Olive Pomace-Derived Compost: Phytotoxicity Assessment and Relevance for Soil Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Evaluation of Olive Pomace Phytotoxicity Using Germination Test
2.2. Germination Index
2.3. Experiment 2: Compost Production, Growth Test, and Assessment of Compost Maturity Composting
2.4. Growth Test
2.5. Statistical Analysis
3. Results
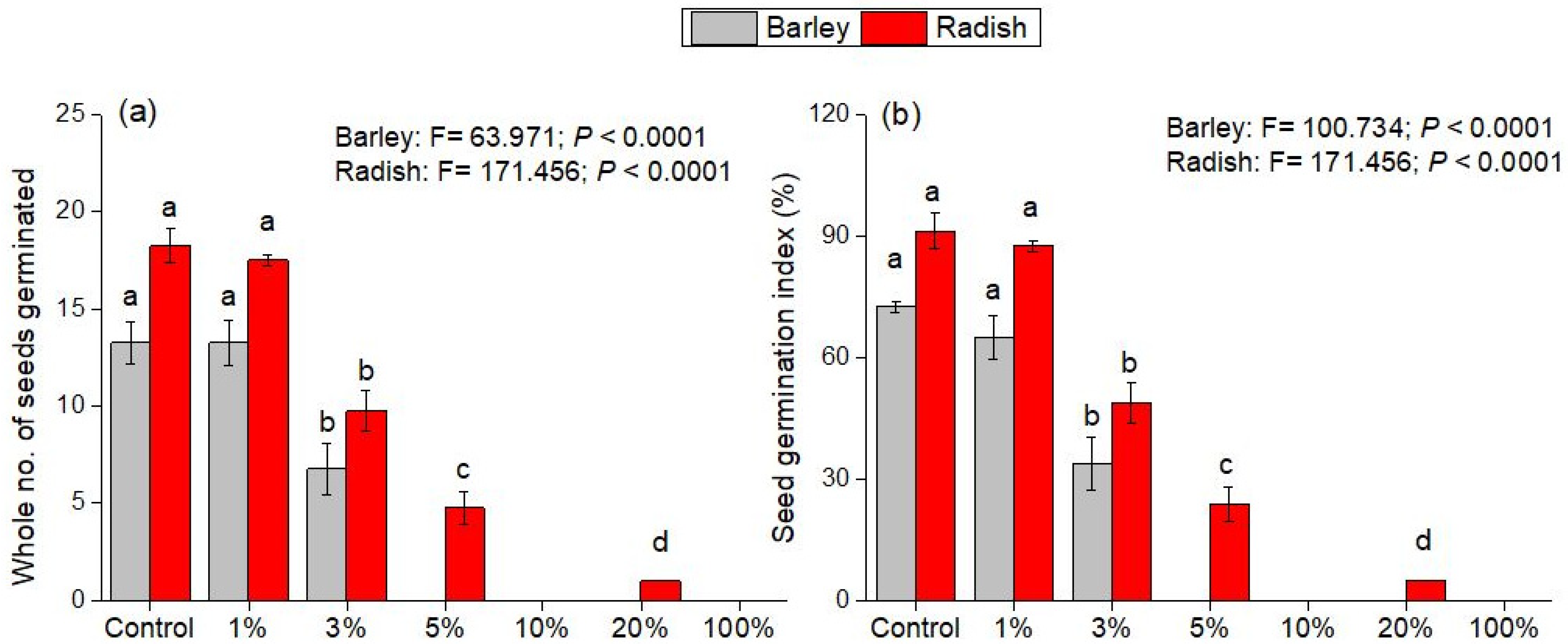
3.1. Effect of Olive Pomace Filtrate on Seed Germination
3.2. Composting Characteristics
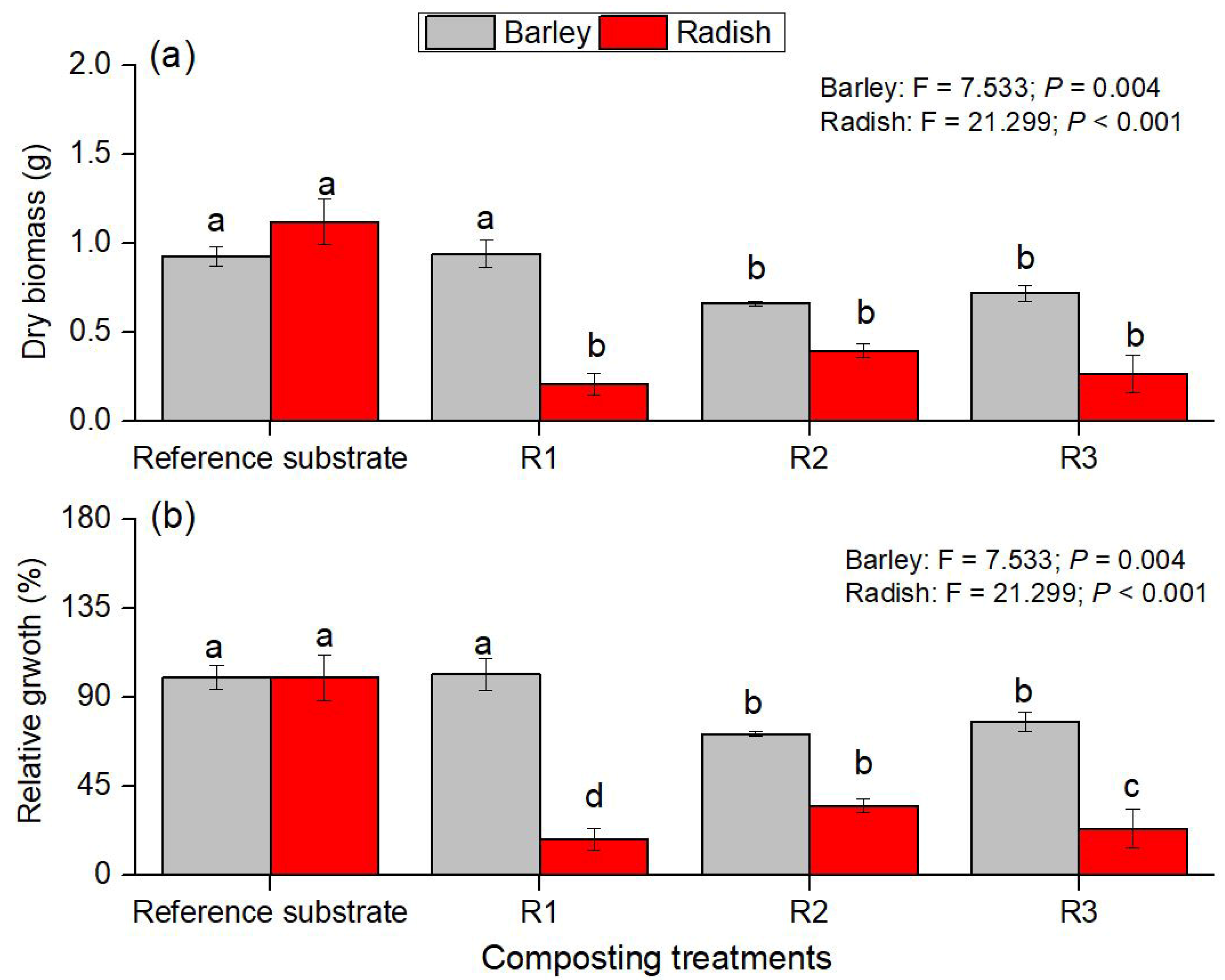
3.3. Effect of Composting Treatments on Plant Growth
4. Discussion
4.1. Phytotoxicity of Olive Pomace
4.2. Effect of Olive Pomace Compost on Plant Growth and Its Applicability in Agriculture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OP | Olive pomace |
| OPF | Olive pomace filtrate |
| COP | Composted olive pomace |
| R1 | Bioreactor-1 |
| R2 | Bioreactor-2 |
| R3 | Bioreactor-3 |
| GI | Germination index |
| RGR | Relative growth rate |
| TPC | Total phenolic content |
References
- Kurtoğlu, S.; Uzundumlu, A.S.; Gövez, E. Olive oil production forecasts for a macro perspective during 2024–2027. Appl. Fruit Sci. 2024, 66, 1089–1100. [Google Scholar] [CrossRef]
- Gentile, E.; Loi, A.; Berisio, S.; Parisi, P.; Gentile, M.; Bruni, M.; Montanari, F.; Christodoulou, M.; Bisonni, M.; Puzniak, J. Study on the Implementation of Conformity Checks in the Olive Oil Sector Throughout the European Union; European Union: Luxembourg, 2020. [Google Scholar]
- Rodríguez-Cohard, J.C.; Sánchez-Martínez, J.D.; Garrido-Almonacid, A. Strategic responses of the European olive-growing territories to the challenge of globalization. Eur. Plan. Stud. 2020, 28, 2261–2283. [Google Scholar] [CrossRef]
- Soyyiğit, S.; Yavuzaslan, K. Complex network analysis of international olive oil market. Tarım Ekon. Derg. 2018, 24, 117–129. [Google Scholar] [CrossRef]
- Gullon, P.; Gullon, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of byproducts from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Afonso, I.S.; Cardoso, B.; Nobrega, G.; Minas, G.; Ribeiro, J.E.; Lima, R.A. Green synthesis of nanoparticles from olive oil waste for environmental and health applications: A review. J. Environ. Chem. Eng. 2024, 12, 114022. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Pantziaros, A.G.; Trachili, X.A.; Zentelis, A.D.; Sygouni, V.; Paraskeva, C.A. A new olive oil production scheme with almost zero waste. Biomass Convers. Biorefinery 2021, 11, 547–557. