Factors Influencing the Impact of Anaerobic Digestates on Soil Properties
Abstract
1. Introduction
2. Methods
3. Effect of Raw Materials on the Quality of Digestates
3.1. Digestates from Animal Waste
3.2. Digestates from Food Waste
3.3. Digestates from Municipal Solid Waste
3.4. Digestates from Sewage Sludge
3.5. Digestates from Green Waste
3.6. Digestates from Mixed Materials—The Result of Co-Digestion
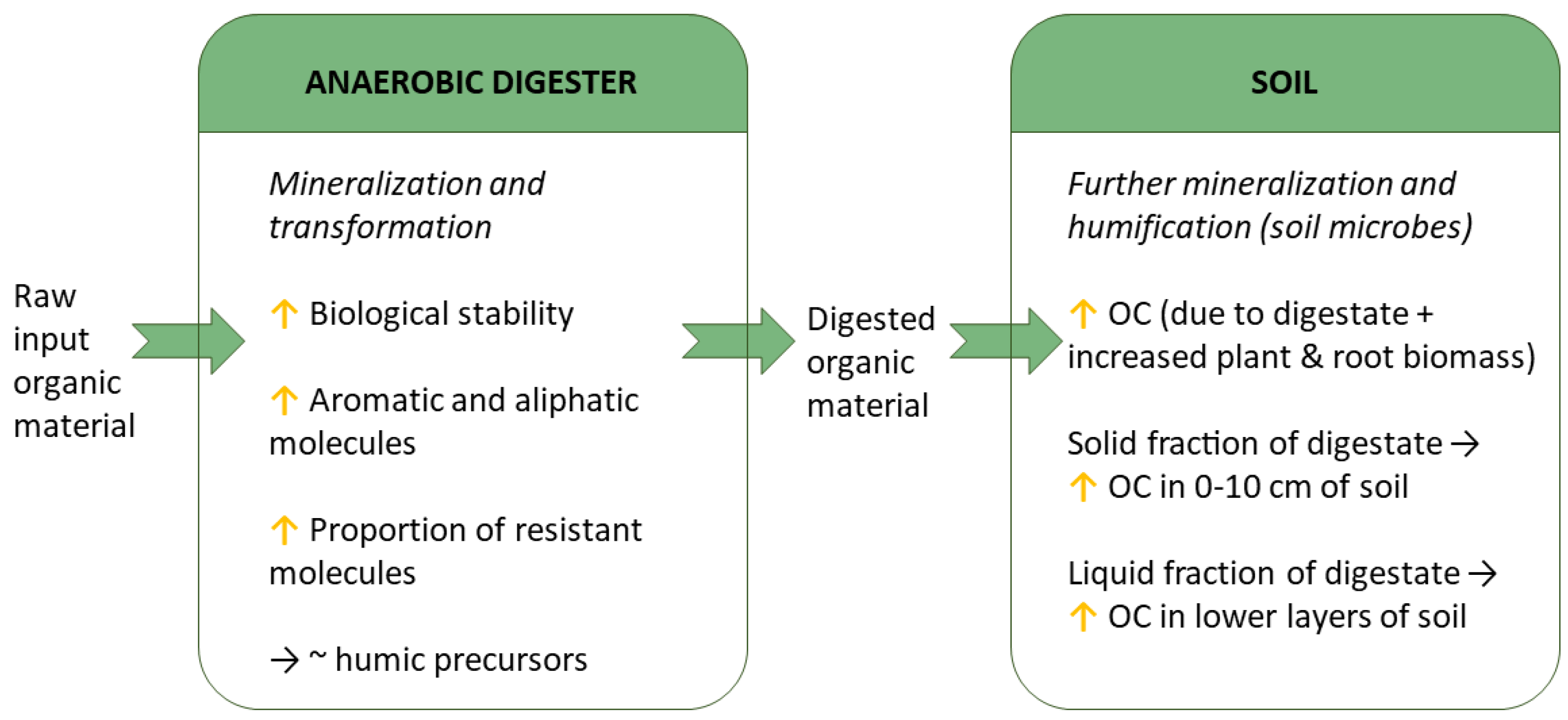
4. Effect of the Digestion Process on the Quality of Digestates
5. Effects of Digestates on Soil Properties
5.1. Effects on Soil Organic Matter (SOM) Content and Quality
5.2. Effects on Element Content and Chemical Properties
5.2.1. Nitrogen Content
5.2.2. Phosphorus, Potassium and Other Nutrient Contents
5.2.3. Electrical Conductivity—Salinity
5.2.4. pH
5.2.5. Cation Exchange Capacity
5.3. Effects on Physical Soil Properties
5.4. Effects on the Biological Properties of Soils
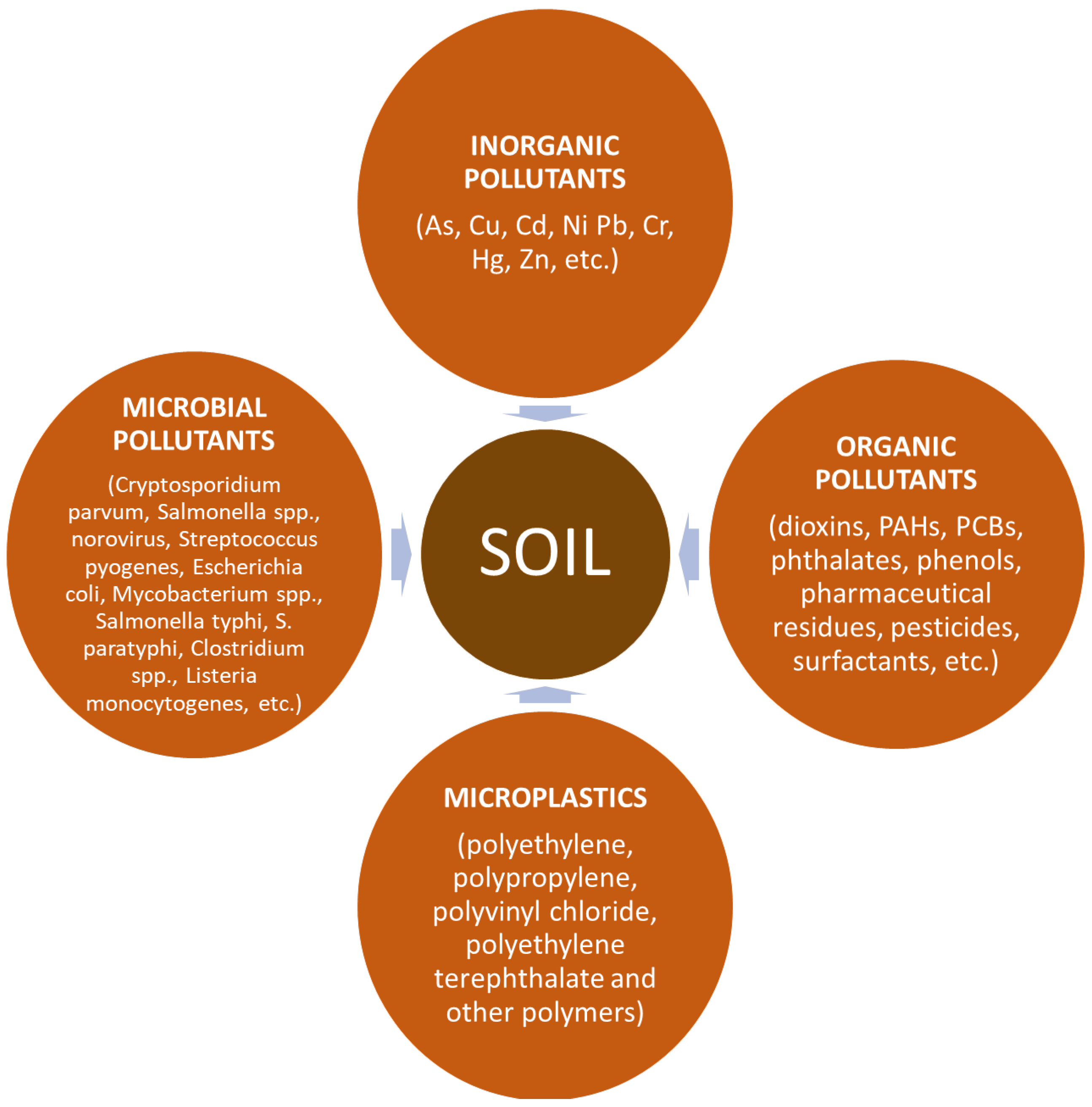
6. Substances Limiting the Application of Digestates
6.1. Inorganic Pollutants
6.2. Organic Pollutants
6.3. Microplastics
6.4. Microbial Pollutants
7. Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEC | cation exchange capacity |
| EC | electrical conductivity |
| MSW | municipal solid waste |
| OC | organic carbon |
| OM | organic matter |
| SOM | soil organic matter |
References
- Maalouf, A.; Mavropoulos, A. Re-Assessing Global Municipal Solid Waste Generation. Waste Manag. Res. 2023, 41, 936–947. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Global Waste Management Outlook 2024: Beyond an Age of Waste—Turning Rubbish into a Resource; UNEP: Nairobi, Kenya, 2024; ISBN 978-92-807-4129-2. [Google Scholar] [CrossRef]
- Eurostat Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 18 June 2025).
