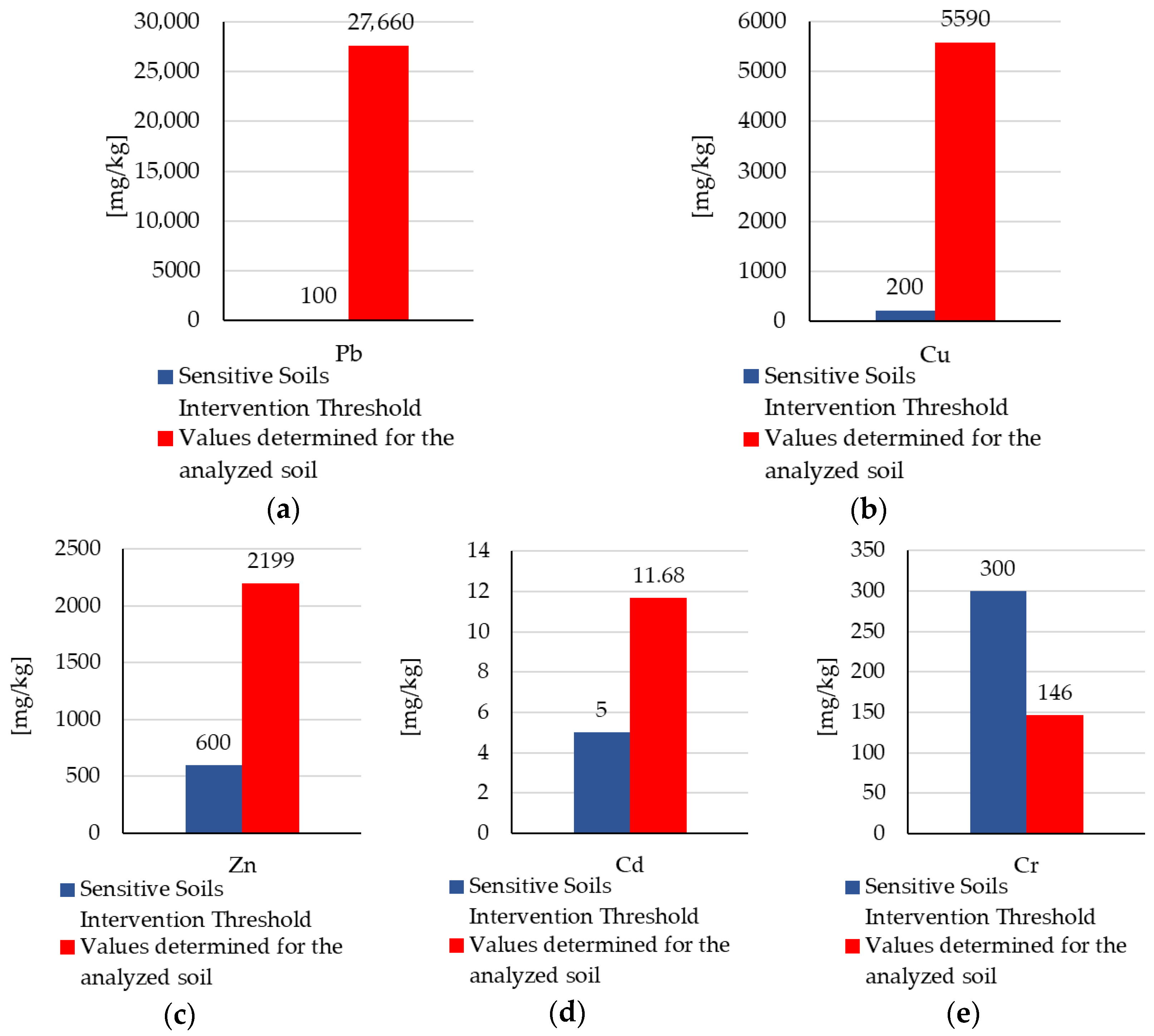
3.1. Soil Characterization
3.2. Metal Extraction from Soil
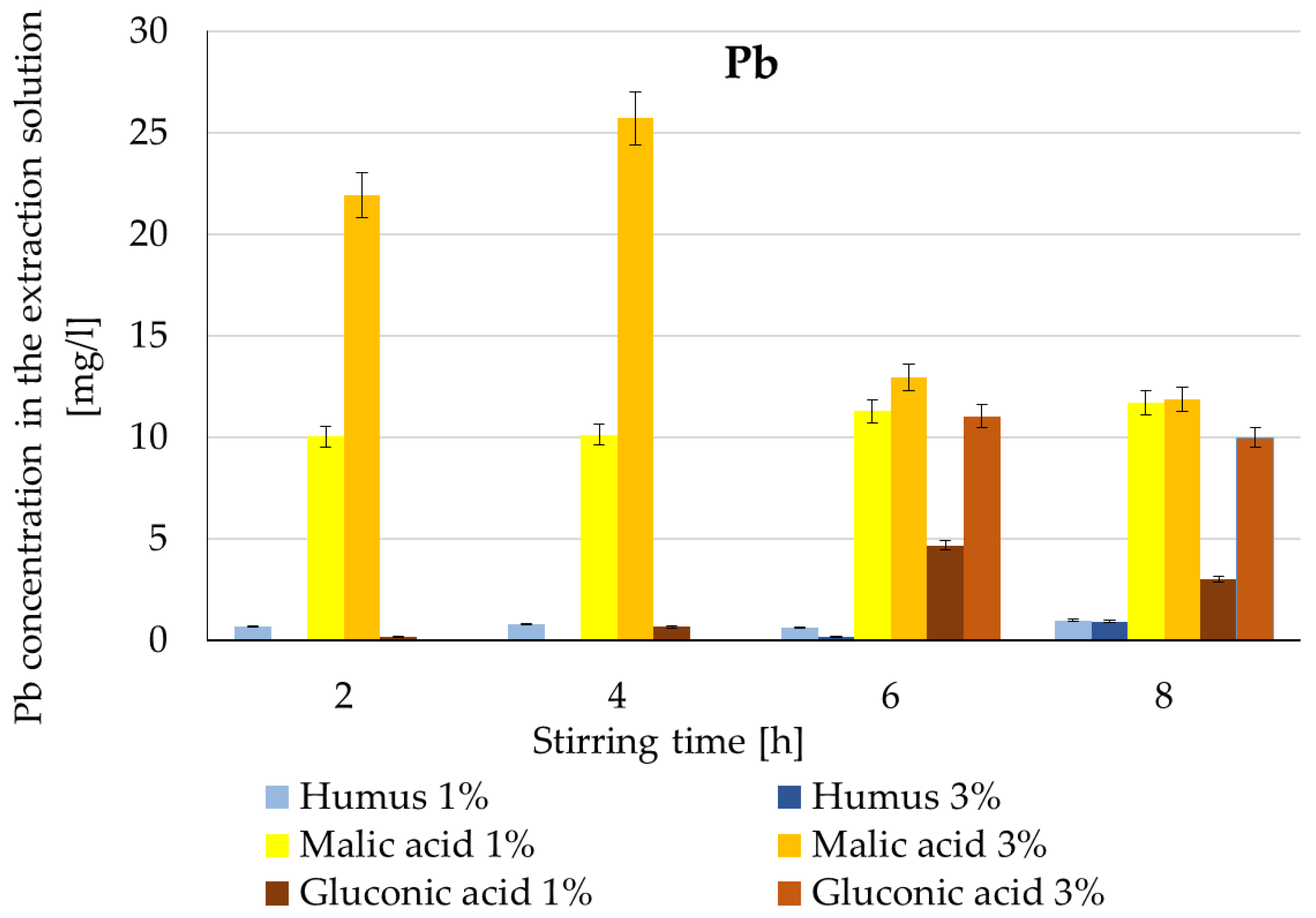
3.2.1. Individual Metal Extraction
3.2.2. Transport Phenomena and Extraction Mechanisms
3.2.3. Chemical Species and pH Influence
3.2.4. Cost–Benefit Analysis
- -
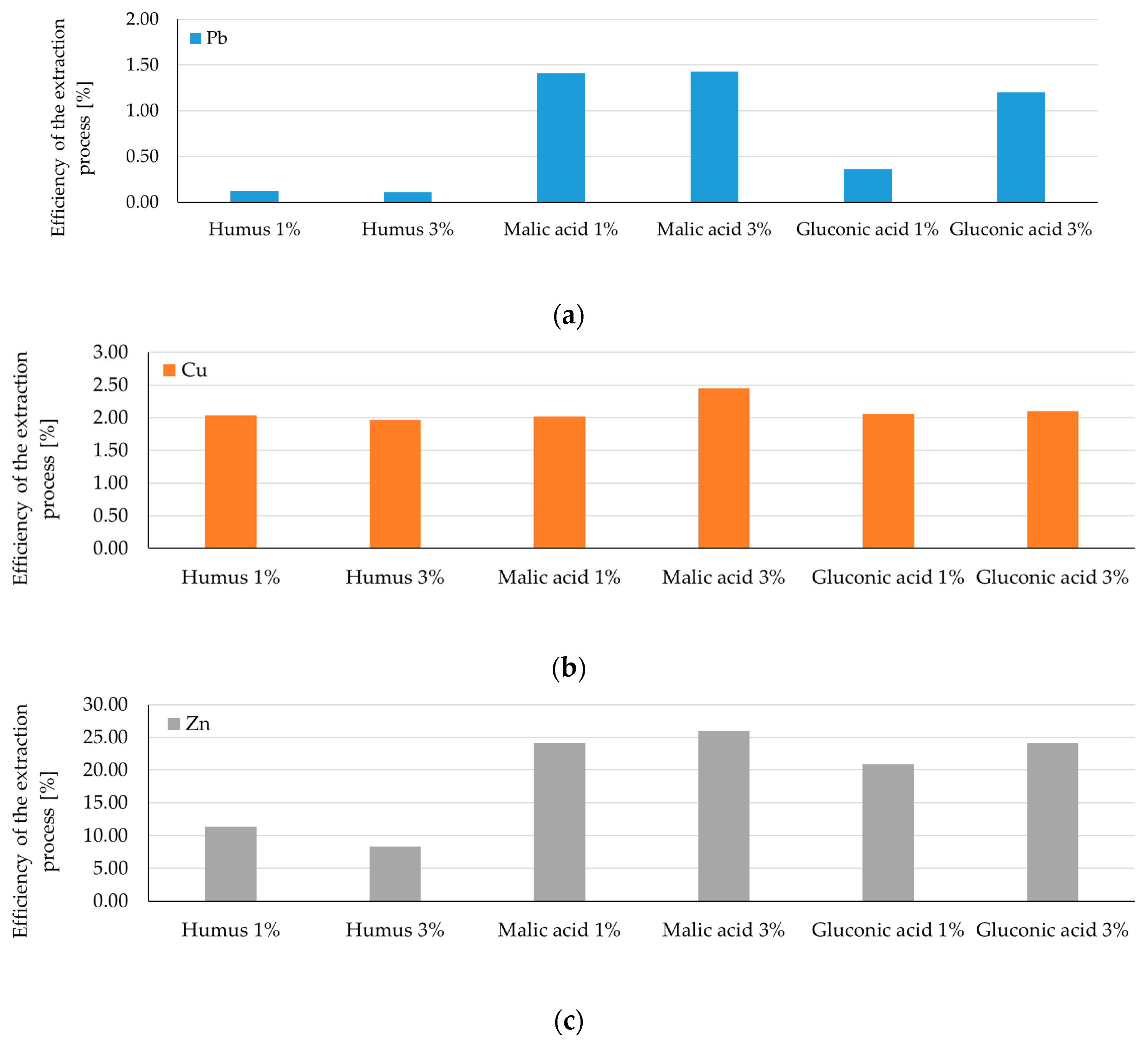
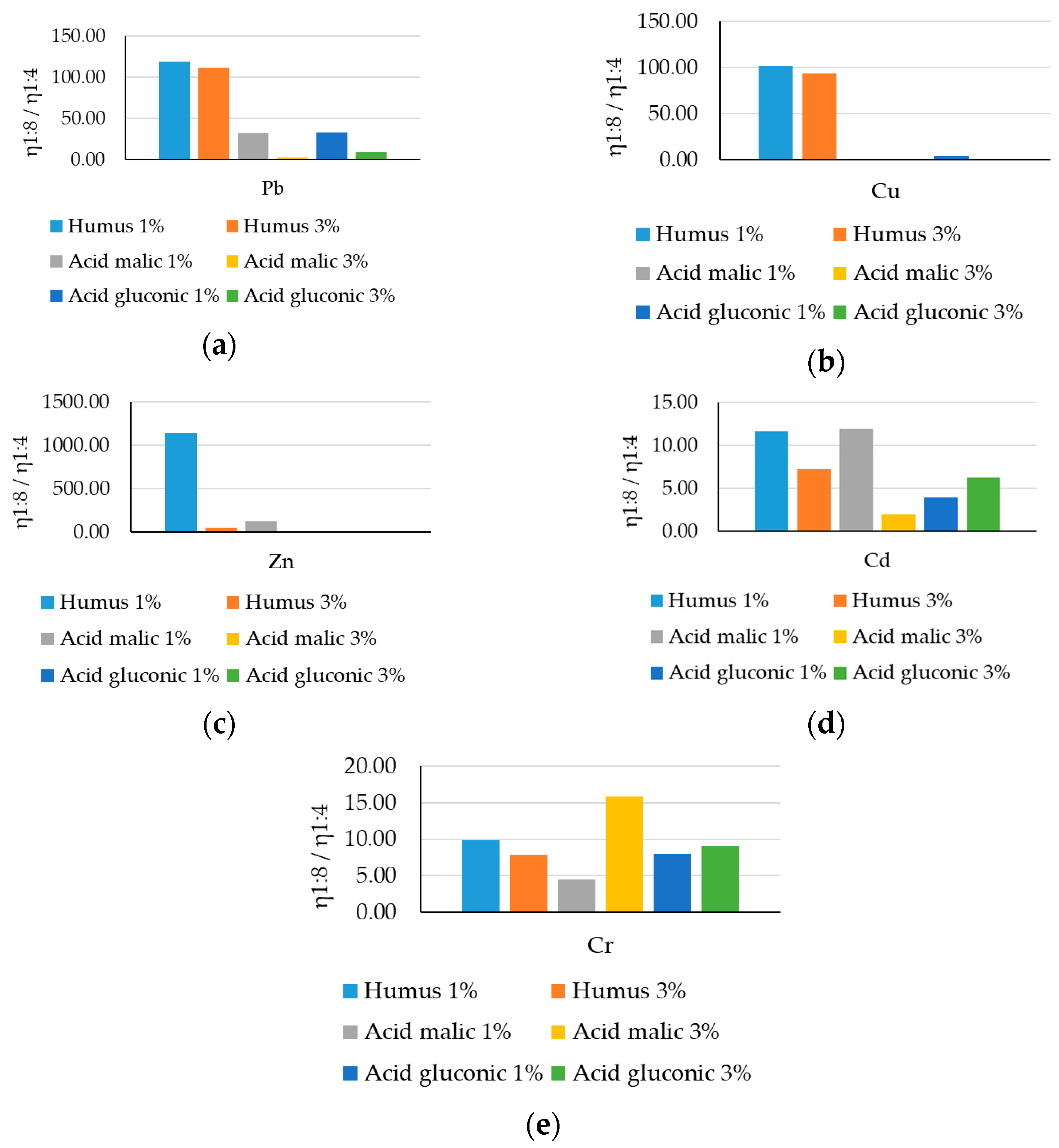