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; García-Pérez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J. Applications of byproducts from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Vagelas, I. Potential use of polyphenolic compounds obtained from olive mill waste waters on plant pathogens and plant parasitic nematodes. In Plant Defence: Biological Control. Progress in Biological Control; Springer: Cham, Switzerland, 2020; Volume 22, pp. 137–177. [Google Scholar]
- Kavvadias, V.; Vavoulidou, E.; Paschalidis, C. Soil degradation in Mediterranean and olive mill wastes. In Bioremediation Science; CRC Press: Boca Raton, FL, USA, 2021; pp. 267–276. [Google Scholar]
- Dahdouh, A.; Khay, I.; Le Brech, Y.; El Maakoul, A.; Bakhouya, M. Olive oil industry: A review of waste stream composition, environmental impacts, and energy valorization paths. Environ. Sci. Pollut. Res. 2023, 30, 45473–45497. [Google Scholar] [CrossRef]
- Alaoui, I.; El Ghadraoui, O.; Tanji, K.; Harrach, A.; Farah, A. The olive mill pomace: A sustainable biofertilizer to improve soil proprieties and plant nutrient uptake. Waste Biomass Valorization 2024, 15, 2575–2590. [Google Scholar]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef]
- Černe, M.; Palčić, I.; Major, N.; Pasković, I.; Perković, J.; Užila, Z.; Lukić, M.; Romić, M.; Ferri, T.Z.; Ban, S.G. Effect of olive-processing technology on the utilization of olive mill pomace as a soil amendment. J. Environ. Qual. 2023, 52, 610–629. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Pereira, S.; Dinis, L.-T.; Brito, C. Enhancing olive cultivation resilience: Sustainable long-term and short-term adaptation strategies to alleviate climate change impacts. Horticulturae 2024, 10, 1066. [Google Scholar] [CrossRef]
- Abdennbi, S.; Chaieb, M.; Mekki, A. Long-term effects of olive mill waste waters spreading on the soil rhizospheric properties of olive trees grown under Mediterranean arid climate. Soil Res. 2023, 62, SR23102. [Google Scholar] [CrossRef]
- Tubeileh, A.M.; Souikane, R.T. Effect of olive vegetation water and compost extracts on seed germination of four weed species. Curr. Plant Biol. 2020, 22, 100150. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Belaqziz, M.; Wichern, M.; Lübken, M. Phytotoxicity assessment of olive mill wastewater treated by different technologies: Effect on seed germination of maize and tomato. Environ. Sci. Pollut. Res. 2020, 27, 8034–8045. [Google Scholar] [CrossRef]