- Günther, S.; Karras, T.; Naegeli de Torres, F.; Semella, S.; Thrän, D. Temporal and Spatial Mapping of Theoretical Biomass Potential across the European Union. Earth Syst. Sci. Data 2024, 16, 59–74. [Google Scholar] [CrossRef]
- Dou, Z.; Toth, J.D. Global Primary Data on Consumer Food Waste: Rate and Characteristics—A Review. Resour. Conserv. Recycl. 2021, 168, 105332. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids Management and Utilizations: A Review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Czekała, W.; Nowak, M.; Bojarski, W. Anaerobic Digestion and Composting as Methods of Bio-Waste Management. Agric. Eng. 2023, 27, 173–186. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Montusiewicz, A. Methods for Enhancing Biogas Production, 1st ed.; Routledge: London, UK, 2024; ISBN 978-1-003-00354-0. [Google Scholar]
- Zupančič, M.; Možic, V.; Može, M.; Cimerman, F.; Golobič, I. Current Status and Review of Waste-to-Biogas Conversion for Selected European Countries and Worldwide. Sustainability 2022, 14, 1823. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The Effect of Digestate Fertilisation on Grass Biogas Yield and Soil Properties in Field-Biomass-Biogas-Field Renewable Energy Production Approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Soha, T.; Papp, L.; Csontos, C.; Munkácsy, B. The Importance of High Crop Residue Demand on Biogas Plant Site Selection, Scaling and Feedstock Allocation—A Regional Scale Concept in a Hungarian Study Area. Renew. Sustain. Energy Rev. 2021, 141, 110822. [Google Scholar] [CrossRef]
- Sobhi, M.; Guo, J.; Gaballah, M.S.; Li, B.; Zheng, J.; Cui, X.; Sun, H.; Dong, R. Selecting the Optimal Nutrients Recovery Application for a Biogas Slurry Based on Its Characteristics and the Local Environmental Conditions: A Critical Review. Sci. Total Environ. 2022, 814, 152700. [Google Scholar] [CrossRef]
- Subbarao, P.M.V.; D’ Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic Digestion as a Sustainable Technology for Efficiently Utilizing Biomass in the Context of Carbon Neutrality and Circular Economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef] [PubMed]
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate Management in Polish Farms as an Element of the Nutrient Cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management Strategies for Anaerobic Digestate of Organic Fraction of Municipal Solid Waste: Current Status and Future Prospects. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 37, 27–39. [Google Scholar] [CrossRef]
- Mukhtiar, A.; Mahmood, A.; Zia, M.A.; Ameen, M.; Dong, R.; Shoujun, Y.; Javaid, M.M.; Khan, B.A.; Nadeem, M.A. Role of Biogas Slurry to Reclaim Soil Properties Providing an Eco-Friendly Approach for Crop Productivity. Bioresour. Technol. Rep. 2024, 25, 101716. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical Aspects of Biowastes Conversion to Fertilizers. Biomass Convers. Biorefinery 2024, 14, 1515–1533. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Martel, J.-L.; Rouez, M.; Crest, M.; Patureau, D. First Fertilizing-Value Typology of Digestates: A Decision-Making Tool for Regulation. Waste Manag. 2019, 86, 67–79. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthigadevi, G.; Bharathiraja, B.; Praveen Kumar, R.; Abo, L.D.; Venkatesa Prabhu, S.; Balachandar, R.; Jayakumar, M. Current and Prognostic Overview on the Strategic Exploitation of Anaerobic Digestion and Digestate: A Review. Environ. Res. 2023, 216, 114526. [Google Scholar] [CrossRef]
- Karimi, B.; Sadet-Bourgeteau, S.; Cannavacciuolo, M.; Chauvin, C.; Flamin, C.; Haumont, A.; Jean-Baptiste, V.; Reibel, A.; Vrignaud, G.; Ranjard, L. Impact of Biogas Digestates on Soil Microbiota in Agriculture: A Review. Environ. Chem. Lett. 2022, 20, 3265–3288. [Google Scholar] [CrossRef]
- van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The Impact of Anaerobic Digestate on Soil Life: A Review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- O’Connor, J.; Mickan, B.S.; Rinklebe, J.; Song, H.; Siddique, K.H.M.; Wang, H.; Kirkham, M.B.; Bolan, N.S. Environmental Implications, Potential Value, and Future of Food-Waste Anaerobic Digestate Management: A Review. J. Environ. Manag. 2022, 318, 115519. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Anaerobic Digestion Fundamentals, Challenges, and Technological Advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of Low pH on Continuous Anaerobic Digestion of Waste Activated Sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef]
- Kovács, A.B.; Kremper, R.; Kincses, I.; Leviczky, Á. Influences of Different Organic Fertilizers on Nutrients of Humic Sandy Soil and on the Growth of Spinach (Spinacia oleracea L.). Acta Agrar. Debreceniensis 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Iocoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of Biogas Digestates Obtained by Anaerobic Digestion and Co-Digestion as Fertilizers: Characterization, Soil Biological Activity and Growth Dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef]
- Pampillón-González, L.; Luna-Guido, M.; Ruíz-Valdiviezo, V.M.; Franco-Hernández, O.; Fernández-Luqueño, F.; Paredes-López, O.; Hernández, G.; Dendooven, L. Greenhouse Gas Emissions and Growth of Wheat Cultivated in Soil Amended with Digestate from Biogas Production. Pedosphere 2017, 27, 318–327. [Google Scholar] [CrossRef]
- Mendonça Costa, M.S.S.D.; Lucas, J.D.; Mendonça Costa, L.A.D.; Orrico, A.C.A. A Highly Concentrated Diet Increases Biogas Production and the Agronomic Value of Young Bull’s Manure. Waste Manag. 2016, 48, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Alfa, M.I.; Adie, D.B.; Igboro, S.B.; Oranusi, U.S.; Dahunsi, S.O.; Akali, D.M. Assessment of Biofertilizer Quality and Health Implications of Anaerobic Digestion Effluent of Cow Dung and Chicken Droppings. Renew. Energy 2014, 63, 681–686. [Google Scholar] [CrossRef]
- Nõlvak, H.; Truu, M.; Kanger, K.; Tampere, M.; Espenberg, M.; Loit, E.; Raave, H.; Truu, J. Inorganic and Organic Fertilizers Impact the Abundance and Proportion of Antibiotic Resistance and Integron-Integrase Genes in Agricultural Grassland Soil. Sci. Total Environ. 2016, 562, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Guillén Fiallos, C.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic Digestate Management, Environmental Impacts, and Techno-Economic Challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy Recovery from Industrial Crop Wastes by Dry Anaerobic Digestion: A Review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential Impact of Salinity on Methane Production from Food Waste Anaerobic Digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef]
- Slepetiene, A.; Ceseviciene, J.; Amaleviciute-Volunge, K.; Mankeviciene, A.; Parasotas, I.; Skersiene, A.; Jurgutis, L.; Volungevicius, J.; Veteikis, D.; Mockeviciene, I. Solid and Liquid Phases of Anaerobic Digestate for Sustainable Use of Agricultural Soil. Sustainability 2023, 15, 1345. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A Comprehensive Review on Food Waste Anaerobic Digestion: Research Updates and Tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Nicholson, F.; Bhogal, A.; Cardenas, L.; Chadwick, D.; Misselbrook, T.; Rollett, A.; Taylor, M.; Thorman, R.; Williams, J. Nitrogen Losses to the Environment Following Food-Based Digestate and Compost Applications to Agricultural Land. Environ. Pollut. 2017, 228, 504–516. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S. Post-Treatment of Food Waste Digestate towards Land Application: A Review. J. Clean. Prod. 2021, 303, 127033. [Google Scholar] [CrossRef]
- O’Connor, J.; Mickan, B.S.; Siddique, K.H.M.; Rinklebe, J.; Kirkham, M.B.; Bolan, N.S. Physical, Chemical, and Microbial Contaminants in Food Waste Management for Soil Application: A Review. Environ. Pollut. 2022, 300, 118860. [Google Scholar] [CrossRef] [PubMed]
- Pawlett, M.; Tibbett, M. Is Sodium in Anaerobically Digested Food Waste a Potential Risk to Soils? Sustain. Environ. Res. 2015, 25, 235–239. [Google Scholar]
- Cheong, J.C.; Lee, J.T.E.; Lim, J.W.; Song, S.; Tan, J.K.N.; Chiam, Z.Y.; Yap, K.Y.; Lim, E.Y.; Zhang, J.; Tan, H.T.W.; et al. Closing the Food Waste Loop: Food Waste Anaerobic Digestate as Fertilizer for the Cultivation of the Leafy Vegetable, Xiao Bai Cai (Brassica rapa). Sci. Total Environ. 2020, 715, 136789. [Google Scholar] [CrossRef]
- Campuzano, R.; González-Martínez, S. Characteristics of the Organic Fraction of Municipal Solid Waste and Methane Production: A Review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic Co-Digestion of Organic Fraction of Municipal Solid Waste (OFMSW): Progress and Challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Patziger, M.; Kiss, K. Towards a Hydrodynamically Enhanced Design and Operation of Primary Settling Tanks—Results of a Long Term in Situ Measurement Investigation Program. Water Environ. J. 2015, 29, 338–345. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage Sludge-to-Energy Approaches Based on Anaerobic Digestion and Pyrolysis: Brief Overview and Energy Efficiency Assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between Biogas Yield and Chemical Composition of Energy Crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef]