- for Pb extraction, the yield increases over 100 times using humus-based extraction solutions, over 30 times when using 1% malic acid and 1% gluconic acid solutions, and approximately 2.8 and 9 times when using h3% malic acid and 3% gluconic acid solutions, respectively.
- -
- for Cu extraction, the yield increases 90–100 times using humus-based extraction solutions, and 4 times using a 1% gluconic acid-based extraction solution, but with unsatisfactory results for the other extraction solutions.
- -
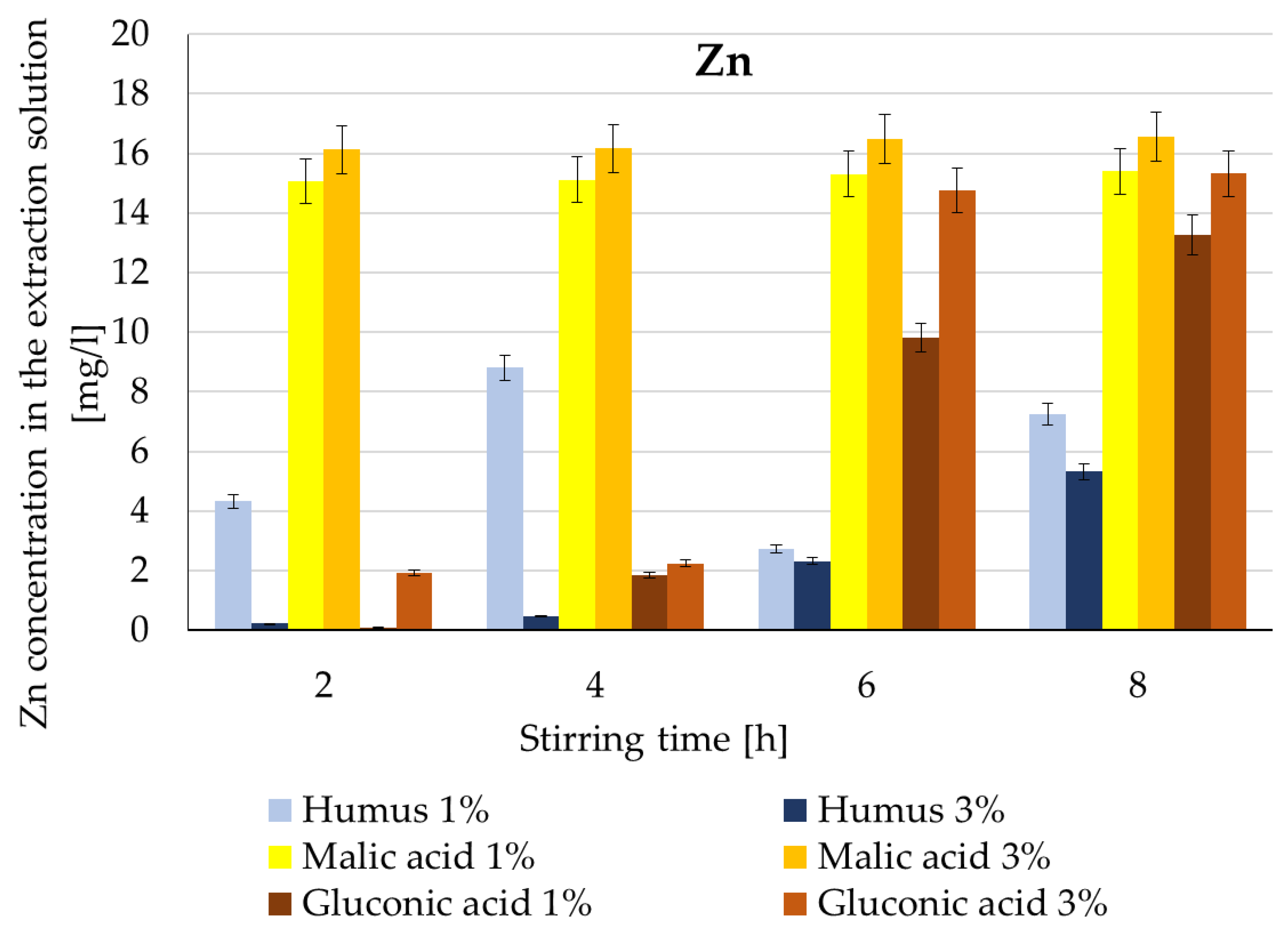
- good and very good values are also recorded in the case of Zn extraction, with humus-based extraction solutions and 1% malic acid again standing out, achieving yields 7–8 times better than the other extraction solutions.
