Soil Solution Viscosity Reduces CO2 Emissions in Tropical Soils: Implications for Climate Change Mitigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
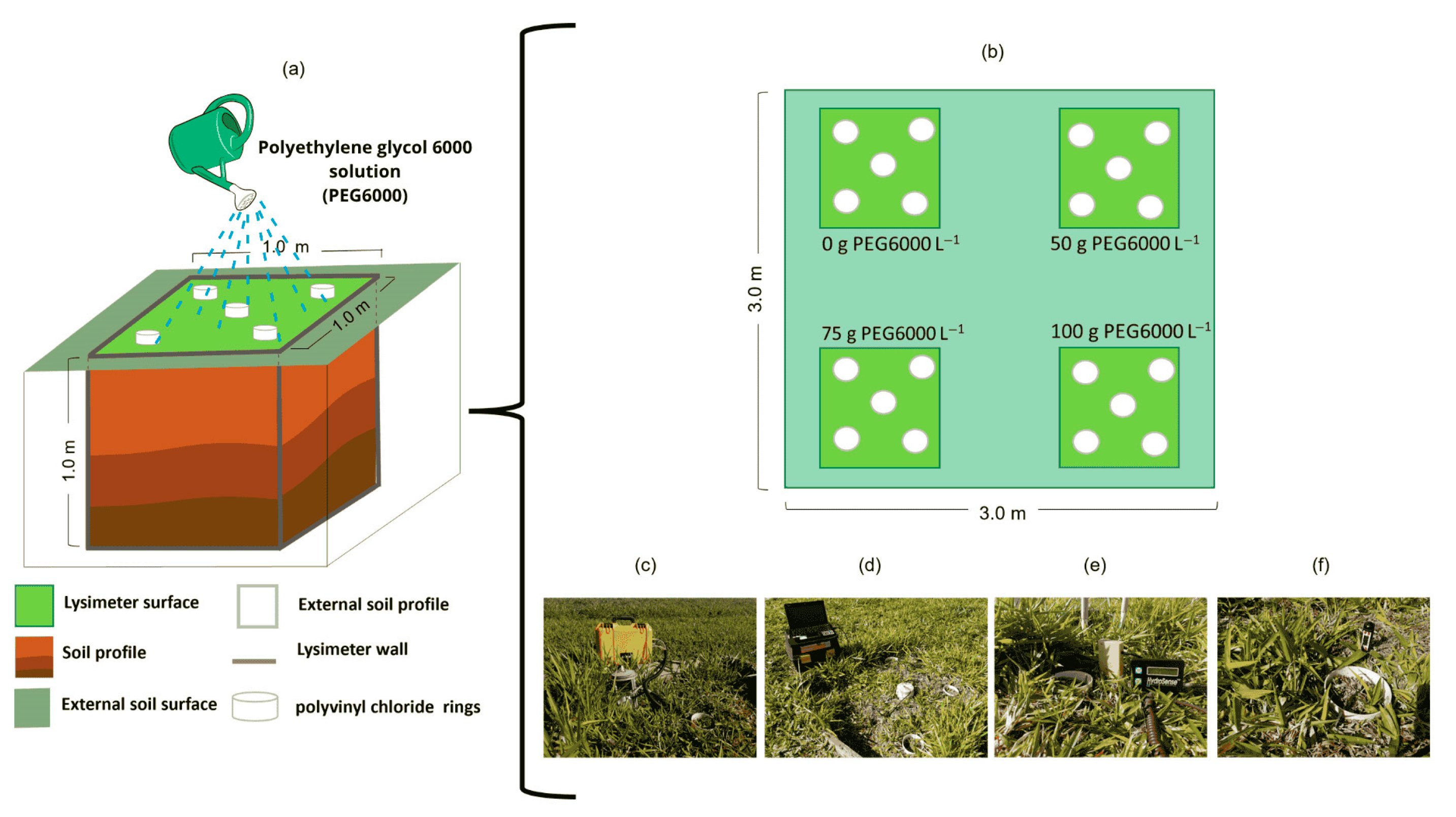
2.2. Experimental Design and Treatments
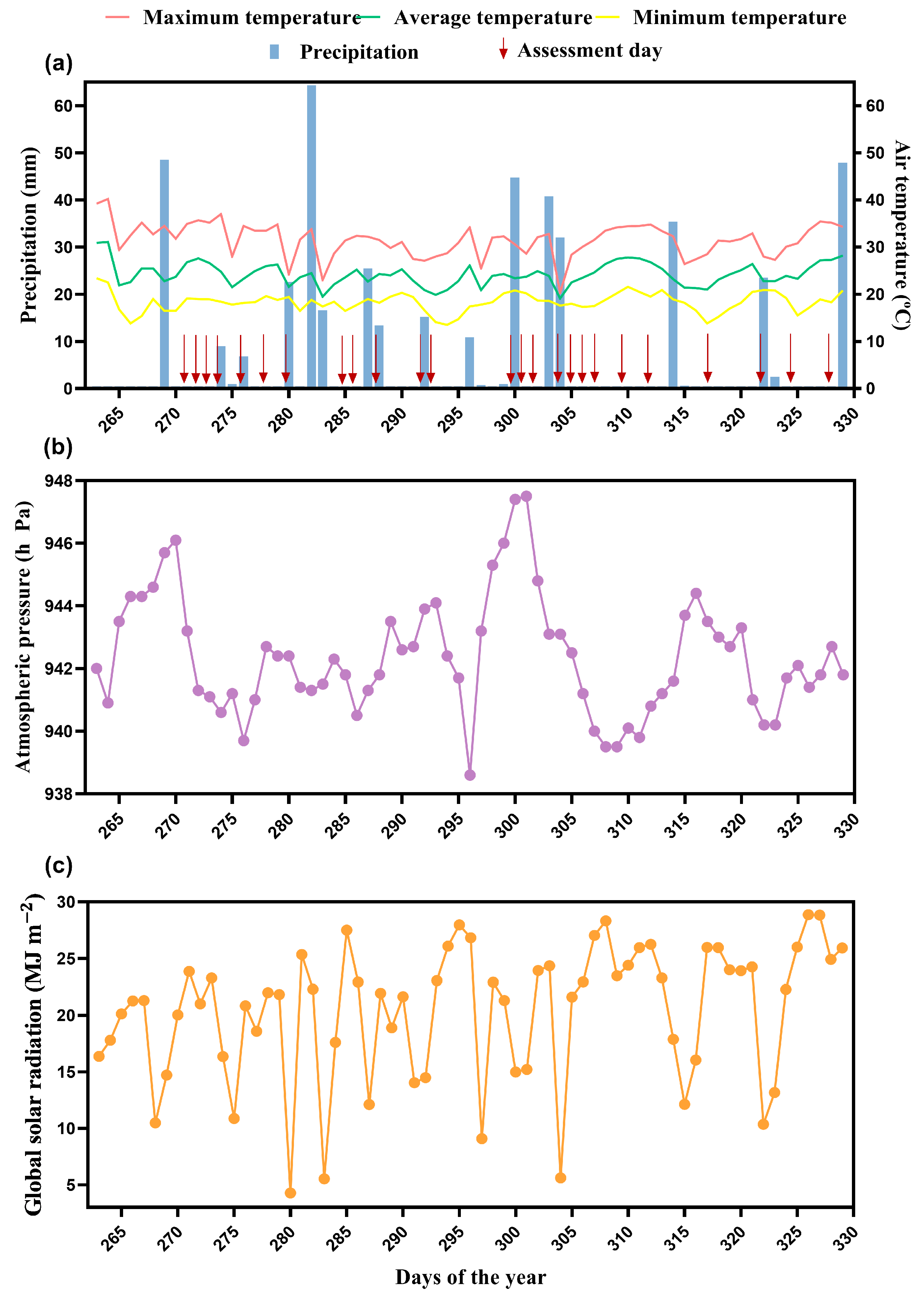
2.3. CO2 Emission, Soil Temperature, and Soil Water Content
2.4. O2 Capture
2.5. Data Processing and Analysis
3. Results
3.1. Viscosity
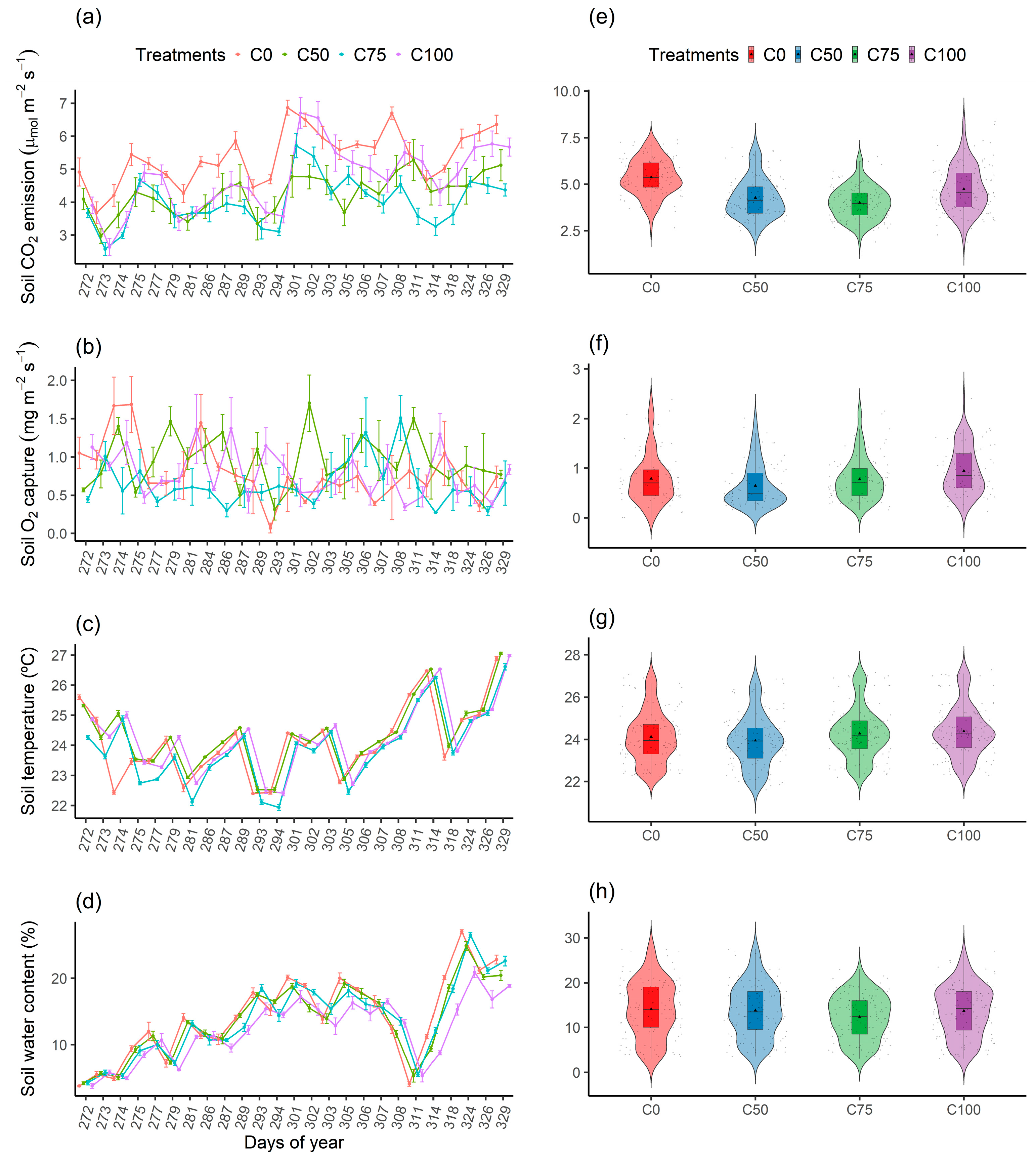
3.2. Temporal Variation of Soil CO2 Emission, Soil O2 Capture, Soil Temperature, and Soil Water Content