- Jaffar, M.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Korai, R.M.; Li, X. Wheat Straw Pretreatment with KOH for Enhancing Biomethane Production and Fertilizer Value in Anaerobic Digestion. Chin. J. Chem. Eng. 2016, 24, 404–409. [Google Scholar] [CrossRef]
- Provenzano, M.R.; Cavallo, O.; Malerba, A.D.; Fabbri, C.; Zaccone, C. Unravelling (Maize silage) Digestate Features throughout a Full-Scale Plant: A Spectroscopic and Thermal Approach. J. Clean. Prod. 2018, 193, 372–378. [Google Scholar] [CrossRef]
- Czakó-Vér, K.; Somfai, D.; Suhajda, E.; Strasszer, M.; Árvay, G.; Dolgosné Kovács, A.; Dittrich, E. The Application of Biogas Fermentation Digestate as Soil Fertilizer. Analecta Tech. Szeged. 2020, 14, 76–81. [Google Scholar] [CrossRef]
- Negri, M.; Bacenetti, J.; Fiala, M.; Bocchi, S. Evaluation of Anaerobic Degradation, Biogas and Digestate Production of Cereal Silages Using Nylon-Bags. Bioresour. Technol. 2016, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Luste, S.; Heinonen-Tanski, H.; Luostarinen, S. Co-Digestion of Dairy Cattle Slurry and Industrial Meat-Processing by-Products—Effect of Ultrasound and Hygienization Pre-Treatments. Bioresour. Technol. 2012, 104, 195–201. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Barbosa, F.G.; Jiménez-Ascencio, J.; Mier-Alba, E.; Singh, A.K.; Dos Santos, J.C.; Balagurusamy, N.; Da Silva, S.S.; Chandel, A.K. Technological Routes for Biogas Production: Current Status and Future Perspectives. In Biogas Production; Balagurusamy, N., Chandel, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–17. ISBN 978-3-030-58826-7. [Google Scholar]
- Qian, S.; Chen, L.; Xu, S.; Zeng, C.; Lian, X.; Xia, Z.; Zou, J. Research on Methane-Rich Biogas Production Technology by Anaerobic Digestion Under Carbon Neutrality: A Review. Sustainability 2025, 17, 1425. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Khoshnevisan, B.; Duan, N. Meta-Analysis of Anaerobic Co-Digestion of Livestock Manure in Last Decade: Identification of Synergistic Effect and Optimization Synergy Range. Appl. Energy 2021, 282, 116128. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic Co-Digestion: Current Status and Perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Grigatti, M. The Impact of Raw and Composted Food Waste Anaerobic Digestates on Soil Organic Carbon Management: A Pot Study. Waste Biomass Valorization 2024, 15, 4915–4925. [Google Scholar] [CrossRef]
- Rigby, H.; Smith, S.R. Nitrogen Availability and Indirect Measurements of Greenhouse Gas Emissions from Aerobic and Anaerobic Biowaste Digestates Applied to Agricultural Soils. Waste Manag. 2013, 33, 2641–2652. [Google Scholar] [CrossRef]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Milc, J.; Pecchioni, N.; Francia, E. Effects of Solid and Liquid Digestate for Hydroponic Baby Leaf Lettuce (Lactuca sativa L.) Cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Koch, K.; Plabst, M.; Schmidt, A.; Helmreich, B.; Drewes, J.E. Co-Digestion of Food Waste in a Municipal Wastewater Treatment Plant: Comparison of Batch Tests and Full-Scale Experiences. Waste Manag. 2016, 47, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Duan, N.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Lu, H.; Dong, T. Co-Digestion of Chicken Manure and Microalgae Chlorella 1067 Grown in the Recycled Digestate: Nutrients Reuse and Biogas Enhancement. Waste Manag. 2017, 70, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of Poultry Manure in Poland—Current State and Future Perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef] [PubMed]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R. Anaerobic Co-Digestion of Recalcitrant Agricultural Wastes: Characterizing of Biochemical Parameters of Digestate and Its Impacts on Soil Ecosystem. Sci. Total Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef]
- Patel, V.; Pandit, S.; Chandrasekhar, K. Basics of Methanogenesis in Anaerobic Digester. In Microbial Applications Vol. 2; Kalia, V.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 291–314. ISBN 978-3-319-52668-3. [Google Scholar]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas Residues as Fertilisers—Effects on Wheat Growth and Soil Microbial Activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Nowak, M.; Czekała, W. Sustainable Use of Digestate from Biogas Plants: Separation of Raw Digestate and Liquid Fraction Processing. Sustainability 2024, 16, 5461. [Google Scholar] [CrossRef]
- Schievano, A.; D’Imporzano, G.; Salati, S.; Adani, F. On-Field Study of Anaerobic Digestion Full-Scale Plants (Part I): An on-Field Methodology to Determine Mass, Carbon and Nutrients Balance. Bioresour. Technol. 2011, 102, 7737–7744. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C. Response of a Continuous Anaerobic Digester to Temperature Transitions: A Critical Range for Restructuring the Microbial Community Structure and Function. Water Res. 2016, 89, 241–251. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Wang, S.; Wu, G.; Zhan, X. A Critical Review on Dry Anaerobic Digestion of Organic Waste: Characteristics, Operational Conditions, and Improvement Strategies. Renew. Sustain. Energy Rev. 2023, 176, 113208. [Google Scholar] [CrossRef]
- Menardo, S.; Gioelli, F.; Balsari, P. The Methane Yield of Digestate: Effect of Organic Loading Rate, Hydraulic Retention Time, and Plant Feeding. Bioresour. Technol. 2011, 102, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Low pH Anaerobic Digestion of Waste Activated Sludge for Enhanced Phosphorous Release. Water Res. 2015, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- De Neve, S.; Sleutel, S.; Hofman, G. Carbon Mineralization from Composts and Food Industry Wastes Added to Soil. Nutr. Cycl. Agroecosyst. 2003, 67, 13–20. [Google Scholar] [CrossRef]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What Is the Potential for Biogas Digesters to Improve Soil Carbon Sequestration in Sub-Saharan Africa? Comparison with Other Uses of Organic Residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing Amendment Properties of Digestate by Studying the Organic Matter Composition and the Degree of Biological Stability during the Anaerobic Digestion of the Organic Fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Evolution of humic substances during anaerobic sludge digestion. Environ. Eng. Manag. J. 2017, 16, 1577–1582. [Google Scholar] [CrossRef]
- Slepetiene, A.; Kochiieru, M.; Jurgutis, L.; Mankeviciene, A.; Skersiene, A.; Belova, O. The Effect of Anaerobic Digestate on the Soil Organic Carbon and Humified Carbon Fractions in Different Land-Use Systems in Lithuania. Land 2022, 11, 133. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, L.; Carswell, A.; Misselbrook, T.; Shen, J.; Han, J. Changes in Soil Organic Carbon Status and Microbial Community Structure Following Biogas Slurry Application in a Wheat-Rice Rotation. Sci. Total Environ. 2021, 757, 143786. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhang, Q.; Shao, H.; Gao, C.; Zhang, X. Effects of Fertilization and Straw Return Methods on the Soil Carbon Pool and CO2 Emission in a Reclaimed Mine Spoil in Shanxi Province, China. Soil Tillage Res. 2019, 195, 104361. [Google Scholar] [CrossRef]
- Sänger, A.; Geisseler, D.; Ludwig, B. Effects of Moisture and Temperature on Greenhouse Gas Emissions and C and N Leaching Losses in Soil Treated with Biogas Slurry. Biol. Fertil. Soils 2011, 47, 249–259. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep Soil Organic Matter—A Key but Poorly Understood Component of Terrestrial C Cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Levin, K.S.; Auerswald, K.; Reents, H.J.; Hülsbergen, K.-J. Effects of Organic Energy Crop Rotations and Fertilisation with the Liquid Digestate Phase on Organic Carbon in the Topsoil. Agronomy 2021, 11, 1393. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W. Effects of Different Manuring Systems with and without Biogas Digestion on Soil Mineral Nitrogen Content and on Gaseous Nitrogen Losses (Ammonia, Nitrous Oxides). Eur. J. Agron. 2009, 30, 1–16. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; De La Fuente, C.; Bernal, M.P. Chemical Properties of Anaerobic Digestates Affecting C and N Dynamics in Amended Soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; De La Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural Use of Digestate for Horticultural Crop Production and Improvement of Soil Properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review. In Biogas; Kumar, S., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Levavasseur, F.; Le Roux, C.; Kouakou, P.; Jean-Baptiste, V.; Houot, S. High Nitrogen Availability but Limited Potential Carbon Storage in Anaerobic Digestates from Cover Crops. J. Soil Sci. Plant Nutr. 2022, 22, 2891–2896. [Google Scholar] [CrossRef]