- -
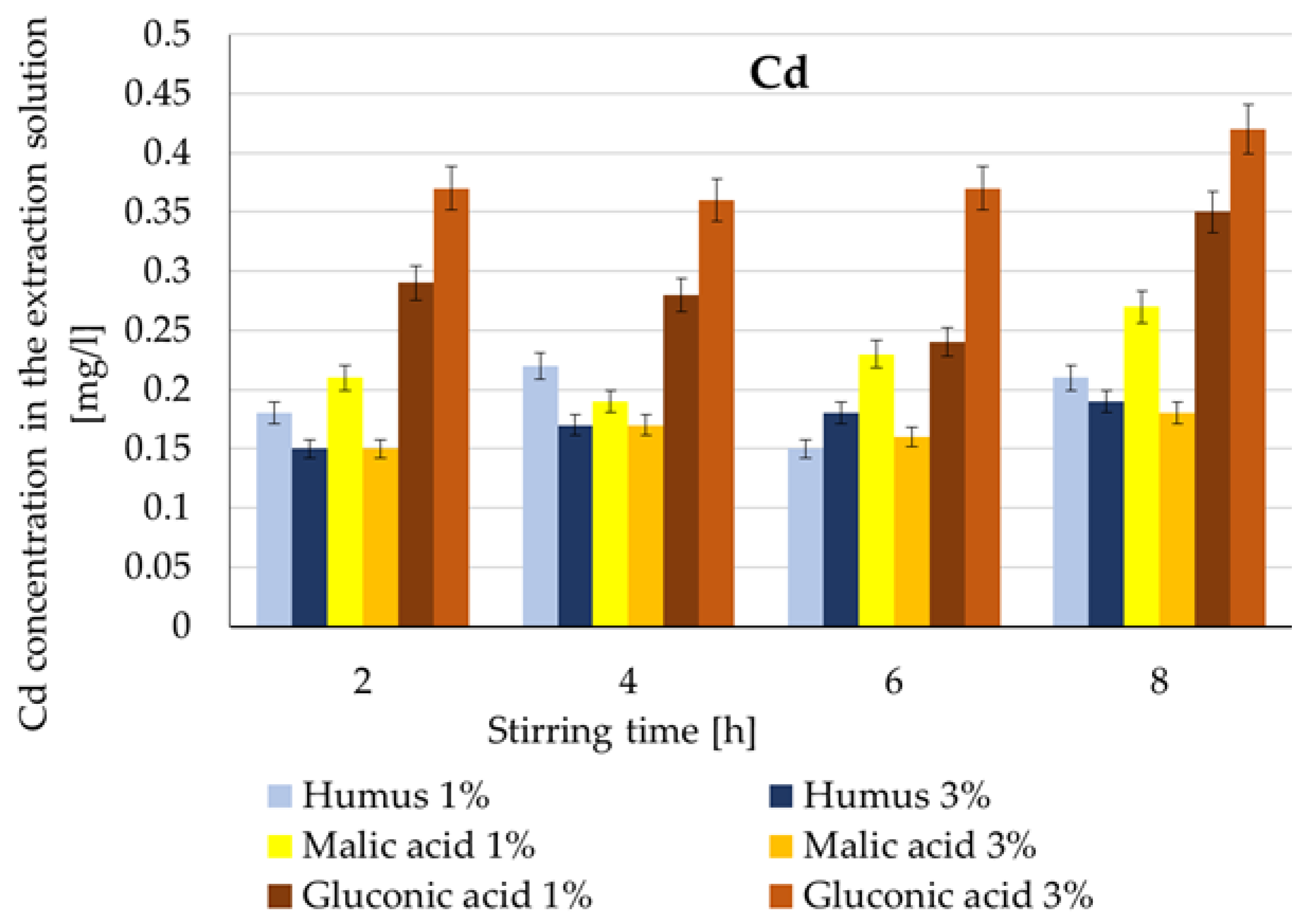
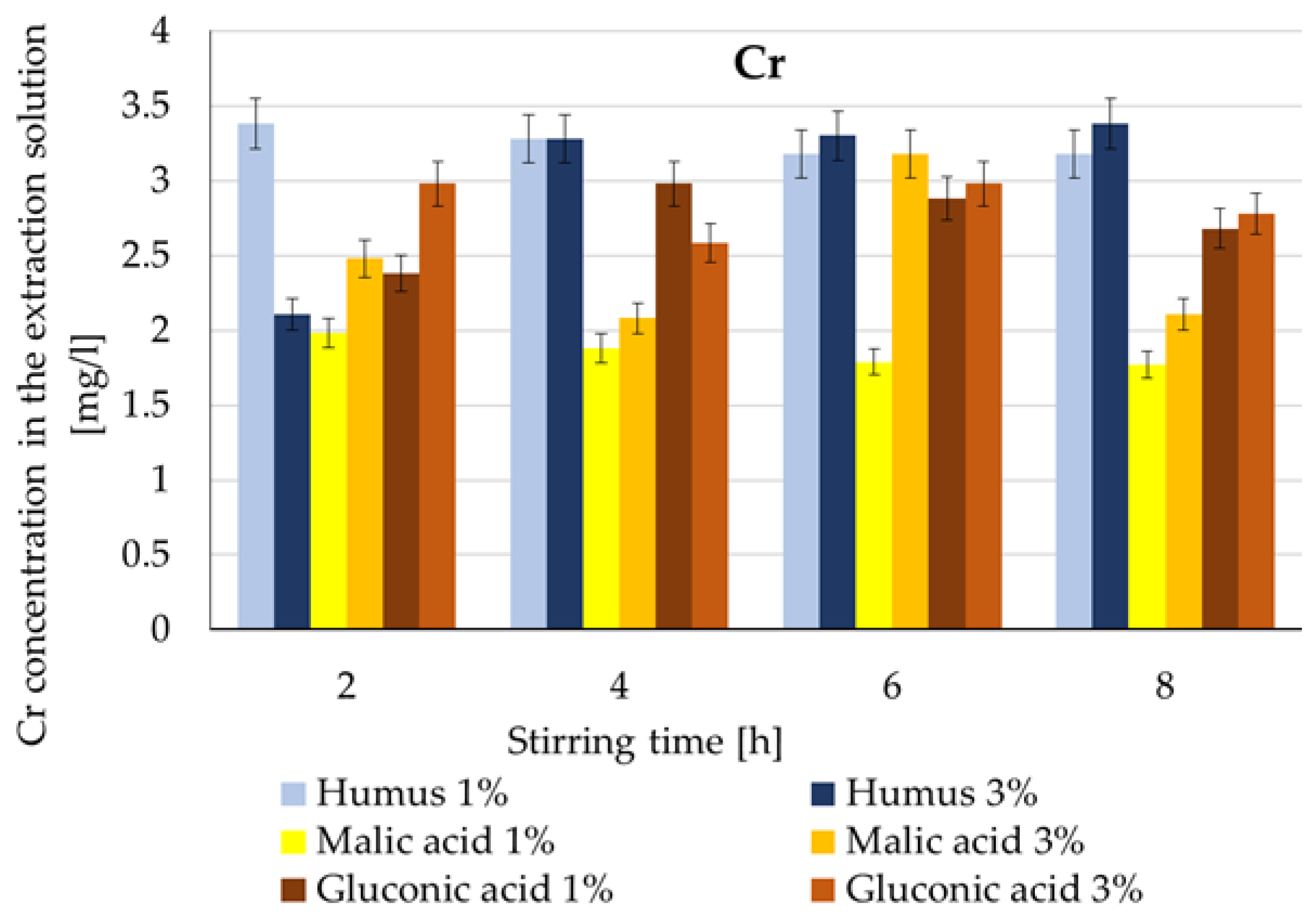
- the extraction yield of Cd and Cr maintains an improving trend more than the increase in costs (>2), within the range of 3.9–15. The yield obtained with a 1:8 ratio was 9 times higher compared to a 1:4 ratio, the only exception being in the case of Cd extraction with a 3% malic acid solution (1.95 times).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Chen, P.; Wang, H.; Yang, Y.; Wu, Y. Remediation of Cu-, Zn-, and Pb-Contaminated Soil Using Different Soil Washing Agents: Removal Efficiencies and Mechanisms. Water Air Soil Pollut. 2023, 234, 476. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Wang, J.P.; Erdenebold, U. A study on reduction of copper smelting slag by carbon for recycling into metal values and cement raw material. Sustainability 2020, 12, 1421. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.-R.; Wazne, M.; Park, J.-H. Assessment of soil washing for Zn contaminated soils using various washing solutions. J. Ind. Eng. Chem. 2012, 18, 822–825. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in a contaminated environmental: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Soleimani, M.; Hajabbasi, M.A.; Afyuni, M.; Akbar, S.; Jensen, J.K.; Holm, P.E.; Borggaard, O.K. Comparison of Natural Humic Substances and Synthetic Ethylenediaminetetraacetic Acid and Nitrilotriacetic Acid as Washing Agents of a Heavy Metal–Polluted Soil. J. Environ. Qual. 2010, 39, 855–862. [Google Scholar] [CrossRef]
- Lestan, D.; Luo, C.; Li, X. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
- Gusiatin, Z.M. Novel and Eco-Friendly Washing Agents to Remove Heavy Metals from Soil by Soil Washing. Environ. Anal. Ecol. Stud. 2018, 2, 133–135. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Hansen, H.C.B.; Holm, P.E.; Jensen, J.K.; Rasmussen, S.B.; Sabiene, N.; Steponkaite, L.; Strobel, B.W. Experimental assessment of using soluble humic substances for remediation of heavy metal polluted soils. Soil Sediment Contam. 2009, 18, 369–382. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Jensen, J.K.; Soleimani, M.; Strobel, B.W. Cleaning heavy metal contaminated soil with soluble humic substances instead of synthetic polycarboxylic acids. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 577–581. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Singh, P.; Singh, S.K.; Prasad, S.M. Plant Responses to Soil Pollution; Springer: Singapore, 2020; Volume 21, p. 248. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.-S.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Li, Q.; Peng, H.; Zhang, J.-X.; Chen, W.-J.; Zhou, B.-C.; Chen, M. Remediation of Heavy Metal-Contaminated Soils with Soil Washing: A Review. Sustainability 2022, 14, 13058. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D.; Klik, B. New-generation washing agents in remediation of metal-polluted soils and methods for washing effluent treatment: A review. Int. J. Environ. Res. Public Health 2020, 17, 6220. [Google Scholar] [CrossRef] [PubMed]
- Race, M.; Marotta, R.; Fabbricino, M.; Pirozzi, F.; Andreozzi, R.; Cortese, L.; Giudicianni, P. Copper and zinc removal from contaminated soils through soil washing process using ethylenediaminedisuccinic acid as a chelating agent: A modeling investigation. J. Environ. Chem. Eng. 2016, 4, 2878–2891. [Google Scholar] [CrossRef]
