Turning Waste into Fertilizer: Aloe vera Leaf Shavings Improve Plant Growth and Support Soil Fertility in Organic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trial
2.1.1. Site Description
2.1.2. Experimental Design
2.1.3. Fertilization Treatments
2.1.4. Aloe Shavings Source and Preparation
2.1.5. Soil Sampling
2.1.6. Monthly Soil Analyses
2.1.7. Monthly Plant Monitoring
2.2. Laboratory Incubations
2.2.1. Sample Collection and Preparation
2.2.2. Quantification of Nitrogen Mineralization
2.2.3. Microbial Respiration
2.3. Statistical Analysis
3. Results and Discussion
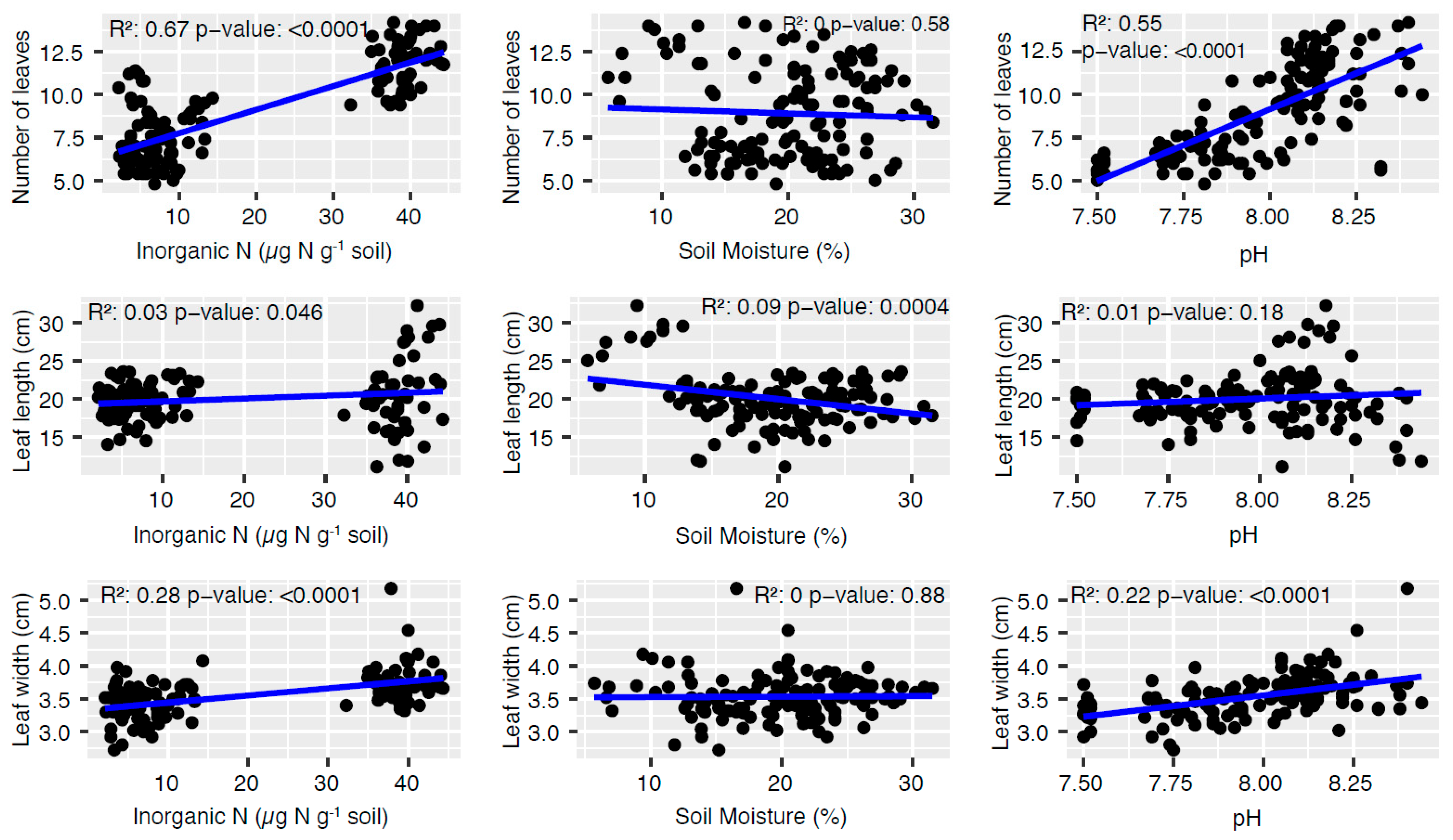
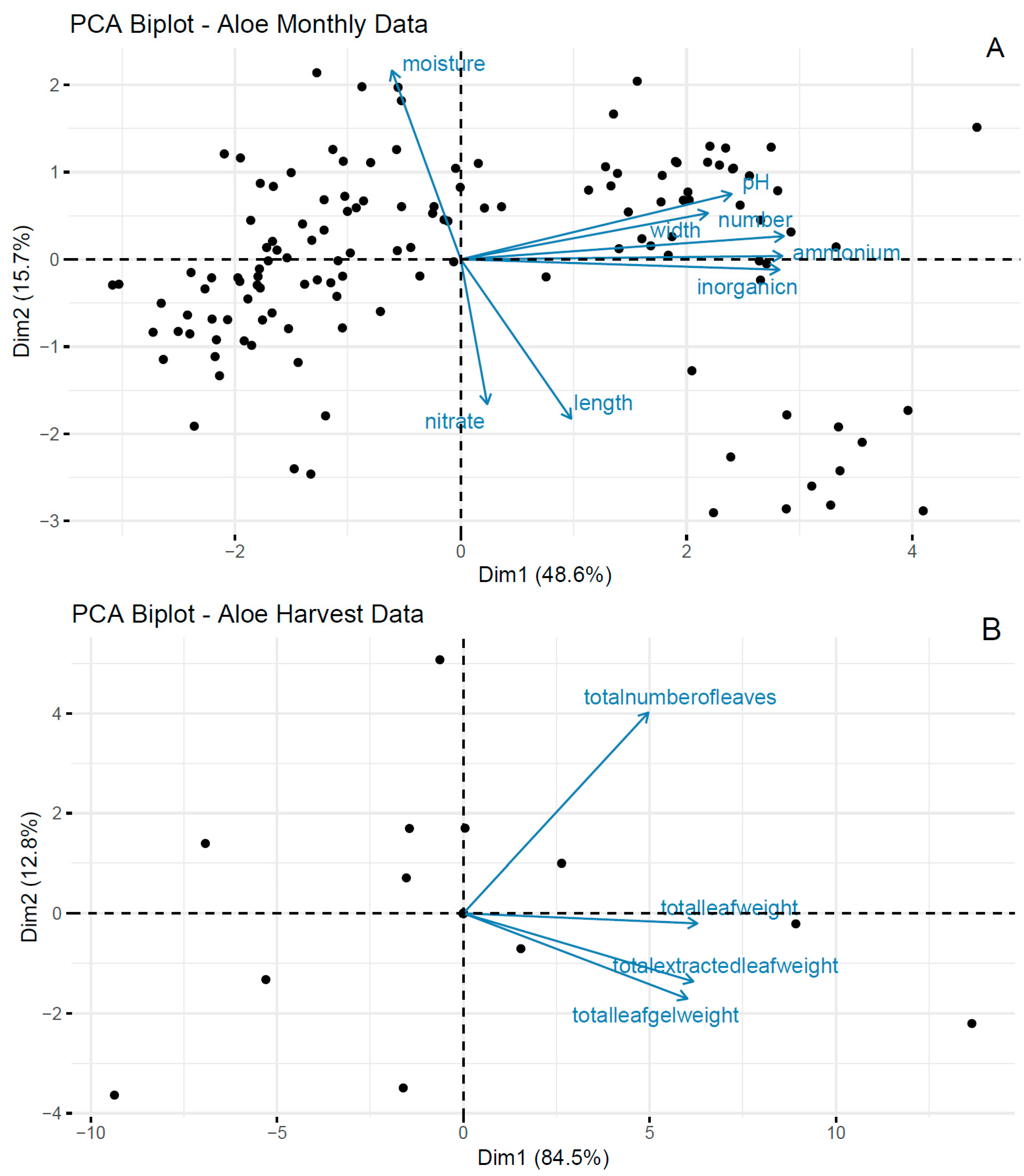
3.1. Monthly Plant Monitoring
3.2. Laboratory Incubations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imarc Group. Aloe vera Market Size, Share, Trends and Forecast by Product, Form, Application, and Region, 2025–2033. 2025. Available online: https://www.imarcgroup.com/aloe-vera-gel-manufacturing-plant (accessed on 24 June 2025).