- Harrat, N.; Kadi, K.; Gueboudji, Z.; Addad, D.; Hamli, S.; Dib, D.; Boukeria, S.; Lekmine, S.; Mekersi, N. Effect of infiltration-percolation treatment of olive mill wastewater on cereal seed germination. Water Air Soil Pollut. 2024, 235, 693. [Google Scholar]
- Bouslimi, H.; Jouili, H.; Caçador, I.; Sleimi, N. Assessment of phenol compound removal from olive oil mill wastewater by using peroxidases extracted from radish and nettle leaves. Rev. Des Sci. De L’Eau 2019, 32, 13–19. [Google Scholar]
- Sdiri Ghidaoui, J.; Bargougui, L.; Chaieb, M.; Mekki, A. Study of the phytotoxic potential of olive mill wastewaters on a leguminous plant ‘Vicia faba L.’. Water Sci. Technol. 2019, 80, 1295–1303. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Lyamlouli, K. Olive mill waste sludge: From permanent pollution to a highly beneficial organic biofertilizer: A critical review and future perspectives. Ecotoxicol. Environ. Saf. 2023, 259, 114997. [Google Scholar] [CrossRef]
- Ameziane, H.; Mabrouki, J.; Benchrifa, M.; Hmouni, D. Review of olive pomace valorization techniques: A sustainable perspective for the olive oil industry. In Advanced Technology for Smart Environment and Energy; Springer: Cham, Switzerland, 2024; pp. 165–183. [Google Scholar]
- Lee, B.H.; Khor, S.M. Biodegradation versus composting. In Handbook of Biodegradable Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–34. [Google Scholar]
- Barthod, J.; Rumpel, C.; Dignac, M.-F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef]
- Guo, X.-X.; Liu, H.-T.; Wu, S.-B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of biomass waste into sustainable organic fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Tüzel, Y.; Ekinci, K.; Öztekin, G.B.; Erdal, I.; Varol, N.; Merken, Ö. Utilization of olive oil processing waste composts in organic tomato seedling production. Agronomy 2020, 10, 797. [Google Scholar] [CrossRef]
- Federici, E.; Massaccesi, L.; Pezzolla, D.; Fidati, L.; Montalbani, E.; Proietti, P.; Nasini, L.; Regni, L.; Scargetta, S.; Gigliotti, G. Short-term modifications of soil microbial community structure and soluble organic matter chemical composition following amendment with different solid olive mill waste and their derived composts. Appl. Soil Ecol. 2017, 119, 234–241. [Google Scholar] [CrossRef]