- Tang, H.; Shuai, W.; Wang, X.; Liu, Y. Extraction of rare earth elements from a contaminated cropland soil using nitric acid, citric acid, and EDTA. Environ. Technol. 2017, 38, 1980–1986. [Google Scholar] [CrossRef]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef]
- Ubner, M. Interaction of Humic Substances with Metal Cations. Ph.D. Thesis, Tallinn University of Technologys, Tallinn, Estonia, 2004; 96p. [Google Scholar]
- Perminova, I.V.; Hatfield, K.; Hertkorn, N. Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice in Humic Substances in the Environment; MacCarthy, P., Clapp, C.E., Malcolm, R.L., Bloom, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 3–36. [Google Scholar]
- Gusiatin, M.Z.; Pasieczna-Patkowska, S.; Bálintová, M.; Kuśmierz, M. Treatment of Wastewater from Soil Washing with Soluble Humic Substances Using Biochars and Activated Carbon. Energies 2023, 16, 4311. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef]
- Huang, G.; You, J.; Zhou, X.; Ren, C.; Islam, M.S.; Hu, H. Effects of low molecular weight organic acids on Cu accumulation by castor bean and soil enzyme activities. Ecotoxicol. Environ. Saf. 2020, 203, 110983. [Google Scholar] [CrossRef]
- Gomez-Garrido, M.; Navarro, J.M.; Navarro, F.J.M.; Cano, A.F. The chelating effect of citric acid, oxalic acid, amino acids and Pseudomonas fluorescens bacteria on phytoremediation of Cu, Zn, and Cr from soil using Suaeda vera. Int. J. Phytoremediat. 2018, 20, 1033–1042. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, H.; Zhang, X. Change in phytoextraction of Cd by rapeseed (Brassica napus L.) with application rate of organic acids and the impact of Cd migration from bulk soil to the rhizosphere. Environ. Pollut. 2020, 267, 115452. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, T.; Zhong, S.; Zhou, F.; Zhang, Y.; Ma, Y.; Fu, Q. Long-term effects of low-molecular-weight organic acids on remobilization of Cd, Cr, Pb, and As in alkaline coastal wetland soil. Environ. Pollut. 2021, 33, 266–277. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, J. Reduction of Cr(VI) by Malic Acid in Aqueous Fe-Rich Soil Suspensions. Chemosphere 2011, 86, 973. [Google Scholar] [CrossRef]
- Han, R.; Dai, H.; Skuza, L.; Wei, S. Comparative study on different organic acids for promoting Solanum nigrum L. hyperaccumulation of Cd and Pb from the contaminated soil. Chemosphere 2021, 278, 130446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Koo, N.; Kim, J.-G.; Lee, S.-H. Effects of Washing Solution, Washing Time, and Solid-Solution Rate on the Maximum Heavy Metals Removal Efficiency. Appl. Sci. 2021, 11, 6398. [Google Scholar] [CrossRef]
- Parimal, P.; Ramesh, K.; Subhamay, B. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Scheglova, N.V.; Popova, T.V.; Druzhinina, A.V.; Smotrina, T.V. Spectrophotometric study of complexation of cobalt (II) with HEDP in aqueous solutions. J. Mol. Liq. 2019, 286, 110909. [Google Scholar] [CrossRef]
- Fischer, K.; Bipp, H.-P. Removal of Heavy Metals from Soil Components and Soils by Natural Chelating Agents. Part II. Soil Extraction by Sugar Acids. Water Air Soil Pollut. 2002, 138, 271–288. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Y.; Zhang, M.; Xu, Y.; Jia, H.; Wei, T.; Guo, J. Effect of organic acids and soil particle size on heavy metal removal from bulk soil with washing. Environ. Geochem. Health 2023, 45, 3187–3198. [Google Scholar] [CrossRef]