- Interfresh F & B. Aloe vera Production. Interfresh. Available online: https://interfresh.com.vn/aloe-vera-production/ (accessed on 18 November 2024).
- Brezosky, L. Aloe vera Puts Small Texas Town on the Map. Herald-Tribune. Available online: https://www.heraldtribune.com/story/news/2002/11/24/aloe-vera-puts-small-texas-town-on-the-map/28730206007 (accessed on 5 October 2025).
- Boukour, R.; Douaik, A.; El Kahkahi, R.; El Halimi, R. Effect of site conditions and fertilization treatments on morphological traits and mineral content of Aloe vera plants. J. Appl. Bot. Food Qual. 2024, 97, 35–44. [Google Scholar] [CrossRef]
- Khater, R.M.; Abd-Allah, W.H.; El Shafay, R.M.M. Effect of organic fertilization and spraying Aloe vera extract on the growth and productivity of Carum carvi L. plant under Shalateen conditions in Egypt. Plant Arch. 2020, 20, 4959–4971. [Google Scholar]
- Nejatzadeh, F. Effect of vermicompost and nitrogen fertilizer on the growth and production of Aloe vera. Int. J. Plant Soil Sci. 2024, 36, 337–345. [Google Scholar] [CrossRef]
- Loncarevic, S.; Johannessen, G.S.; Rorvik, L.M. Bacteriological quality of organically grown leaf lettuce in Norway. Lett. Appl. Microbiol. 2005, 41, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture: Sources, Impacts and Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–41. [Google Scholar]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://websoilsurvey.nrcs.usda.gov/app/ (accessed on 21 July 2024).
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kempers, A.J.; Zweers, A. Ammonium determination in soil extracts by the salicylate method. Commun. Soil Sci. Plant Anal. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Cristiano, G.; Murillo-Amador, B.; De Lucia, B. Propagation techniques and agronomic requirements for the cultivation of Barbados aloe (Aloe vera (L.) Burm. f.)—A review. Front. Plant Sci. 2016, 7, 1410. [Google Scholar] [CrossRef]
- Chowdhury, T.; Chowdhury, M.A.H.; Rahman, M.A.; Nahar, K.; Chowdhury, M.T.I.; Khan, M.S.I. Response of Aloe vera to inorganic and organic fertilization in relation to leaf biomass yield and post harvest fertility of soil. Bulg. J. Agric. Sci. 2020, 26, 346–354. [Google Scholar]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Chauhan, S.K. Effect of nitrogen, salinity and water regimes on crop growth and yield of Aloe in semi-arid regions. New Ser. 2018, 39, 425–429. [Google Scholar]