- Chowdhury, A.K.M.M.B.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting: A review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Angeloni, G.; Spadi, A.; Corti, F.; Calcaprina, M.; Carpi, G.; Maioli, F.; Parenti, A.; Masella, P. Advancing circular economy in olive oil production: Comparing maturation systems for vermicompost creation from olive pomace. Biomass 2024, 4, 1178–1190. [Google Scholar] [CrossRef]
- Khdair, A.; Abu-Rumman, G. Sustainable environmental management and valorization options for olive mill byproducts in the Middle East and North Africa (MENA) region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Rose, P.K.; Kidwai, M.K.; Kantiwal, P. Green approaches for the valorization of olive mill wastewater. In Green Chemistry Approaches to Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2024; pp. 313–336. [Google Scholar]
- Xie, Y.; Wu, P.; Qu, Y.; Guo, X.; Zheng, J.; Xing, Y.; Zhang, X.; Liu, Q. The evolution of nutrient and microbial composition and maturity during the composting of different plant-derived wastes. Biology 2025, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Li, G.; Ma, R.; Kong, Y.; Yuan, J. Selection of sensitive seeds for evaluation of compost maturity with the seed germination index. Waste Manag. 2021, 136, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Diacono, M.; Ferri, D.; Ciaccia, C.; Tittarelli, F.; Ceglie, F.; Verrastro, V.; Ventrella, D.; Vitti, C.; Montemurro, F. Bioassays and application of olive pomace compost on emmer: Effects on yield and soil properties in organic farming. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2012, 62, 510–518. [Google Scholar] [CrossRef]
- Lončarić, Z.; Galić, V.; Nemet, F.; Perić, K.; Galić, L.; Ragályi, P.; Uzinger, N.; Rékási, M. The evaluation of compost maturity and ammonium toxicity using different plant species in a germination test. Agronomy 2024, 14, 2636. [Google Scholar] [CrossRef]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of heavy metals on seed germination and seedling growth of wheat, pea, and tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar]
- Bremner, J.; Mulvaney, C. Nitrogen-total, principles of kjeldahl methods. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 2015; pp. 595–624. Available online: https://acsess.onlinelibrary.wiley.com/doi/book/10.2134/agronmonogr9.2.2ed (accessed on 5 August 2025).