- Meignant, I.; Stancampiano, L.M.; Verrillo, M.; Zohreh Barzgar, Z.; Caporale, A.G.; Brun, C.; Riccardo Spaccini, R.; Bridoux, M.C. Remediation of heavy metal-contaminated soils by washing with green compost humic substances. Environ. Chem. Lett. 2025. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Li, W.; Deng, Z.; Wu, J. A Biotic Strategy for Enhanced Hexavalent Chromium Removal by Zero-Valent Iron under the Interference of Humic Acid. Water 2024, 16, 1475. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, F.J.; Zhou, Y. Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 2020, 255, 126690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, T.; Han, X.; Yang, X.; He, Z. Effects of pH and low molecular weight organic acids on competitive adsorption and desorption of cadmium and lead in paddy soils. Environ. Monit. 2012, 184, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, L.; Tan, W.; Liu, C.; Dang, Z.; Qiu, G. Remediation of heavy metal contaminated soils by organic acid extraction and electrochemical adsorption. Environ. Pollut. 2020, 264, 114745. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, X.; Wei, M.; Zeng, G.; Hou, S.; Li, D.; Xu, H. Elucidation of the mechanisms into effects of organic acids on soil fertility, cadmium speciation and ecotoxicity in contaminated soil. Chemosphere 2020, 239, 124706. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Polyak, E.T.; Gabor, T. Assessment of Soil Quality Status and the Ecological Risk in the Baia Mare, Romania Area. Sustainability 2022, 14, 3739. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Gabor, T. The influence of polluted soil aeration in the process of in situ bioleaching. Stud. Univ. Babes-Bolyai Chem. 2016, 61. Available online: https://chem.ubbcluj.ro/~studiachemia/issues/chemia2016_3/tom2/06_Sur_etal_355_364.pdf (accessed on 16 September 2025).
- STAS 7184/1-84; Soils. Sample Collection for Soil and Agrochemical Studies. ASRO: Bucharest, Romania, 1984. (In Romanian)
- SR ISO 11464:1998; Soil Quality. Pretreatment of Samples for Psysico-Chemical Analysis. ASRO: Bucharest, Romania, 1998. (In Romanian)
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils, 3rd ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Ministry of Waters, Forests and Environmental Protection. Order No. 756 of 3 November 1997 for the Approval of the Regulation on Environmental Pollution, Assesment. In Official Gazzate No. 303 Bis of 6 November 1997; Ministry of Waters, Forests and Environmental Protection: Bucharest, Romania, 1997. Available online: https://legislatie.just.ro/Public/DetaliiDocument/13572 (accessed on 28 August 2023). (In Romanian)
- Sur, I.M.; Hegyi, A.; Micle, V.; Gabor, T.; Lazarescu, A.-V. Influence of the Extraction Solution on the Removal of Heavy Metals from Polluted Soils. Materials 2023, 16, 6189. [Google Scholar] [CrossRef]
- Bahemmat, M.; Farahbakhsh, M.; Kianirad, M. Humic Substances-enhanced Electroremediation of Heavy Metals Contaminated Soil. J. Hazard. Mater. 2016, 312, 307. [Google Scholar] [CrossRef] [PubMed]
- Marx, E.S.; Hart, J.; Stevens, R.G. Soil Test Interpretation Guide; Oregon State University Extension Service: Corvallis, OR, USA, 1996; Available online: https://ir.library.oregonstate.edu/downloads/w9505065q?utm_source=chatgpt.com (accessed on 28 August 2025).