- Fouda, S.; Von Tucher, S.; Lichti, F.; Schmidhalter, U. Nitrogen Availability of Various Biogas Residues Applied to Ryegrass. J. Plant Nutr. Soil Sci. 2013, 176, 572–584. [Google Scholar] [CrossRef]
- Follett, R.F. Chapter 2—Transformation and Transport Processes of Nitrogen in Agricultural Systems. In Nitrogen in the Environment, 2nd ed.; Hatfield, J.L., Follett, R.F., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 19–50. ISBN 978-0-12-374347-3. [Google Scholar]
- Ren, A.-T.; Abbott, L.K.; Chen, Y.; Xiong, Y.-C.; Mickan, B.S. Nutrient Recovery from Anaerobic Digestion of Food Waste: Impacts of Digestate on Plant Growth and Rhizosphere Bacterial Community Composition and Potential Function in Ryegrass. Biol. Fertil. Soils 2020, 56, 973–989. [Google Scholar] [CrossRef]
- Makádi, M.; Szegi, T.; Tomócsik, A.; Orosz, V.; Michéli, E.; Ferenczy, A.; Posta, K.; Biró, B. Impact of Digestate Application on Chemical and Microbiological Properties of Two Different Textured Soils. Commun. Soil Sci. Plant Anal. 2016, 47, 167–178. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Juárez, M.F.-D.; Zangerle, M.; Insam, H. Effects of Digestate on Soil Chemical and Microbiological Properties: A Comparative Study with Compost and Vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tsachidou, B.; Scheuren, M.; Gennen, J.; Debbaut, V.; Toussaint, B.; Hissler, C.; George, I.; Delfosse, P. Biogas Residues in Substitution for Chemical Fertilizers: A Comparative Study on a Grassland in the Walloon Region. Sci. Total Environ. 2019, 666, 212–225. [Google Scholar] [CrossRef]
- Capra, F.; Abalos, D.; Maris, S.C.; Ardenti, F.; Lommi, M.; Tabaglio, V.; Fiorini, A. Towards Efficient N Cycling in Intensive Maize: Role of Cover Crops and Application Methods of Digestate Liquid Fraction. GCB Bioenergy 2023, 15, 867–885. [Google Scholar] [CrossRef]
- Haraldsen, T.K.; Andersen, U.; Krogstad, T.; Sørheim, R. Liquid Digestate from Anaerobic Treatment of Source-Separated Household Waste as Fertilizer to Barley. Waste Manag. Res. J. Sustain. Circ. Econ. 2011, 29, 1271–1276. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer Performance of Liquid Fraction of Digestate as Synthetic Nitrogen Substitute in Silage Maize Cultivation for Three Consecutive Years. Sci. Total Environ. 2017, 599–600, 1885–1894. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing Inorganic Fertilizer with Anaerobic Digestate May Maintain Agricultural Productivity at Less Environmental Cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Goglio, A.; Tambone, F.; Giordano, A.; Maretto, L.; Squartini, A.; Stevanato, P.; Meers, E.; et al. Nitrogen Dynamics in Soils Fertilized with Digestate and Mineral Fertilizers: A Full Field Approach. Sci. Total Environ. 2023, 868, 161500. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Sharma, P.; Xu, S.; Bu, J.; An, S.; Foo, J.B.L.; Wee, B.K.; Wang, Y.; Lee, J.T.E.; Zhang, J.; et al. Enhancing Sustainable Crop Cultivation: The Impact of Renewable Soil Amendments and Digestate Fertilizer on Crop Growth and Nutrient Composition. Environ. Pollut. 2024, 342, 123132. [Google Scholar] [CrossRef]
- Kovács, A.B.; Kremper, R.; Kincses, İ.; Szabó, A. Influences of Ammonium-Nitrate, Food Waste Compost and Bacterial Fertilizer on Soluble Soil Nitrogen Forms and on the Growth of Carrot (Daucus carota L.). Eurasian J. Soil Sci. 2014, 3, 95–100. [Google Scholar] [CrossRef]
- Hupfauf, S.; Bachmann, S.; Fernández-Delgado Juárez, M.; Insam, H.; Eichler-Löbermann, B. Biogas Digestates Affect Crop P Uptake and Soil Microbial Community Composition. Sci. Total Environ. 2016, 542, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; Hazarika, S.; Baruah, D.C. By-Products of Bioenergy Systems (Anaerobic Digestion and Gasification) as Sources of Plant Nutrients: Scope of Processed Application and Effect on Soil and Crop. J. Mater. Cycles Waste Manag. 2019, 21, 556–572. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Cavani, L.; Porfido, C.; Terzano, R.; Pii, Y.; Cesco, S.; Marzadori, C.; Mimmo, T. The Fertilising Potential of Manure-Based Biogas Fermentation Residues: Pelleted vs. Liquid Digestate. Heliyon 2020, 6, e03325. [Google Scholar] [CrossRef]
- Yu, F.-B.; Luo, X.-P.; Song, C.-F.; Zhang, M.-X.; Shan, S.-D. Concentrated Biogas Slurry Enhanced Soil Fertility and Tomato Quality. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The Potential of Digestate as a Biofertilizer in Eroded Soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural Use of Biogas Digestate as a Replacement Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Kovács, A.B.; Kincses, I.; Kremper, R.; Szofilkanics, A. Effects of Organic Fertilizers on the Growth and Nutrient Uptake of Cucumber (Cucumis sativus L.) Seedlings. Ann. Acad. Romanian Sci. Ser. Agric. Silvic. Vet. Med. Sci. 2017, 6, 136–143. [Google Scholar]
- Vanden Nest, T.; Ruysschaert, G.; Vandecasteele, B.; Cougnon, M.; Merckx, R.; Reheul, D. P Availability and P Leaching after Reducing the Mineral P Fertilization and the Use of Digestate Products as New Organic Fertilizers in a 4-Year Field Trial with High P Status. Agric. Ecosyst. Environ. 2015, 202, 56–67. [Google Scholar] [CrossRef]
- Weimers, K.; Bergstrand, K.-J.; Hultberg, M.; Asp, H. Liquid Anaerobic Digestate as Sole Nutrient Source in Soilless Horticulture—Or Spiked with Mineral Nutrients for Improved Plant Growth. Front. Plant Sci. 2022, 13, 770179. [Google Scholar] [CrossRef]
- Grigatti, M.; Boanini, E.; Cavani, L.; Ciavatta, C.; Marzadori, C. Phosphorus in Digestate-Based Compost: Chemical Speciation and Plant-Availability. Waste Biomass Valorization 2015, 6, 481–493. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Paredes, C.; Zhang, H.; Giles, C.D.; Darch, T.; Stutter, M.; George, T.S.; Shand, C.; Lumsdon, D.; Cooper, P.; et al. Organic Acids Regulation of Chemical–Microbial Phosphorus Transformations in Soils. Environ. Sci. Technol. 2016, 50, 11521–11531. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Suman, A.; Singh, P.N.; Srivastava, T.K. Improving Quality of Sugarcane-Growing Soils by Organic Amendments under Subtropical Climatic Conditions of India. Biol. Fertil. Soils 2007, 44, 367–376. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from Renewable Energy Production: Their Value for Fertilizing Pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A.; Szostek, R.; Wyżlic, K.; Borowski, M. Digestate from an Agricultural Biogas Plant as a Factor Shaping Soil Properties. Agronomy 2024, 14, 1528. [Google Scholar] [CrossRef]
- Brtnicky, M.; Kintl, A.; Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kucerik, J.; Vitez, T.; Prichystalova, J.; Baltazar, T.; Elbl, J. EFFECT of Digestates Derived from the Fermentation of Maize-Legume Intercropped Culture and Maize Monoculture Application on Soil Properties and Plant Biomass Production. Chem. Biol. Technol. Agric. 2022, 9, 43. [Google Scholar] [CrossRef]
- Meng, X.; Zeng, B.; Wang, P.; Li, J.; Cui, R.; Ren, L. Food Waste Anaerobic Biogas Slurry as Fertilizer: Potential Salinization on Different Soil Layer and Effect on Rhizobacteria Community. Waste Manag. 2022, 144, 490–501. [Google Scholar] [CrossRef]
- Jin, K.; Ran, Y.; Alengebawy, A.; Yang, G.; Jia, S.; Ai, P. Agro-Environmental Sustainability of Using Digestate Fertilizer for Solanaceous and Leafy Vegetables Cultivation: Insights on Fertilizer Efficiency and Risk Assessment. J. Environ. Manag. 2022, 320, 115895. [Google Scholar] [CrossRef]
- Voelkner, A.; Holthusen, D.; Horn, R. Determination of Soil Dispersion Caused by Anaerobic Digestates: Interferences of pH and Soil Charge with Regard to Soil Texture and Water Content. J. Soils Sediments 2015, 15, 1491–1499. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in Soil Chemical and Microbiological Properties during 4 Years of Application of Various Organic Residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]
- Pastorelli, R.; Valboa, G.; Lagomarsino, A.; Fabiani, A.; Simoncini, S.; Zaghi, M.; Vignozzi, N. Recycling Biogas Digestate from Energy Crops: Effects on Soil Properties and Crop Productivity. Appl. Sci. 2021, 11, 750. [Google Scholar] [CrossRef]
- Lončarić, Z.; Vukobratović, M.; Ragalyi, P.; Filep, T.; Popovic, B.; Krunoslav, K.; Vukobratović, Ž. Computer Model for Organic Fertilizer Evaluation. Poljopr. Osijek 2009, 15, 38–46. [Google Scholar]
- Shilpi, S.; Lamb, D.; Bolan, N.; Seshadri, B.; Choppala, G.; Naidu, R. Waste to Watt: Anaerobic Digestion of Wastewater Irrigated Biomass for Energy and Fertiliser Production. J. Environ. Manag. 2019, 239, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Voelkner, A.; Ohl, S.; Holthusen, D.; Hartung, E.; Dörner, J.; Horn, R. Impact of Mechanically Pre-Treated Anaerobic Digestates on Soil Properties. J. Soil Sci. Plant Nutr. 2015, 15, 882–895. [Google Scholar] [CrossRef]
- Voelkner, A.; Holthusen, D.; Horn, R. Influence of Homogenized Residues of Anaerobic Digestate on the Physicochemical Properties of Differently Textured Soils. J. Plant Nutr. Soil Sci. 2015, 178, 261–269. [Google Scholar] [CrossRef]