- Moschou, C.E.; Papadimitriou, D.M.; Galliou, F.; Markakis, N.; Papastefanakis, N.; Daskalakis, G.; Sabathianakis, M.; Stathopoulou, E.; Bouki, C.; Daliakopoulos, I.N. Grocery waste compost as an alternative hydroponic growing medium. Agronomy 2022, 12, 789. [Google Scholar] [CrossRef]
- Nelson, P. Index to EPA Test Methods. United States Environmental Protection Agency, Region I; EPA: Boston, MA, USA, 2003. [Google Scholar]
- Pascual, J.A.; Garcia, C.; Hernandez, T. Comparison of fresh and composted organic waste in their efficacy for the improvement of arid soil quality. Bioresour. Technol. 1999, 68, 255–264. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brkić, K.; Radulović, M.; Sladonja, B.; Lukić, I.; Šetić, E. Application of Soxtec Apparatus for Oil Content Determination in Olive Fruit. Riv. Ital. Delle Sostanze Grasse 2006, 8, 115–119. [Google Scholar]
- Černe, M.; Palčić, I.; Major, N.; Pasković, I.; Perković, J.; Užila, Z.; Filipović, V.; Romić, M.; Ban, S.G.; Heath, D.J. Effect of sewage sludge-derived amendments on the nutrient uptake by Chinese cabbage from Mediterranean soils. J. Plant Nutr. 2023, 46, 1421–1445. [Google Scholar] [CrossRef]
- Itävaara, M.; Vikman, M.; Liisa, M.; Vuorinen, A. Maturity tests for composts—Verification of a test scheme for assessing maturity. Compos. Sci. Util. 2010, 18, 174–183. [Google Scholar]
- Kasmi, E.H.; Khattach, Y.; Makaoui, A.; Essadek, A.; Terroufi, S.; Mzabri, I.; Aboukhalid, K.; Maatougui, A.; Neffa, M. Agronomic impact and phytotoxicity of olive mill wastewater as a biofertilizer on Vicia faba L. Ecol. Eng. Environ. Technol. 2024, 5, 119–136. [Google Scholar] [CrossRef]
- Mekersi, N.; Kadi, K.; Addad, D.; Amari, A.; Lekmine, S. Soil amendment by olive pomace improving soil quality. Int. J. Nat. Resour. Environ. 2022, 4, 17–22. [Google Scholar]
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E. Olive mill wastes: A source of bioactive molecules for plant growth and protection against pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef]
- Rusan, M.J.; Albalasmeh, A.A.; Zuraiqi, S.; Bashabsheh, M. Evaluation of phytotoxicity effect of olive mill wastewater treated by different technologies on seed germination of barley (Hordeum vulgare L.). Environ. Sci. Pollut. Res. 2015, 22, 9127–9135. [Google Scholar] [CrossRef]
- Belaqziz, M.; El-Abbassi, A.; Agrafioti, E.; Galanakis, C.M. Agronomic application of olive mill wastewater: Effects on maize production and soil properties. J. Environ. Manag. 2016, 171, 158–165. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Albalasmeh, A.A.; Malkawi, H.I. Treated olive mill wastewater effects on soil properties and plant growth. Water Air Soil Pollut. 2016, 227, 135. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Zhang, B.; Li, H.; Wang, Z.; Si, J.; Fan, S.; Feng, B. Does energy cost constitute the primary cause of ammonium toxicity in plants? PLANTA 2022, 256, 62. [Google Scholar] [CrossRef]
- Maenhout, P.; Van den Bulcke, J.; Van Hoorebeke, L.; Cnudde, V.; De Neve, S.; Sleutel, S. Nitrogen limitations on microbial degradation of plant substrates are controlled by soil structure and moisture content. Front. Microbiol. 2018, 9, 1433. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of high soil C/N ratio and nitrogen limitation caused by the long-term combined organic-inorganic fertilization on the soil microbial community structure and its dominated soc decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compos. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Shannon, M.; Grieve, C. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Nemet, F.; Perić, K.; Lončarić, Z. Microbiological activities in the composting process–a review. COLUMELLA–J. Agric. Environ. Sci. 2021, 8, 41–53. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Ran, X.; Xu, Y.; Chen, Y.; Wu, C.; Tang, J. Nitrogen transformation mechanisms and compost quality assessment in sustainable mesophilic aerobic composting of agricultural waste. Sustainability 2025, 17, 575. [Google Scholar] [CrossRef]