- Staben, M.L.; Ellsworth, J.W.; Sullivan, D.M.; Horneck, D.A.; Brown, B.D.; Stevens, R.G. Monitoring Soil Nutrients Using a Management Unit Approach; Oregon State University Extension Service: Corvallis, OR, USA, 2003. [Google Scholar]
- Xing, Z.; Tian, K.; Du, C.; Li, C.; Zhou, J.; Chen, Z. Agricultural soil characterization by FTIR spectroscopy at micrometer scales: Depth profiling by photoacoustic spectroscopy. Geoderma 2019, 335, 94–103. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J. Mater. Sci. Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef]
- Qiu, S.R.; Wood, B.C.; Ehrmann, P.R.; Demos, S.G.; Miller, P.E.; Schaffers, K.I.; Suratwala, T.I.; Brow, R.K. Origins of optical absorption characteristics of Cu2+ complexes in aqueous solutions. Phys. Chem. Chem. Phys. 2015, 17, 18913–18923. [Google Scholar] [CrossRef]
- Xu, L.; Dai, H.; Skuza, L.; Xu, J.; Shi, J.; Wei, S. Co-high-efficiency washing agents for simultaneous removal of Cd, Pb and As from smelting soil with risk assessment. Chemosphere 2022, 300, 134581. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, D.; Kim, S.; Wang, S.; Cho, W.; Lee, M. As, Pb and Cu Stabilization By a Mixture Type of Mg-Fe Layered Double Hydroxide (LDH) with Oyster Shell: Laboratory and Field Evaluations. Water Air Soil Pollut. 2024, 235, 669. [Google Scholar] [CrossRef]
- Nie, X.; Huang, X.; Li, M.; Lu, Z.; Ling, X. Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics 2024, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; An, J.; Park, S.H.; Koutsospyros, A. Remediation of Heavy Metal (Cu, Pb) Contaminated Fine Soil Using Stabilization with Limestone and Livestock Bone Powder. Sustainability 2023, 15, 11244. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 2003. [Google Scholar] [CrossRef]
- Yao, W.; Huang, L.; Yang, Z.; Zhao, F. Effects of organic acids on heavy metal release or immobilization in contaminated soil. Trans. Nonferrous Met. Soc. China 2022, 32, 1277–1289. [Google Scholar] [CrossRef]
- Klik, B.; Kulikowska, D.; Gusiatin, Z.M.; Pasieczna-Patkowska, S. Washing agents from sewage sludge: Efficiency of Cd removal from highly contaminated soils and effect on soil organic balance. J. Soils Sediments 2020, 20, 284–296. [Google Scholar] [CrossRef]
- Zou, Q.; Gao, Y.; Yi, S.; Jiang, J.; Aihemaiti, A.; Li, D.; Yang, M. Multi-step column leaching using low-molecular-weight organic acids for remediating vanadium- and chromium-contaminated soil. Environ. Sci. Pollut. Res. 2019, 26, 15406–15413. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Zhang, P.; Pan, Y.; Huang, Z.; Xiao, H. Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. React. Funct. Polym. 2022, 173, 105210. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef] [PubMed]
- Damian, G.E.; Micle, V.; Sur, I.M. Removal of heavy metals from contaminated soil using chitosan as washing agent—A preliminary study. J. Environ. Prot. Ecol. 2020, 21, 823–829. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, J.; Wu, J.; Li, B. Effects of Inorganic and Organic Acids on Heavy Metals Leaching from Contaminated Sediment. In An Interdisciplinary Response to Mine Water Challenges; Sun, S., Wang, W., Eds.; China University of Mining and Technology Press: Xuzhou, China, 2014; pp. 406–411. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 402647. [Google Scholar] [CrossRef]
- Ash, C.; Tejnecký, V.; Borůvka, L.; Drábek, O. Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrol. 2016, 187, 18–30. [Google Scholar] [CrossRef]