3.3. Quadratic Regression Analysis of Soil CO2 Emission, Soil O2 Capture, Soil Temperature, and Soil Water Content
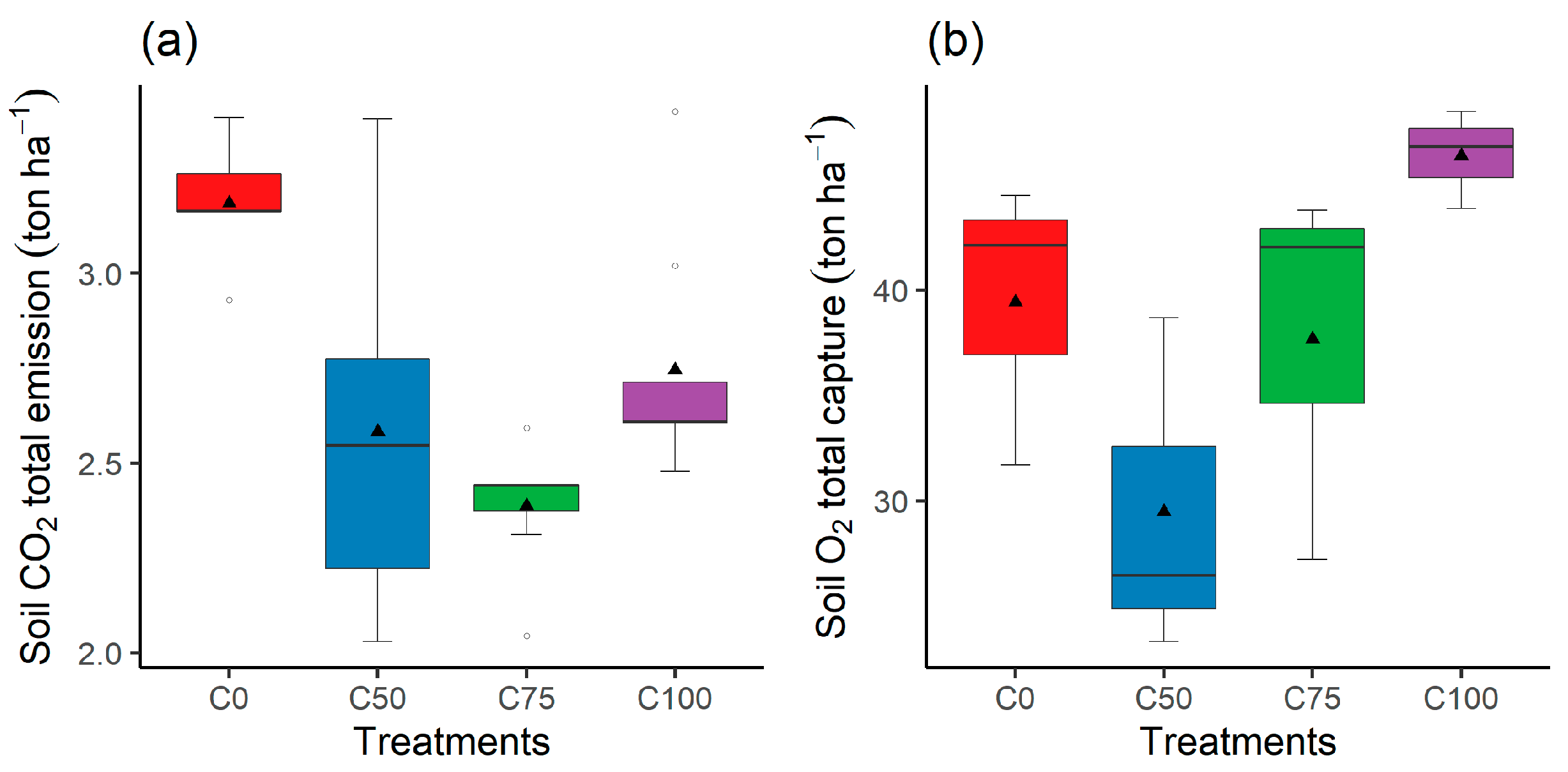
3.4. Quadratic Regression Analysis of Total Soil CO2 Emission and Total Soil O2 Capture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | carbon dioxide |
| CH4 | methane |
| N2O | nitrous oxide |
| AFOLU | Agriculture, forestry, and other land uses |
| RECSOIL | Recarbonization of Global Agricultural Soils |
| SOC | Soil organic carbon |
| OM | Organic matter |
| PEG6000 | polyethylene glycol 6000 |