- Di, D.; Wang, S.; Chen, G.; Wang, Q.; Zhang, J.; Niu, X.; Huang, D. NH4+-N and low ratios of NH4+-N/NO3−-N promote the remediation efficiency of Salix linearistipularis in Cd-and Pb-contaminated soil. Forests 2024, 15, 419. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, L.; Dai, J.; Chen, J.; Yang, X.; Wang, X.; Wang, Z.; Feng, L. Effects of the C/N ratio on the microbial community and lignocellulose degradation, during branch waste composting. Bioprocess Biosyst. Eng. 2022, 45, 1163–1174. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms And Perspectives I: General Consequences And Plant Responses; Springer: Singapore, 2020; pp. 117–145. [Google Scholar]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Chaudhari, S.; Upadhyay, A.; Kulshreshtha, S. Influence of organic amendments on soil properties, microflora and plant growth. Sustain. Agric. Rev. 2021, 52, 147–191. [Google Scholar]
- Zhu, X.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of different no 3−: Nh 4+ ratios on the photosynthesis and ultrastructure of lettuce seedlings. Hortic. Environ. Biotechnol. 2020, 61, 459–472. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Zhang, Y.; Li, D.; Chen, L.; Wei, Z.; Chen, X.; Pan, C.; Song, Y. The active role of metabolic regulators in nitrogen loss reduction and organic nitrogen transformation during different materials composting. J. Clean. Prod. 2022, 345, 131134. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- Kamal, M.Z.U.; Sarker, U.; Roy, S.K.; Alam, M.S.; Azam, M.G.; Miah, M.Y.; Hossain, N.; Ercisli, S.; Alamri, S. Manure-biochar compost mitigates the soil salinity stress in tomato plants by modulating the osmoregulatory mechanism, photosynthetic pigments, and ionic homeostasis. Sci. Rep. 2024, 14, 21929. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Younis, A.; Abideen, Z.; Naveed, M.; Koyro, H.-W.; Siddique, K.H. Biochar, compost, and biochar–compost blend applications modulate growth, photosynthesis, osmolytes, and antioxidant system of medicinal plant alpinia zerumbet. Front. Plant Sci. 2021, 12, 707061. [Google Scholar] [CrossRef]
- Irin, I.J.; Hasanuzzaman, M. Organic amendments: Enhancing plant tolerance to salinity and metal stress for improved agricultural productivity. Stresses 2024, 4, 185–209. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Zhou, J.; Muhoza, B.; Wang, F.; Herzberger, A.; Yu, X. Integrated eco-strategies towards sustainable carbon and nitrogen cycling in agriculture. J. Environ. Manag. 2021, 293, 112856. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Tamborrino, A.; Beneduce, L.; Gagliardi, A.; Giuliani, M.; Gatta, G. Composting of olive mill pomace, agro-industrial sewage sludge and other residues: Process monitoring and agronomic use of the resulting composts. Foods 2021, 10, 2143. [Google Scholar] [CrossRef]
- Selim, M.M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int. J. Agron. 2020, 2020, 2821678. [Google Scholar] [CrossRef]