| Washing Agent | Reported Removal Efficiency (%) | Optimal pH Range | Observations/Remarks | References |
|---|---|---|---|---|
| Humic substances | Zn, Pb: 15% Cu, Cd, Cr: 20–40% | 6–8 | high affinity for Pb2+ and Cu2+; adsorption capacity increases with pH | [7,10,16,20,21,29,35,36,37] |
| Malic acid | Zn, Pb, Cr: 50–88% Cd: 1.9–60% Cu: 5% | 3–7 | efficient, biodegradable; pH strongly affects metal mobility; low cost | [1,14,23,24,25,26,27,28,29,33,38,39,40,41] |
| Gluconic acid | Pb, Cu: 62–80% Zn, Cd, Cr: 34–60% | 7–9 | non-toxic polyhydroxycarboxylic acid; effective at alkaline pH | [1,29,30,31,32,33] |
| pH [-] | Ammonium Nitrogen N [ppm] | Potassium K [ppm] | Phosphorus P [ppm] | Humus [%] | Organic Carbon [%] | Electrical Conductivity [μs/cm] | Salinity [PSU] |
|---|---|---|---|---|---|---|---|
| 6.5 ± 0.1 | 14.87 ± 0.8 | 14.45 ± 0.7 | 23.06 ± 1.0 | 2.5 ± 0.2 | 1.7 ± 0.1 | 1100 ± 50 | 2.1 ± 0.1 |
| Metal | Washing Agent | Observations | Optimal Time | Maximum Yield (%) | Yield (%) Other Authors |
|---|---|---|---|---|---|
| Pb | Humus | Lowest extraction; reduced solubilization | n/a | 0.1% | 19% [29] |
| Malic acid | Rapid extraction; 3% max ~26 mg/L at 4 h | 4 h | 1.4% | 50–89% [14,29] | |
| Gluconic acid | Slower extraction; 3% significant after 6 h (~11 mg/L) | 6–8 h | 0.3–1.2% | 30% [29] 66–80% [33] | |
| Cu | Humus | 1% constant ~3.4 mg/L; 3% slightly higher after 6–8 h | 8 h | 2% | 20–40% [9,10,16] 15% [35] 5–28% [36] |
| Malic acid | 3% most effective ~4.1 mg/L; Progressive mobilization | 8 h | 2.45% | 3–5% [49] | |
| Gluconic acid | Gradual increase; 6–8 h ~3.5–3.6 mg/L | 8 h | 2% | 62–80% [33] | |
| Zn | Humus | 1%: 4 → 8 mg/L; 3%: slower, max ~6 mg/L | 8 h | 8.3–11.4% | 13% [35] |
| Malic acid | Most efficient; stable ~16–17 mg/L | 8 h | 24–26% | 60% [33] | |
| Gluconic acid | Increase after 6–8 h; 3% max ~14–15 mg/L | 8 h | 20–24% | 45% [33] | |
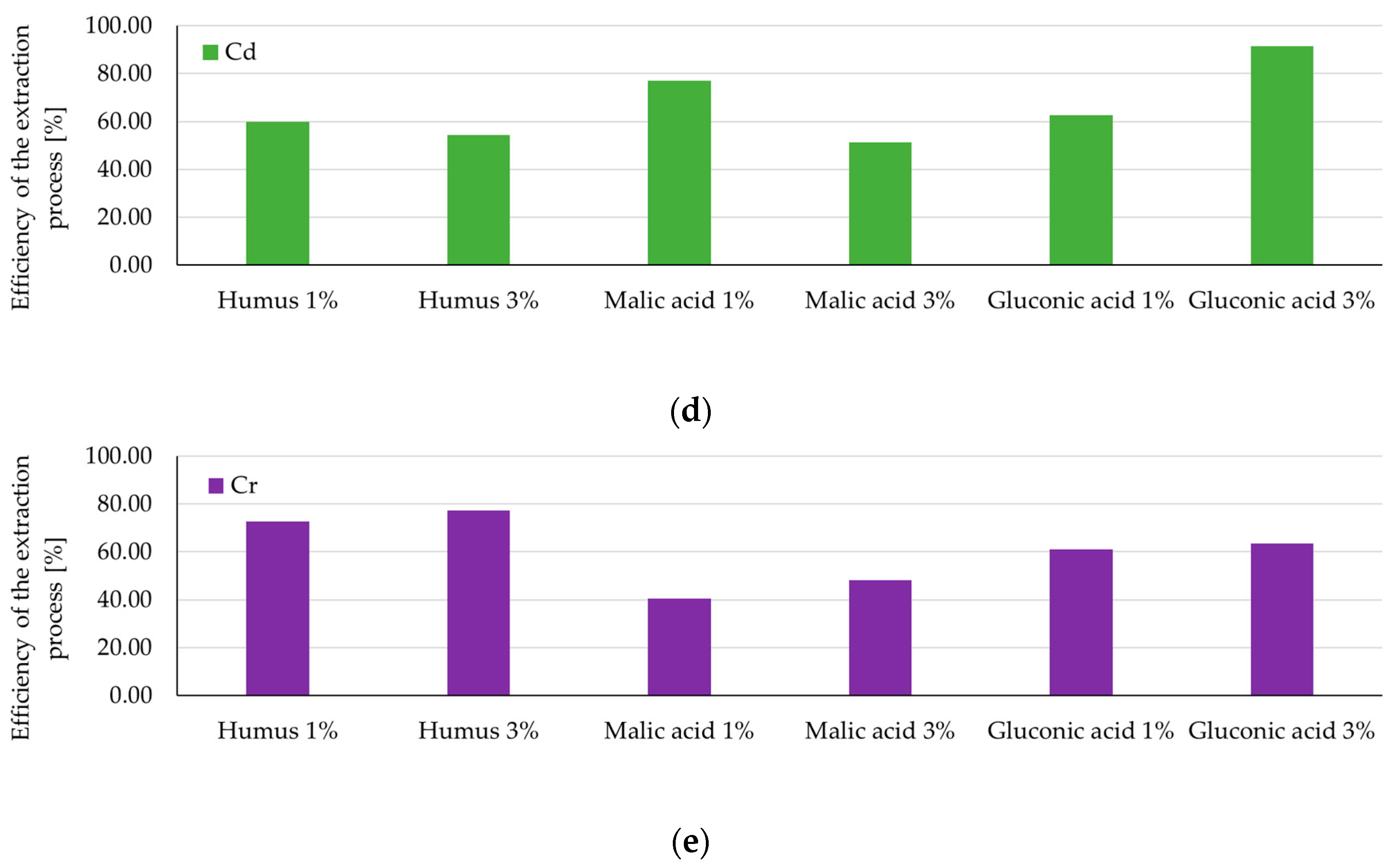
| Cd | Humus | Low mobility; ≤0.22 mg/L | 8 h | 54–60% | 25% [10] 20–70% [36] 35–75% [62] |
| Malic acid | Moderate extraction; 1% > 3% | 8 h | 51–77% | 60% [33] 1.9–60% [40,41] | |
| Gluconic acid | Most favorable; 3% max 0.42 mg/L | 8 h | 63–91% | 34% [29] 48–63% [33] | |
| Cr | Humus | Stable ~3.2–3.4 mg/L | 8 h | 75–78% | 23–31% [37] |
| Malic acid | Lowest values (~3.2 mg/L) | 6 h | 40–48% | 70% [63] | |
| Gluconic acid | High initial extraction (~2.5–3 mg/L) | 8 h | 61–63% | 39–60% [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sur, I.M.; Prodan, V.C.; Micle, V.; Nasui, M.; Hegyi, A.; Pop, V.S.; Scurtu, L.I. Washing Procedure with Several Reagents for Ecological Rehabilitation of Soil Polluted with Heavy Metals. Soil Syst. 2025, 9, 130. https://doi.org/10.3390/soilsystems9040130
Sur IM, Prodan VC, Micle V, Nasui M, Hegyi A, Pop VS, Scurtu LI. Washing Procedure with Several Reagents for Ecological Rehabilitation of Soil Polluted with Heavy Metals. Soil Systems. 2025; 9(4):130. https://doi.org/10.3390/soilsystems9040130
Chicago/Turabian StyleSur, Ioana Monica, Vasile Calin Prodan, Valer Micle, Mircea Nasui, Andreea Hegyi, Veronica Simona Pop, and Liviu Iacob Scurtu. 2025. "Washing Procedure with Several Reagents for Ecological Rehabilitation of Soil Polluted with Heavy Metals" Soil Systems 9, no. 4: 130. https://doi.org/10.3390/soilsystems9040130
APA StyleSur, I. M., Prodan, V. C., Micle, V., Nasui, M., Hegyi, A., Pop, V. S., & Scurtu, L. I. (2025). Washing Procedure with Several Reagents for Ecological Rehabilitation of Soil Polluted with Heavy Metals. Soil Systems, 9(4), 130. https://doi.org/10.3390/soilsystems9040130