- Singh, B.; Chatterjee, A.; Chatterjee, R.; Chatterjee, M.; Banerjee, S. Molecular Mechanisms of Calcium Inducing Salt Tolerance in Rice: Ameliorative Interaction between CBL4 and P5CR Proteins. Agrokém. És Talajt. 2024, 73, 20–41. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica Juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic Characteristics of Five Different Urban Waste Digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
- Mortola, N.; Romaniuk, R.; Cosentino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.; Bres, P.; Riera, N.; Roba, M.; Butti, M.; et al. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca Sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate pH Influences the Nutrient Absorption and Rhizosphere Microbiome of Huanglongbing-Affected Grapefruit Plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar] [CrossRef]
- Simon, F.W.; de Castilhos Junior, A.B.; Loss, A.; Malinowski, C.; Matias, M.S. Effects of Food Waste Digested Materials on Lactuva Sativa Growth and Soil Composition. Int. J. Environ. Sci. Technol. 2023, 20, 9013–9028. [Google Scholar] [CrossRef]
- Liu, W.; Yao, B.; Xu, Y.; Dai, S.; Wang, M.; Ma, J.; Ye, Z.; Liu, D. Biogas Digestate as a Potential Nitrogen Source Enhances Soil Fertility, Rice Nitrogen Metabolism and Yield. Field Crops Res. 2024, 318, 109568. [Google Scholar] [CrossRef]
- Bloom, P.R.; Skyllberg, U. Soil pH and pH Buffering. In Handbook of Soil Sciences: Properties and Processes; CRC Press: Boca Raton, FL, USA, 2011; pp. 19-1–19-14. ISBN 978-0-429-09598-6. [Google Scholar]
- Garg, R.N.; Pathak, H.; Das, D.K.; Tomar, R.K. Use of Flyash and Biogas Slurry for Improving Wheat Yield and Physical Properties of Soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Bakhshawan, M.K.; Minyawi, M.; Aboukarima, A.M. Impact of adding biogas liquid slurry on soil surface on penetration resistance. Misr J. Agric. Eng. 2017, 34, 525–540. [Google Scholar] [CrossRef]
- Jaša, S.; Badalíková, B.; Červinka, J. Influence of Digestate on Physical Properties of Soil in ZD Budišov. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 75–83. [Google Scholar] [CrossRef]
- Malav, L.C.; Khan, S.; Gupta, N.; Kumar, S.; Bhattacharyya, R.; Malav, M.K. Effect of Biogas Slurry and Urea on Soil Health. J. Agric. Phys. 2015, 15, 55–62. [Google Scholar]
- Mayerová, M.; Šimon, T.; Stehlík, M.; Madaras, M.; Koubová, M.; Smatanová, M. Long-Term Application of Biogas Digestate Improves Soil Physical Properties. Soil Tillage Res. 2023, 231, 105715. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, J.; Xu, L.; Zhou, J. Effects of Combined Application of Biogas Slurry and Chemical Fertilizer on Soil Aggregation and C/N Distribution in an Ultisol. PLoS ONE 2017, 12, e0170491. [Google Scholar] [CrossRef]
- Tang, J.; Yin, J.; Davy, A.J.; Pan, F.; Han, X.; Huang, S.; Wu, D. Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain. Sustainability 2022, 14, 15099. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Liu, K.; Li, Z.; Qian, X.; Zang, H.; Yang, X.; Zhao, Y.; Shen, Y.; Li, Z.; et al. Water-Stable Aggregates and Carbon Accumulation in Barren Sandy Soil Depend on Organic Amendment Method: A Three-Year Field Study. J. Clean. Prod. 2019, 212, 393–400. [Google Scholar] [CrossRef]
- Koch, S.; Liu, H.; Lenz, C.; Eichler-Löbermann, B.; Vogel, H.-J.; Lennartz, B. Soil Structure and Solute Transport Pathways in Biogas Digestate-Amended Soils. Soil Tillage Res. 2024, 240, 106074. [Google Scholar] [CrossRef]
- Palupi, C.C.; Ngadisih, N.; Karyadi, J.N.W.; Tirtalistyani, R.; Heikal Ismail, M.; Mawandha, H.G. Effect of Biogas Slurry Fertilizer on Dynamics of Soil Consistency and Tillage Power Requirement. J. Ilm. Rekayasa Pertan. Dan Biosist. 2022, 10, 14–23. [Google Scholar] [CrossRef]
- Musse, Z.A.; Yoseph Samago, T.; Beshir, H.M. Effect of Liquid Bio-Slurry and Nitrogen Rates on Soil Physico-Chemical Properties and Quality of Green Bean (Phaseolus vulgaris L.) at Hawassa Southern Ethiopia. J. Plant Interact. 2020, 15, 207–212. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or Microbial-Derived? A Review on the Molecular Composition of Stabilized Soil Organic Matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J.; Ma, J.; Ye, J.; Yu, Q.; Zou, P.; Sun, W.; Lin, H.; Wang, F.; Zhao, X.; et al. Long-Term Biogas Slurry Application Increases Microbial Necromass but Not Plant Lignin Contribution to Soil Organic Carbon in Paddy Soils as Regulated by Fungal Community. Waste Manag. 2024, 175, 254–264. [Google Scholar] [CrossRef]
- Ernst, G.; Müller, A.; Göhler, H.; Emmerling, C. C and N Turnover of Fermented Residues from Biogas Plants in Soil in the Presence of Three Different Earthworm Species (Lumbricus Terrestris, Aporrectodea Longa, Aporrectodea Caliginosa). Soil Biol. Biochem. 2008, 40, 1413–1420. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Olesen, J.E.; Møller, H.B.; Sørensen, P.; Christensen, B.T. Carbon Dynamics and Retention in Soil after Anaerobic Digestion of Dairy Cattle Feed and Faeces. Soil Biol. Biochem. 2013, 58, 82–87. [Google Scholar] [CrossRef]
- Ahlberg-Eliasson, K.; Liu, T.; Nadeau, E.; Schnürer, A. Forage Types and Origin of Manure in Codigestion Affect Methane Yield and Microbial Community Structure. Grass Forage Sci. 2018, 73, 740–757. [Google Scholar] [CrossRef]
- Wentzel, S.; Joergensen, R.G. Quantitative Microbial Indices in Biogas and Raw Cattle Slurries. Eng. Life Sci. 2016, 16, 231–237. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Peterka, J.; Štindl, P.; Plát, V. Agrochemical Value of Organic Matter of Fermenter Wastes in Biogas Production. Plant Soil Environ. 2008, 54, 321–328. [Google Scholar] [CrossRef]
- Coban, H.; Miltner, A.; Elling, F.J.; Hinrichs, K.-U.; Kästner, M. The Contribution of Biogas Residues to Soil Organic Matter Formation and CO2 Emissions in an Arable Soil. Soil Biol. Biochem. 2015, 86, 108–115. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-Based Biogas Fermentation Residues—Friend or Foe of Soil Fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Stumpe, B.; Werner, S.; Jung, R.; Heinze, S.; Jüschke, E.; Strippel, C.; Marschner, B. Organic Carbon Dynamics and Enzyme Activities in Agricultural Soils Amended with Biogas Slurry, Liquid Manure and Sewage Sludge. Agric. Sci. 2012, 3, 104–113. [Google Scholar] [CrossRef]
- Sapp, M.; Harrison, M.; Hany, U.; Charlton, A.; Thwaites, R. Comparing the Effect of Digestate and Chemical Fertiliser on Soil Bacteria. Appl. Soil Ecol. 2015, 86, 1–9. [Google Scholar] [CrossRef]
- Jothi, G.; Pugalendhi, S.; Poornima, K.; Rajendran, G. Management of Root-Knot Nematode in Tomato Lycopersicon Esculentum, Mill., with Biogas Slurry. Bioresour. Technol. 2003, 89, 169–170. [Google Scholar] [CrossRef]
- Westphal, A.; Kücke, M.; Heuer, H. Soil Amendment with Digestate from Bio-Energy Fermenters for Mitigating Damage to Beta Vulgaris Subspp. by Heterodera Schachtii. Appl. Soil Ecol. 2016, 99, 129–136. [Google Scholar] [CrossRef]
- Moinard, V.; Redondi, C.; Etiévant, V.; Savoie, A.; Duchene, D.; Pelosi, C.; Houot, S.; Capowiez, Y. Short- and Long-Term Impacts of Anaerobic Digestate Spreading on Earthworms in Cropped Soils. Appl. Soil Ecol. 2021, 168, 104149. [Google Scholar] [CrossRef]
- Garbini, G.L.; Grenni, P.; Rauseo, J.; Patrolecco, L.; Pescatore, T.; Spataro, F.; Barra Caracciolo, A. Insights into Structure and Functioning of a Soil Microbial Community Amended with Cattle Manure Digestate and Sulfamethoxazole. J. Soils Sediments 2022, 22, 2158–2173. [Google Scholar] [CrossRef]
- Garcia-Sánchez, M.; Garcia-Romera, I.; Cajthaml, T.; Tlustoš, P.; Száková, J. Changes in Soil Microbial Community Functionality and Structure in a Metal-Polluted Site: The Effect of Digestate and Fly Ash Applications. J. Environ. Manag. 2015, 162, 63–73. [Google Scholar] [CrossRef]
- Meng, X.; Ma, C.; Petersen, S.O. Sensitive Control of N2O Emissions and Microbial Community Dynamics by Organic Fertilizer and Soil Interactions. Biol. Fertil. Soils 2022, 58, 771–788. [Google Scholar] [CrossRef]
- Cattin, M.; Semple, K.T.; Stutter, M.; Romano, G.; Lag-Brotons, A.J.; Parry, C.; Surridge, B.W.J. Changes in Microbial Utilization and Fate of Soil Carbon Following the Addition of Different Fractions of Anaerobic Digestate to Soils. Eur. J. Soil Sci. 2021, 72, 2398–2413. [Google Scholar] [CrossRef]
- Baldasso, V.; Tomasino, M.P.; Sayen, S.; Guillon, E.; Frunzo, L.; Gomes, C.A.R.; Alves, M.J.; Castro, R.; Mucha, A.P.; Almeida, C.M.R. Effects of Digestate Soil Amendment on the Fate of Trace Metals and on the Soil Microbial Community. Environ. Pollut. 2025, 371, 125961. [Google Scholar] [CrossRef] [PubMed]
- Madegwa, Y.M.; Uchida, Y. Liming Improves the Stability of Soil Microbial Community Structures against the Application of Digestate Made from Dairy Wastes. J. Environ. Manag. 2021, 297, 113356. [Google Scholar] [CrossRef] [PubMed]
- Mora-Salguero, D.; Montenach, D.; Gilles, M.; Jean-Baptiste, V.; Sadet-Bourgeteau, S. Long-Term Effects of Combining Anaerobic Digestate with Other Organic Waste Products on Soil Microbial Communities. Front. Microbiol. 2025, 15, 1490034. [Google Scholar] [CrossRef]