- Rathore, R.; Mathur, A. Scope of cultivation and value chain perspectives of medicinal herbs in India: A case study on Aloe vera and Isabgol. J. Pharmacogn. Phytochem. 2019, 8, 243–246. [Google Scholar]
- Chowdhury, T.; Rahman, M.A.; Nahar, K.; Chowdhury, M.A.H.; Khan, M.S.I. Growth and yield performance of Aloe vera grown in different soil types of Bangladesh: Yield performance of Aloe vera in different soils. J. Bangladesh Agric. Univ. 2018, 16, 448–456. [Google Scholar] [CrossRef]
- Tyler, G.; Olsson, T. Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant Soil 2001, 230, 307–321. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Laghari, A.H.; Bhabhan, G.M.; Talpur, K.H. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Usharani, K.V.; Roopashree, K.M.; Naik, D. Role of soil physical, chemical and biological properties for soil health improvement and sustainable agriculture. J. Pharmacogn. Phytochem. 2019, 8, 1256–1267. [Google Scholar]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.D.; Alves, P.R.L.; de Paula, A.M.; Nakatani, A.S.; Pereira, J.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Mamatha, B.; Mudigiri, C.; Ramesh, G.; Saidulu, P.; Meenakshi, N.; Prasanna, C.L. Enhancing soil health and fertility management for sustainable agriculture: A review. Asian J. Soil Sci. Plant Nutr. 2024, 10, 182–190. [Google Scholar] [CrossRef]
- Liptzin, D.; Rieke, E.L.; Cappellazzi, S.B.; Bean, G.M.; Cope, M.; Greub, K.L.H.; Norris, C.E.; Tracy, P.W.; Aberle, E.; Ashworth, A.; et al. An evaluation of nitrogen indicators for soil health in long-term agricultural experiments. Soil Sci. Soc. Am. J. 2023, 87, 868–884. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The soil organic matter in connection with soil properties and soil inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Gondal, A.H.; Hussain, I.; Ijaz, A.B.; Zafar, A.; Ch, B.I.; Zafar, H.; Sohail, M.D.; Niazi, H.; Touseef, M.; Khan, A.A.; et al. Influence of soil pH and microbes on mineral solubility and plant nutrition: A review. Int. J. Agric. Biol. Sci. 2021, 5, 71–81. [Google Scholar]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840. [Google Scholar] [CrossRef]
- Souza, M.S.; Silva, T.G.F.; Souza, L.S.B.; Ferraz Jardim, A.M.D.R.; Araújo Júnior, G.D.N.; Nunes Alves, H.K.M. Practices for the improvement of the agricultural resilience of the forage production in semiarid environment: A review. Amazon. J. Plant Res. 2019, 3, 417–430. [Google Scholar] [CrossRef]
- Maluf, H.J.G.M.; Soares, E.M.B.; Silva, I.R.; Neves, J.C.L.; Silva, L.O.G. Decomposition of crop residues and nutrient mineralization in soils with different textures. Rev. Bras. Cienc. Solo. 2015, 39, 1681–1689. [Google Scholar] [CrossRef]