- Oyetunji, O.; Bolan, N.; Hancock, G. A comprehensive review on enhancing nutrient use efficiency and productivity of broadacre (arable) crops with the combined utilization of compost and fertilizers. J. Environ. Manag. 2022, 317, 115395. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Zeng, C.; Li, Y.; Zhu, L.; Wu, J.; Chen, J.; Wei, Z. Assessment contributions of physicochemical properties and bacterial community to mitigate the bioavailability of heavy metals during composting based on structural equation models. Bioresour. Technol. 2019, 289, 121657. [Google Scholar] [CrossRef]
- Garau, M.; Garau, G.; Diquattro, S.; Roggero, P.P.; Castaldi, P. Mobility, bioaccessibility and toxicity of potentially toxic elements in a contaminated soil treated with municipal solid waste compost. Ecotoxicol. Environ. Saf. 2019, 186, 109766. [Google Scholar] [CrossRef]
- Ciadamidaro, L.; Puschenreiter, M.; Santner, J.; Wenzel, W.W.; Madejón, P.; Madejón, E. Assessment of trace element phytoavailability in compost amended soils using different methodologies. J. Soils Sediments 2017, 17, 1251–1261. [Google Scholar]
- Gong, Q.; Chen, P.; Shi, R.; Gao, Y.; Zheng, S.-A.; Xu, Y.; Shao, C.; Zheng, X. Health assessment of trace metal concentrations in organic fertilizer in northern China. Int. J. Environ. Res. Public Health 2019, 16, 1031. [Google Scholar]
- The Commission of the European Communities. Commission decision of 3 November 2006 establishing revised ecological criteria and the related assessment and verification requirements for the award of the community eco-label to soil improvers. Off. J. Eur. Union 2006, 325, 28–34. [Google Scholar]
- Ait-El-Mokhtar, M.; Fakhech, A.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Wahbi, S.; Meddich, A. Compost as an eco-friendly alternative to mitigate salt-induced effects on growth, nutritional, physiological and biochemical responses of date palm. Int. J. Recycl. Org. Waste Agric. 2022, 11, 85. [Google Scholar]
- Lerma-Moliz, R.; López-González, J.A.; Suárez-Estrella, F.; Martínez-Gallardo, M.R.; Jurado, M.; Estrella-González, M.J.; Toribio, A.; Jiménez, R.; López, M. Mitigation of phytotoxic effect of compost by application of optimized aqueous extraction protocols. Sci. Total Environ. 2023, 873, 162288. [Google Scholar]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy metals in source-separated compost and digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef]
- Nunes, N.; Ragonezi, C.; Gouveia, C.S.; de Carvalho, M.Â.P. Review of sewage sludge as a soil amendment in relation to current international guidelines: A heavy metal perspective. Sustainability 2021, 13, 2317. [Google Scholar] [CrossRef]
- Černe, M.; Palčić, I.; Major, N.; Pasković, I.; Perković, J.; Užila, Z.; Filipović, V.; Romić, M.; Ban, S.G.; Jaćimović, R. Effect of sewage sludge derived compost or biochar amendment on the phytoaccumulation of potentially toxic elements and radionuclides by Chinese cabbage. J. Environ. Manag. 2021, 293, 112955. [Google Scholar] [CrossRef]

| Material | Analysis | Measured Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | EC [µS/cm] | Ash [%] | Organic Matter [%] | TPC [%] | TN | TC | C/N | Dry Matter [%] | Moisture Content [%] | Oil Content [%] | NO3-N [mg/kg] | NH4-N [mg/kg] | ||
| Straw | Mean | 5.54 | 122 | 6.16 | 93.8 | 4.72 | 0.30 | 37.7 | 127 | / | / | / | / | / |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | / | / | / | / | / | |
| SE | 0.08 | 4 | 0.04 | 0.04 | 0.31 | 0.02 | 1.3 | 6.8 | / | / | / | / | / | |
| Raw olive pomace | Mean | 4.96 | 2692 | 3.18 | 96.8 | 12.18 | 0.97 | 48.4 | 50 | 33.0 | 67.0 | 14.79 | 1.9 | <0.38 |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| SE | 0.01 | 89 | 0.04 | 0.04 | 0.33 | 0.03 | 0.2 | 1.6 | 0.5 | 0.5 | 0.15 | 0.3 | / | |
| Sheep litter | Mean | 7.15 | 8162 | 18.05 | 81.9 | 4.91 | 2.51 | 34.4 | 14 | 43.8 | 56.2 | / | 0.6 | 10,303 |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | / | 5 | 5 | |