- Nikolaidou, C.; Mola, M.; Papakostas, S.; Aschonitis, V.G.; Monokrousos, N.; Kougias, P.G. The Effect of Anaerobic Digestate as an Organic Soil Fertilizer on the Diversity and Structure of the Indigenous Soil Microbial and Nematode Communities. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Pezzolla, D.; Marconi, G.; Turchetti, B.; Zadra, C.; Agnelli, A.; Veronesi, F.; Onofri, A.; Benucci, G.M.N.; Buzzini, P.; Albertini, E.; et al. Influence of Exogenous Organic Matter on Prokaryotic and Eukaryotic Microbiota in an Agricultural Soil. A Multidisciplinary Approach. Soil Biol. Biochem. 2015, 82, 9–20. [Google Scholar] [CrossRef]
- Barduca, L.; Wentzel, S.; Schmidt, R.; Malagoli, M.; Joergensen, R.G. Mineralisation of Distinct Biogas Digestate Qualities Directly after Application to Soil. Biol. Fertil. Soils 2021, 57, 235–243. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Zhu, Y.-J.; Jin, L.; Wang, B.-T.; Xu, X.; Zou, X.; Ruan, H.-H.; Jin, F.-J. Contrasting Responses of Fungal and Bacterial Communities to Biogas Slurry Addition in Rhizospheric Soil of Poplar Plantations. Appl. Soil Ecol. 2022, 175, 104427. [Google Scholar] [CrossRef]
- Xu, M.; Xian, Y.; Wu, J.; Gu, Y.; Yang, G.; Zhang, X.; Peng, H.; Yu, X.; Xiao, Y.; Li, L. Effect of Biogas Slurry Addition on Soil Properties, Yields, and Bacterial Composition in the Rice-Rape Rotation Ecosystem over 3 Years. J. Soils Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Ma, J.; Chapman, S.; Zou, P.; Ye, J.; Yu, Q.; Sun, W.; Lin, H.; Jiang, L. Soil Microbial Activity and Community Composition as Influenced by Application of Pig Biogas Slurry in Paddy Field in Southeast China. Paddy Water Environ. 2020, 18, 15–25. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Wang, C.; Zhao, X.; Wu, K.; Yang, B.; Yin, F.; Zhang, W. Biogas Slurry Application Alters Soil Properties, Reshapes the Soil Microbial Community, and Alleviates Root Rot of Panax Notoginseng. PeerJ 2022, 10, e13770. [Google Scholar] [CrossRef] [PubMed]
- Czatzkowska, M.; Rolbiecki, D.; Korzeniewska, E.; Harnisz, M. Heavy Metal and Antimicrobial Residue Levels in Various Types of Digestate from Biogas Plants—A Review. Sustainability 2025, 17, 416. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace Metal Speciation and Bioavailability in Anaerobic Digestion: A Review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, L.; Carswell, A.; Misselbrook, T.; Shen, J.; Han, J. Fate and Transfer of Heavy Metals Following Repeated Biogas Slurry Application in a Rice-Wheat Crop Rotation. J. Environ. Manag. 2020, 270, 110938. [Google Scholar] [CrossRef]
- Derehajło, S.; Tymińska, M.; Skibko, Z.; Borusiewicz, A.; Romaniuk, W.; Kuboń, M.; Olech, E.; Koszel, M. Heavy Metal Content in Substrates in Agricultural Biogas Plants. Agric. Eng. 2023, 27, 315–329. [Google Scholar] [CrossRef]
- Bian, B.; Wu, H.S.; Zhou, L.J. Contamination and Risk Assessment of Heavy Metals in Soils Irrigated with Biogas Slurry: A Case Study of Taihu Basin. Environ. Monit. Assess. 2015, 187, 155. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, L.; Chen, D.; Toyota, K.; Strong, P.J.; Wang, H.; Hirasawa, T. Decontamination of Anaerobically Digested Slurry in a Paddy Field Ecosystem in Jiaxing Region of China. Agric. Ecosyst. Environ. 2012, 146, 13–22. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhao, J.; Ma, J.; Yu, Q.; Zou, P.; Lin, H.; Ma, J. Fate of Heavy Metals and Bacterial Community Composition Following Biogas Slurry Application in a Single Rice Cropping System. J. Soils Sediments 2022, 22, 968–981. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Q.; Wang, J.; Li, H.; Qin, J.; Li, X.; Gouda, S.G.; Liu, Y.; Liu, Q.; Guo, G.; et al. Ecological Circular Agriculture: A Case Study Evaluating Biogas Slurry Applied to Rice in Two Soils. Chemosphere 2022, 301, 134628. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Liu, J. Comparative Characterization of Sewage Sludge Compost and Soil: Heavy Metal Leaching Characteristics. J. Hazard. Mater. 2016, 310, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Martín, M.J.; García-Delgado, M.; Lorenzo, L.F.; Rodríguez-Cruz, M.S.; Arienzo, M. Heavy Metals in Sewage Sludge Amended Soils Determined by Sequential Extractions as a Function of Incubation Time of Soils. Geoderma 2007, 142, 262–273. [Google Scholar] [CrossRef]
- Pasquini, L.; Munoz, J.-F.; Pons, M.-N.; Yvon, J.; Dauchy, X.; France, X.; Le, N.D.; France-Lanord, C.; Görner, T. Occurrence of Eight Household Micropollutants in Urban Wastewater and Their Fate in a Wastewater Treatment Plant. Statistical Evaluation. Sci. Total Environ. 2014, 481, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N.; Yakovlev, A.S. Contamination of Soils and Groundwater with New Organic Micropollutants: A Review. Eurasian Soil Sci. 2016, 49, 560–569. [Google Scholar] [CrossRef]
- García-Santiago, X.; Franco-Uría, A.; Omil, F.; Lema, J.M. Risk Assessment of Persistent Pharmaceuticals in Biosolids: Dealing with Uncertainty. J. Hazard. Mater. 2016, 302, 72–81. [Google Scholar] [CrossRef]
- Chenxi, W.; Spongberg, A.L.; Witter, J.D. Determination of the Persistence of Pharmaceuticals in Biosolids Using Liquid-Chromatography Tandem Mass Spectrometry. Chemosphere 2008, 73, 511–518. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef]
- Clarke, B.O.; Smith, S.R. Review of ‘Emerging’ Organic Contaminants in Biosolids and Assessment of International Research Priorities for the Agricultural Use of Biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Tadeo, J.L. Influence of Moisture on the Availability and Persistence of Clotrimazole and Fluconazole in Sludge-Amended Soil. Environ. Toxicol. Chem. 2012, 31, 501–507. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, M.D.; Santos, J.L.; Aparicio, I.; Alonso, E. Distribution and Temporal Evolution of Pharmaceutically Active Compounds alongside Sewage Sludge Treatment. Risk Assessment of Sludge Application onto Soils. J. Environ. Manag. 2012, 102, 18–25. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical Antibiotic Compounds in Soils—A Review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- León, C.; Henríquez, C.; López, N.; Sanchez, G.; Pastén, B.; Baeza, P.; Ojeda, J. Inhibitory Effect of the Ascorbic Acid on Photodegradation of Pharmaceuticals Compounds Exposed to UV-B Radiation. J. Photochem. Photobiol. 2021, 7, 100035. [Google Scholar] [CrossRef]
- Chen, W.-L.; Lin, S.-C.; Huang, C.-H.; Peng, S.-Y.; Ling, Y.S. Wide-Scope Screening for Pharmaceutically Active Substances in a Leafy Vegetable Cultivated under Biogas Slurry Irrigation. Sci. Total Environ. 2021, 750, 141519. [Google Scholar] [CrossRef] [PubMed]
- Widyasari-Mehta, A.; Hartung, S.; Kreuzig, R. From the Application of Antibiotics to Antibiotic Residues in Liquid Manures and Digestates: A Screening Study in One European Center of Conventional Pig Husbandry. J. Environ. Manag. 2016, 177, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Bloem, E. Antibiotic Residues in Substrates and Output Materials from Biogas Plants—Implications for Agriculture. Chemosphere 2021, 278, 130425. [Google Scholar] [CrossRef]
- Ali, A.M.; Nesse, A.S.; Eich-Greatorex, S.; Sogn, T.A.; Aanrud, S.G.; Bunæs, J.A.A.; Lyche, J.L.; Kallenborn, R. Organic Contaminants of Emerging Concern in Norwegian Digestates from Biogas Production. Environ. Sci. Process. Impacts 2019, 21, 1498–1508. [Google Scholar] [CrossRef]
- Rodriguez-Navas, C.; Björklund, E.; Halling-Sørensen, B.; Hansen, M. Biogas Final Digestive Byproduct Applied to Croplands as Fertilizer Contains High Levels of Steroid Hormones. Environ. Pollut. 2013, 180, 368–371. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Hadi, M.; Lin, C.; Nguyen, H.-L.; Thai, V.-B.; Hoang, H.-G.; Vo, D.-V.N.; Tran, H.-T. Microplastics in Sewage Sludge: Distribution, Toxicity, Identification Methods, and Engineered Technologies. Chemosphere 2022, 308, 136455. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in Soils: Analytical Methods, Pollution Characteristics and Ecological Risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent Trends in Degradation of Microplastics in the Environment: A State-of-the-Art Review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Ashrafy, A.; Liza, A.A.; Islam, M.N.; Billah, M.M.; Arafat, S.T.; Rahman, M.M.; Rahman, S.M. Microplastics Pollution: A Brief Review of Its Source and Abundance in Different Aquatic Ecosystems. J. Hazard. Mater. Adv. 2023, 9, 100215. [Google Scholar] [CrossRef]