- Uwamahoro, H.; Kpomblekou-A, K.; Mortley, D.; Quarcoo, F. Organic vegetable crop residue decomposition in soils. Heliyon 2023, 9, e14529. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Salazar, J.; Kang, J.J.; Parsons, J.; Cheng, C.L.; Castillo, A.; Pujol Pereira, E.I. Compost and biochar to promote soil biological activities under sweet potatoes cultivation in a subtropical semiarid region. Appl. Environ. Soil Sci. 2020, 7230595. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Li, X.; Li, X.; Lou, J.; Wei, M. Effect of vegetable residues incorporation on soil fertility, rhizosphere microbial community structure, and plant growth of continuously cropped cucumber in a solar greenhouse. Ann. Microbiol. 2022, 72, 32. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The function of root exudates in the root colonization by beneficial soil rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]

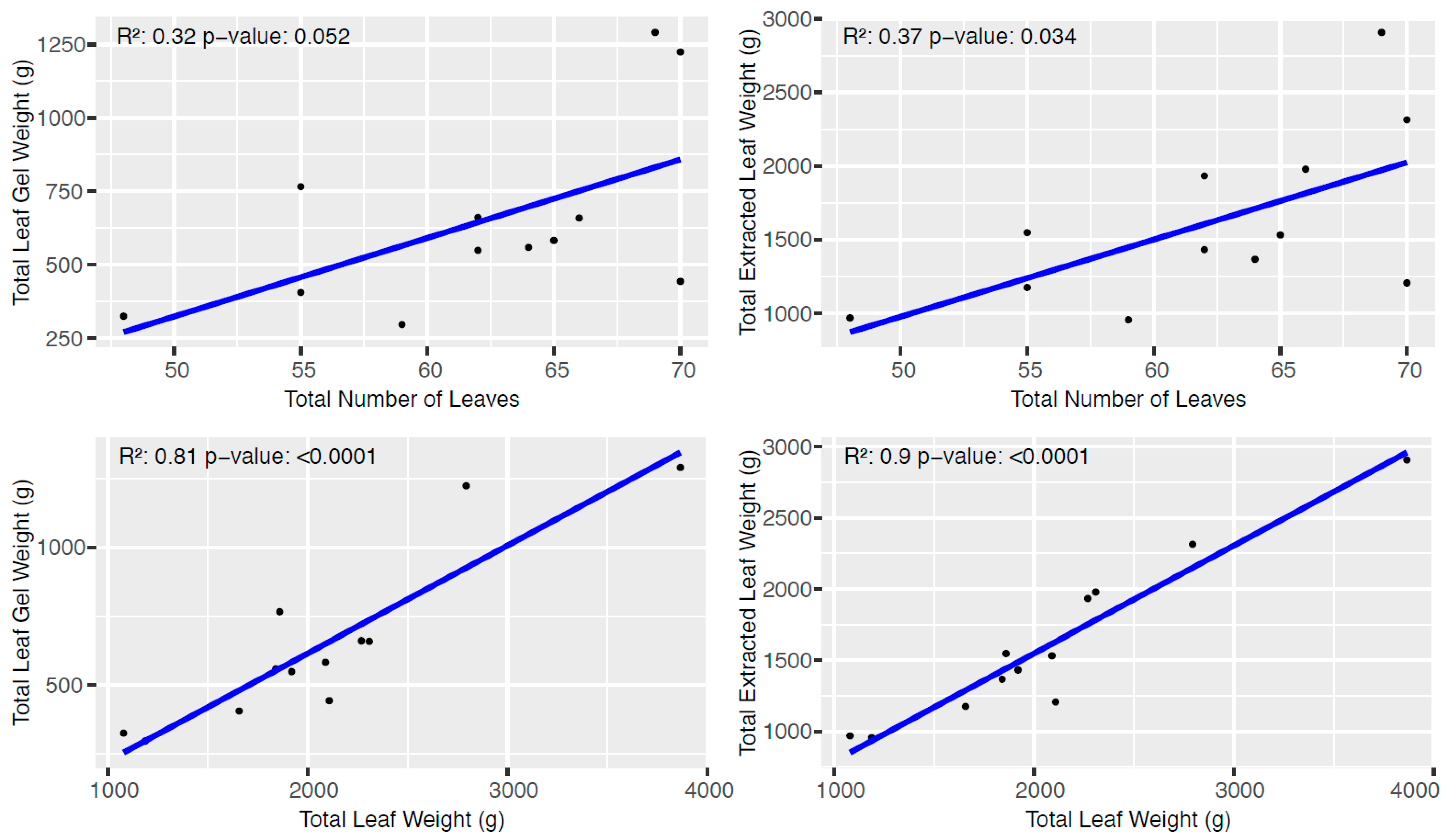

| Fertilizer | Leaf Weight | Leaf Gel Weight | Extracted Leaf Weight |
|---|---|---|---|
| Grams Per Plant | Grams Per Plant | Grams Per Plant | |
| Control | 374.9 ± 45.1 | 107.8 ± 39.6 | 261.9 ± 41.4 |
| Aloe Shavings | 455.5 ± 75.7 | 150.8 ± 63.4 | 362.8 ± 81.9 |
| Organic Compost | 452.6 ± 235.2 | 141.8 ± 82.5 | 358.8 ± 168.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, I.E.; Cocco, C.; Kang, J.J.; Cheng, C.-L.; Pereira, E. Turning Waste into Fertilizer: Aloe vera Leaf Shavings Improve Plant Growth and Support Soil Fertility in Organic Systems. Soil Syst. 2025, 9, 113. https://doi.org/10.3390/soilsystems9040113
Jaramillo IE, Cocco C, Kang JJ, Cheng C-L, Pereira E. Turning Waste into Fertilizer: Aloe vera Leaf Shavings Improve Plant Growth and Support Soil Fertility in Organic Systems. Soil Systems. 2025; 9(4):113. https://doi.org/10.3390/soilsystems9040113
Chicago/Turabian StyleJaramillo, Isaiah E., Carine Cocco, James Jihoon Kang, Chu-Lin Cheng, and Engil Pereira. 2025. "Turning Waste into Fertilizer: Aloe vera Leaf Shavings Improve Plant Growth and Support Soil Fertility in Organic Systems" Soil Systems 9, no. 4: 113. https://doi.org/10.3390/soilsystems9040113
APA StyleJaramillo, I. E., Cocco, C., Kang, J. J., Cheng, C.-L., & Pereira, E. (2025). Turning Waste into Fertilizer: Aloe vera Leaf Shavings Improve Plant Growth and Support Soil Fertility in Organic Systems. Soil Systems, 9(4), 113. https://doi.org/10.3390/soilsystems9040113