| SE | 0.05 | 199 | 0.35 | 0.3 | 0.16 | 0.18 | 0.3 | 1.0 | 0.8 | 0.8 | / | 0.2 | 634 | |
| Tr. | Analysis | Ash [%] | Organic Matter [%] | Moisture [%] | Dry Matter [%] | EC [μS/cm] | pH | TC [%] | TN [%] | C/N | TPC [%] | Oil Contents [%] | NO3-N [mg/kg] | NH4-N [mg/kg] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioreactor-1 | Mean | 14.5 b | 85.4 a | 51.7 a | 48.3 ab | 4670 c | 6.93 a | 37.8 a | 1.22 b | 31 a | 2.93 a | 0.09 a | <0.40 | 293 b |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 0.60 | 0.60 | 1.004 | 1.00 | 413 | 0.08 | 0.10 | 0.02 | 0.70 | 0.31 | 0.01 | / | 26 | |
| Bioreactor-2 | Mean | 14.2 b | 85.8 a | 46.1 b | 53.9 a | 5958 b | 7.13 a | 37.7 a | 1.96 a | 19 b | 3.05 a | 0.04 b | <0.40 | 396 a |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 0.20 | 0.20 | 0.40 | 0.40 | 448 | 0.13 | 0.30 | 0.01 | 0.10 | 0.26 | 0.02 | / | 10 | |
| Bioreactor-3 | Mean | 19.6 a | 80.4 a | 46.3 b | 53.7 a | 9632 a | 7.74 a | 35.3 b | 2.11 a | 17 b | 3.42 a | 0.05 b | <0.40 | 222 b |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 0.10 | 0.10 | 0.40 | 0.40 | 112 | 0.05 | 0.10 | 0.02 | 0.20 | 0.30 | 0.02 | / | 10 |
| Tr. | Analysis | Ca [mg/kg] | Mg [mg/kg] | K [mg/kg] | P [mg/kg] | S [mg/kg] | Al [mg/kg] | Cr [mg/kg] | Cu [mg/kg] | Fe [mg/kg] | Li [mg/kg] | Mn [mg/kg] | Zn [mg/kg] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioreactor-1 | Mean | 21,714.2 c | 1192.9 c | 30,843.1 c | 1498.07 c | 3000.6 c | 3272.5 a | <0.5 | <0.5 | 1256.3 a | 29.0 a | 53.3 | 26.6 c |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 3235.5 | 19.9 | 433.8 | 686.8 | 73.0 | 53.4 | 0.0 | 0.0 | 182.6 | 0.8 | 5.5 | 3.0 | |
| Bioreactor-2 | Mean | 29,483.0 a | 2235.5 b | 38,794.4 b | 2900.8 b | 4035.3 a | 1772.9 c | <0.5 | <0.5 | 1108.5 b | 29.2 a | 81.0 | 76.5 b |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 1162.5 | 431.7 | 3219.7 | 32.3 | 444.1 | 20.9 | 0.0 | 0.0 | 101.1 | 0.6 | 15.5 | 22.7 | |
| Bioreactor-3 | Mean | 27,111.3 b | 2869.9 a | 40,430.2 a | 6022.68 a | 3849.8 b | 2248.5 b | 15.2 | 13.2 | 1231.4 a | 24.0 b | 97.2 | 119.5 a |
| N | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | |
| SE | 900.1 | 32.7 | 773.9 | 132.1 | 503.0 | 50.4 | 6.2 | 5.4 | 31.2 | 2.0 | 7.7 | 2.2 | |
| EU Limit values for soil improvers 1 | 100 | 100 | / | / | / | 300 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, Q.; Bouhadi, M.; Palčić, I.; Anđelini, D.; Cvitan, D.; Major, N.; Lukić, M.; Goreta Ban, S.; Ban, D.; Heath, D.; et al. Olive Pomace-Derived Compost: Phytotoxicity Assessment and Relevance for Soil Systems. Soil Syst. 2025, 9, 107. https://doi.org/10.3390/soilsystems9040107
Javed Q, Bouhadi M, Palčić I, Anđelini D, Cvitan D, Major N, Lukić M, Goreta Ban S, Ban D, Heath D, et al. Olive Pomace-Derived Compost: Phytotoxicity Assessment and Relevance for Soil Systems. Soil Systems. 2025; 9(4):107. https://doi.org/10.3390/soilsystems9040107
Chicago/Turabian StyleJaved, Qaiser, Mohammed Bouhadi, Igor Palčić, Dominik Anđelini, Danko Cvitan, Nikola Major, Marina Lukić, Smiljana Goreta Ban, Dean Ban, David Heath, and et al. 2025. "Olive Pomace-Derived Compost: Phytotoxicity Assessment and Relevance for Soil Systems" Soil Systems 9, no. 4: 107. https://doi.org/10.3390/soilsystems9040107
APA StyleJaved, Q., Bouhadi, M., Palčić, I., Anđelini, D., Cvitan, D., Major, N., Lukić, M., Goreta Ban, S., Ban, D., Heath, D., Rijavec, T., Lapanje, A., & Černe, M. (2025). Olive Pomace-Derived Compost: Phytotoxicity Assessment and Relevance for Soil Systems. Soil Systems, 9(4), 107. https://doi.org/10.3390/soilsystems9040107