- Nanthini Devi, K.; Raju, P.; Santhanam, P.; Perumal, P. Impacts of Microplastics on Marine Organisms: Present Perspectives and the Way Forward. Egypt. J. Aquat. Res. 2022, 48, 205–209. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, Y.; Zhang, L.; Zhang, X. Inhibition of Aged Microplastics and Leachates on Methane Production from Anaerobic Digestion of Sludge and Identification of Key Components. J. Hazard. Mater. 2023, 446, 130717. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xiao, K.; Zhu, Y.; Ou, B.; Yu, W.; Liang, S.; Hou, H.; Yuan, S.; Gan, F.; Mi, R.; et al. Deciphering the Role of Microplastic Size on Anaerobic Sludge Digestion: Changes of Dissolved Organic Matter, Leaching Compounds and Microbial Community. Environ. Res. 2022, 214, 114032. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Demirer, G.N. Microplastics in Anaerobic Digestion: Occurrence, Impact, and Mitigation Strategies. J. Environ. Health Sci. Eng. 2024, 22, 397–411. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Chand, R.; Rasmussen, L.A.; Tumlin, S.; Vollertsen, J. The Occurrence and Fate of Microplastics in a Mesophilic Anaerobic Digester Receiving Sewage Sludge, Grease, and Fatty Slurries. Sci. Total Environ. 2021, 798, 149287. [Google Scholar] [CrossRef]
- Alvim, C.B.; Ferrer-Polonio, E.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Fernández-Navarro, J.; Alonso-Molina, J.L.; Amorós-Muñoz, I. Effect of Polystyrene Nanoplastics on the Activated Sludge Process Performance and Biomass Characteristics. A Laboratory Study with a Sequencing Batch Reactor. J. Environ. Manag. 2023, 329, 117131. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senathirajah, K.; Subashchandrabose, S.R.; Evans, G.; Thavamani, P. Transport and Fate of Microplastics in Wastewater Treatment Plants: Implications to Environmental Health. Rev. Environ. Sci. Biotechnol. 2018, 17, 637–653. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the Degradation of (Micro)Plastics: Degradation Methods, Influencing Factors, Environmental Impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Aitken, M.D.; Sobsey, M.D.; Van Abel, N.A.; Blauth, K.E.; Singleton, D.R.; Crunk, P.L.; Nichols, C.; Walters, G.W.; Schneider, M. Inactivation of Escherichia Coli O157:H7 during Thermophilic Anaerobic Digestion of Manure from Dairy Cattle. Water Res. 2007, 41, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.; Toze, S. Human Pathogens and Their Indicators in Biosolids: A Literature Review. Environ. Int. 2009, 35, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic Bacteria and Mineral N in Soils Following the Land Spreading of Biogas Digestates and Fresh Manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Bonetta, S.; Ferretti, E.; Bonetta, S.; Fezia, G.; Carraro, E. Microbiological Contamination of Digested Products from Anaerobic Co-Digestion of Bovine Manure and Agricultural by-Products: Microbial Contamination of Digestate. Lett. Appl. Microbiol. 2011, 53, 552–557. [Google Scholar] [CrossRef]
- Islam, M.; Biswas, P.; Sabuj, A.; Haque, Z.; Saha, C.; Alam, M.; Rahman, M.; Saha, S. Microbial Load in Bio-Slurry from Different Biogas Plants in Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 376. [Google Scholar] [CrossRef]
- Nag, R.; Whyte, P.; Markey, B.K.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; Cummins, E. Ranking Hazards Pertaining to Human Health Concerns from Land Application of Anaerobic Digestate. Sci. Total Environ. 2020, 710, 136297. [Google Scholar] [CrossRef]
- Massé, D.; Gilbert, Y.; Topp, E. Pathogen Removal in Farm-Scale Psychrophilic Anaerobic Digesters Processing Swine Manure. Bioresour. Technol. 2011, 102, 641–646. [Google Scholar] [CrossRef]
- Pu, C.; Liu, H.; Ding, G.; Sun, Y.; Yu, X.; Chen, J.; Ren, J.; Gong, X. Impact of Direct Application of Biogas Slurry and Residue in Fields: In Situ Analysis of Antibiotic Resistance Genes from Pig Manure to Fields. J. Hazard. Mater. 2018, 344, 441–449. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Meng, J.; Zhang, J.; Zhuang, H.; Zheng, G.; Xie, W.; Ping, L.; Shan, S. Long-Term Biogas Slurry Application Increased Antibiotics Accumulation and Antibiotic Resistance Genes (ARGs) Spread in Agricultural Soils with Different Properties. Sci. Total Environ. 2021, 759, 143473. [Google Scholar] [CrossRef]

| Element (Form) | Change of Content | Soil | Raw Materials of Digestate | Digestate Rate | Reference |
|---|---|---|---|---|---|
| N (total) | 5.4–45% increase | Silt loam | Swine manure | Corresponding to 170–680 kg N ha−1 | [124] |
| 7% decrease–16% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] | |
| 41–55% increase | Sandy loam | Cattle and pig manure | Corresponding to 2.56 g N per plant | [111] | |
| 39–58% increase | Sand | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| 6.4–13% increase | Loam | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| 14–19% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] | |
| (available) | 52–61% increase | Sandy loam | Cattle and pig manure | Corresponding to 2.56 g N per plant | [111] |
| P (total) | 11% decrease–62% increase | Silt loam | Swine manure | Corresponding to 170–680 kg N ha−1 | [124] |
| 19% increase | Texture not specified | Green waste, maize silage, beet pulp, stillage, whey | 36,000 L ha−1 | [113] | |
| 12–20% increase | Sandy loam | Cattle and pig manure | Corresponding to 55.8 g P per plant | [111] | |
| (available) | 212–218% increase | Sandy loam | Cattle and pig manure | Corresponding to 55.8 g P per plant | [111] |
| 50–54% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] | |
| 26–44% increase | Sand | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| 10–34% increase | Loam | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| K (total) | 2.4–17% increase | Silt loam | Swine manure | Corresponding to 170–680 kg N ha−1 | [124] |
| 128% increase | Texture not specified | Green waste, maize silage, beet pulp, stillage, whey | 36,000 L ha−1 | [113] | |
| (exchangeable) | 4% decrease–22% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 0–36% increase | Loamy sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| 3.8 decrease–62% increase | Sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| (available) | 59–67% increase | Sandy loam | Cattle and pig manure | Corresponding to 7.56 g K per plant | [111] |
| 39–73% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] | |
| 25–94% increase | Texture not specified | Corn silage, straw, chicken manure, pig slurry, distiller’s grain | Corresponding to 28–112 mg N kg−1 | [121] | |
| 49–90% increase | Sand | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| 1% decrease–26% increase | Loam | Cow and poultry manure, plant residues, other organic residues, sterilized animal residues | Corresponding to 170 kg N ha−1 | [98] | |
| Mg (total) | 3% increase | Texture not specified | Green waste, maize silage, beet pulp, stillage, whey | 36,000 L ha−1 | [113] |
| (exchangeable) | 4% decrease–13% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 0–19% increase | Loamy sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| 4.4% decrease–20% increase | Sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| (available) | 47–53% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] |
| Zn (exchangeable) | 11% decrease–16% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| (available) | 3.6–32% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] |
| Ca (exchangeable) | 5% decrease–2% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 6.3% decrease–2.2% increase | Loamy sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| 1.6–28% increase | Sand | Sugar beet and maize in different ratios | 30 m3 ha−1 | [125] | |
| Cu (available) | 9% decrease–4% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 7.1% decrease–36% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] | |
| Fe (available) | 9% decrease–2% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 2.9% decrease–1.2% increase | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] | |
| Mn (available) | 23% decrease–15% increase | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] |
| 9.5–12% decrease | Silty loam | Corn silage, sugar bagasse beet, pomace of fruit, dairy waste, manure | 30–60 m3 ha−1 | [80] |
| pH Change | Raw Materials of Digestate | Digestate pH | Change in Soil pH Compared to Control Treatment (as a Result of the Applied Digestate Rate) | Reference |
|---|---|---|---|---|
| pH increased | Chicken manure | 7.62 | pH(H2O) from 5.63 to 5.71 (digestate replacing 25% N fertilizer: 27,167 kg ha−1 digestate)–5.86 (digestate replacing 75% N fertilizer: 81,333 kg ha−1 digestate) | [138] |
| 70% corn silage, 15% sugar bagasse beet, 5% pomace of fruit, 5% dairy waste, 5% manure | 7.8–8.5 | pH(KCl) from 4.5 to 4.6 (30 m3 ha−1 digestate) and 4.9 (60 m3 ha−1 digestate) | [80] | |
| Diluted chicken manure, food waste, maize and beetroot waste | 8.1–8.74 (solid) 7.94–8.17 (liquid) | pH(KCl) from 7.68 to 7.82 (solid or liquid digestate corresponding to 85 kg N ha−1) and 7.77 (liquid digestate) and 7.80 (solid digestate corresp. to 170 kg N ha−1) | [11] | |
| Green waste, maize silage, beet pulp, stillage, whey | 8.73 | from 7.56 to 7.63 (36,000 L ha−1 digestate) | [113] | |
| Liquid: pig slurry, corn-based distiller’s grain, cattle slurry Solid: corn silage, straw, chicken manure | 7.90 (liquid) 8.25 (solid) | pH(KCl) from 4.52 to 4.67 (liquid digestate corresponding to 112 kg N ha−1) and 4.62 (solid digestate corresponding to 84 kg N ha−1) | [121] | |
| 95% food waste (restaurant), 5% gardening waste | 6.82 (liquid) 8.86 (dried) | pH(KCl) in winter/spring season from 4.73 to 4.98 (dried) and 5.46 (liquid) in spring/summer season from 4.88 to 5.36 (dried) and 5.56 (liquid) (corresponding to 140 kg N ha−1) | [137] | |
| Cow dung (CD) or Ipomoea carnea leaves + cow dung (ICD) or Rice straw + green gram stover + cow dung (RGC) | 7.3 (CD), 7.8 (ICD), 8.0 (RGC) | pH(H2O) from 5.43 to 5.60 (CD), 5.48 (ICD), 5.51 (RGC) (digestate corresponding to 60 kg N ha−1) | [109] | |
| M100: maize or SB100: sugar beet or SB80M20: 80% sugar beet and 20% maize or M80SB20: 80% maize and 20% sugar beet | M100: 8.25–7.93 SB100: 8.21–7.85 SB80M20: 7.74–7.82 M80SB20: 7.75–7.85 | pH(CaCl2) loamy soil from 6.64 to 7.24–7.24 (M100); 7.23–7.28 (SB100); 7.16–7.23 (SB80M20); 7.16–7.21 (M80SB20) sandy soil from 5.19 to 5.74–5.82 (M100); 5.65–5.71 (SB100); 5.76–5.83 (SB80M20); 5.81–5.89 (M80SB20) (30 m3 ha−1 digestate) | [130] | |
| pH decreased | Not defined | 7.5 | pH(CaCl2) from 5.78 to 5.69 (digestate corresponding to 190 kg N ha−1) | [120] |
| Swine manure | For eggplant: 8.1 (solid–SD) 8.3 (liquid –LD) For cabbage: 8.1 (SD) 9.2 (LD) | pH(H2O) Eggplant: from 6.99 to 6.62 (13,375 kg ha−1 SD + 90,785 kg ha−1 LD)–6.36 (53,500 kg ha−1 SD + 224,800 kg ha−1 LD) Shanghai cabbage: from 7.15 to 6.90 (13,375 kg ha−1 SD + 90,785 kg ha−1 LD)–6.65 (53,500 kg ha−1 SD + 224,800 kg ha−1 LD) | [124] | |
| pH both increased and decreased | 42.4% animal residues, 22.3% cow manure, 18.9% organic residues, 12.0% plant residues, 4.4% poultry manure | 8.03 | 1st year: sandy soil: from 6.27 to 6.29; loamy soil: no change (8.13); 2nd year: sandy soil: from 6.40 to 6.25; loamy soil: from 7.64 to 7.67 (digestate corresp. to 170 kg N ha−1 year−1) | [98] |
| Parameter | Change | Soil Texture | Raw Materials of Digestate | Digestate Rate | Reference |
|---|---|---|---|---|---|
| Water-holding | Increased | Loamy soil | Pig manure + cleansing water | 120 m3 ha−1 | [146] |
| capacity | Sandy loam | Agricultural waste | 15 t ha−1 | [140] | |
| No change | Sandy soil | Dairy slurry, maize silage, wheat grain | 30 m3 ha−1 year−1 | [148] | |
| Bulk density | Decreased | Sandy loam | Cattle dung | Corresponding to 150 kg ha−1 N | [143] |
| Loamy soil | Pig manure + cleansing water | 120 m3 ha−1 | [146] | ||
| Sandy soil | Dairy slurry, maize silage, wheat grain | 30 m3 ha−1 year−1 | [148] | ||
| Silt loam and sandy loam | Corn silage, pig slurry, farmyard manure, hay | Corresponding to 120–150 kg ha−1 N | [144] | ||
| Silty clay loam | Pig manure and urine | Corresponding to 18–120 kg ha−1 N | [145] | ||
| Sandy loam | Agricultural waste | 4 and 15 t ha−1 | [140] | ||
| No change | Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] | |
| Sandy loam | Cow dung and brewery factory residue | Up to 61.8 m3 ha−1 | [150] | ||
| Increased | Sandy clay | Agricultural by-products | 20–40 t ha−1 | [142] | |
| Aggregate stability | Increased | Silt loam and sandy loam | Digestate I: corn silage, cattle slurry; Digestate II: corn silage, pig slurry, farmyard manure, hay | Corresponding to 120–150 kg ha−1 N | [144] |
| Silty clay | Energy crops, agricultural by-products, cattle slurry | 34,700 L ha−1 | [127] | ||
| Silty clay loam | Pig manure and urine | Corresponding to 36–54 kg ha−1 N | [145] | ||
| Loamy soil | Pig manure + cleansing water | 120 m3 ha−1 | [146] |
| Digestate Raw Material | Dose | Soil | Test Plant | Change in Microbial Community | Reference |
|---|---|---|---|---|---|
| Sewage sludge | 147 kg N ha−1 | No data | Spring wheat | - Decline in diversity and richness, but effects on soil bacteria appears low - Digestate bacteria did not strongly alter community patterns in the soil | [162] |
| Dairy cattle manure + maize silage + agro-food waste | No data | Clay | No plant | - Digestate induced a shift in the soil microbial community - Digestate favoured some fungal groups like Basidiomycota - Increased abundance and number of bacterial species involved in N metabolism | [166] |
| Sugar beet pulp + fruit marc + maize silage | 100 g dw kg soil−1 | Loam | Wheat | - Increase in the functional diversity of soil microbial communities - Abundance enhancement of bacteria, actinobacteria and fungal communities - Increased the Gram+/Gram− ratio | [167] |
| Cow manure + silage | 100 kg N ha−1 | Sandy loam, clay loam | No plant | - Increased microbial biomass | [168] |
| Livestock and poultry manure + food waste | 170 kg N ha−1 | High and low nutrient soil | No plant | - Decreased microbial biomass C - Negative values of carbon use efficiency | [169] |
| Livestock and poultry manure + food waste | 170 kg N ha−1 | High and low nutrient soil | No plant | - Increased microbial biomass C - Increased fungal-to-bacterial ratio - Increased of carbon use efficiency | [169] |
| Municipal solid biowaste | Soil:digestate ratio was 14:1 | Loamy sand | No plant | - Increase in microbial abundance and diversity - Stabilization of the karyotic community - The Firmicutes dominated community changed into a Proteobacteria and Bacteroidota dominated community | [170] |
| Cattle slurry + maize silage | 33 mg P kg soil−1 | Loamy sand | Amaranth, Sorghum | - Increased the microbial biomass, not detailed community structure changes | [108] |
| Maize + grass + whole plant silage | 33 mg P kg soil−1 | Loamy sand | Amaranth, Sorghum | - Increased the microbial biomass, not detailed community structure changes | [108] |
| Dairy cow manure | 50 kg NH4+ ha−1 | Andosol | No plant | - Negative effect on ammonia oxidizing archaea - Positive effect on ammonia oxidizing bacteria if lime was also applied | [171] |
| Restaurant and agri-food waste + livestock effluents + plant matter | 170 kg N ha−1 | Loam | Maize, w. wheat, sugarbeet, spr. barley | - Increase in the abundance of the prokaryotic Firmicutes and the fungal Mucoromycota | [172] |
| Agricultural and livestock waste | 5 N unit 2 L soil−1 | Clayey loam | No plant | - No substantial alterations in the soil microbial structure - Increased number of genera - Increased relative abundance of Myxococcota - New genera appeared and involved in nutrient cycling and C storage - Microbial diversity remained stable - Increased the complexity of microbial community and bacterial interactions | [173] |
| Pig slurry | 340 kg N ha−1 | Silty loam | No plant | - Increase in Gram-negative bacteria - Decrease in fungi to bacteria ratio - Decreasing trend in both bacterial and yeast and fungal richness | [174] |
| Food waste | 25 and 50 kg N ha−1 | Yellow sandy duplex | Annual ryegrass | - Increase in AM fungal hyphae density at 50 kg N ha−1 dose - Shifted the bacterial community composition - Digestate affected bacterial, C and N cycling genes community composition | [97] |
| Cattle manure + clover/grass; chicken manure + cow and pig manure + ensiled energy crops | 75 kg N ha−1 | Silty clay loam | No plant | - Growth of saprotrophic soil fungi after solid and composted solid digestate - No effect of liquid fraction on microbial activity | [175] |
| No data | 250–375 m3 ha−1 yr−1 | Sandy loam | Poplar | - Increased Shannon diversity and species richness of fungal communities, but not that of bacteria | [176] |
| Pig manure | 150 kg N ha−1 | Gleyic Solonchak, loam | Wheat, rice | - Increased the relative abundance of Gammaproteobacteria and Hyphomicrobiaceae - More complex bacterial community and decrease of the complexity of fungal network | [85] |
| Pig manure | 59.9–264.4 t ha−1 | Silt loam | Rice and rape | - Increased the bacterial diversity and richness at digestate dose 165.1 t ha−1 - Decreased relative abundance of Actinobacteria - Increased relative abundance of Nitrospirae - Increased bacterial diversity | [177] |
| Pig manure | 270–540 kg N ha−1 | Mollic Endoaquept | Rice | - Increased the relative abundance of Nitrospirae | [178] |
| Vegetable juice waste | 0.6 g N kg soil−1 | Latosols | No plant | - Decreased relative abundance of potential fungal pathogens (Fusarium, Cylindrocarpon, Alternaria, and Phoma) - Enriching in bacteria and increasing the phylogenetic relatedness of the bacterial community. | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragályi, P.; Szécsy, O.; Uzinger, N.; Magyar, M.; Szabó, A.; Rékási, M. Factors Influencing the Impact of Anaerobic Digestates on Soil Properties. Soil Syst. 2025, 9, 78. https://doi.org/10.3390/soilsystems9030078
Ragályi P, Szécsy O, Uzinger N, Magyar M, Szabó A, Rékási M. Factors Influencing the Impact of Anaerobic Digestates on Soil Properties. Soil Systems. 2025; 9(3):78. https://doi.org/10.3390/soilsystems9030078
Chicago/Turabian StyleRagályi, Péter, Orsolya Szécsy, Nikolett Uzinger, Marianna Magyar, Anita Szabó, and Márk Rékási. 2025. "Factors Influencing the Impact of Anaerobic Digestates on Soil Properties" Soil Systems 9, no. 3: 78. https://doi.org/10.3390/soilsystems9030078
APA StyleRagályi, P., Szécsy, O., Uzinger, N., Magyar, M., Szabó, A., & Rékási, M. (2025). Factors Influencing the Impact of Anaerobic Digestates on Soil Properties. Soil Systems, 9(3), 78. https://doi.org/10.3390/soilsystems9030078