| FCO2 | Soil CO2 emissions (µmol m−2 s−1) |
| FO2 | Soil O2 capture (mg m−2 s−1) |
| Ts | Soil temperature (°C) |
| SWC | Soil water content (%) |
| TFCO2 | Total soil CO2 emissions (tons ha−1) |
| TFO2 | Total soil O2 capture (tons ha−1) |
Appendix A
| N | Mean | SD | Median | IQR | Min | Max | MSE | CV% | Curtose | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 emission (µmol m−2 s−1) | C0 | 125 | 5.38 | 0.96 | 5.37 | 1.31 | 2.65 | 7.39 | ±0.08 | 17.88 | −0.24 |
| C50 | 125 | 4.26 | 1.07 | 4.14 | 1.42 | 2.24 | 7.19 | ±0.09 | 25.23 | 0.13 | |
| C75 | 125 | 4.00 | 0.87 | 3.95 | 1.14 | 1.90 | 6.47 | ±0.08 | 21.81 | 0.03 | |
| C100 | 125 | 4.72 | 1.24 | 4.51 | 1.87 | 1.90 | 8.38 | ±0.11 | 26.21 | −0.02 | |
| O2 capture (mg m−2 s−1) | C0 | 75 | 0.79 | 0.51 | 0.70 | 0.51 | 0.003 | 2.38 | ±0.06 | 64.30 | 1.38 |
| C50 | 75 | 0.65 | 0.43 | 0.49 | 0.57 | 0.15 | 2.01 | ±0.06 | 66.92 | 0.91 | |
| C75 | 75 | 0.77 | 0.43 | 0.70 | 0.56 | 0.16 | 2.15 | ±0.05 | 55.99 | 1.18 | |
| C100 | 75 | 0.95 | 0.48 | 0.86 | 0.71 | 0.13 | 2.40 | ±0.05 | 50.79 | −0.31 | |
| Ts (°C) | C0 | 125 | 24.10 | 1.20 | 24.0 | 1.40 | 22.20 | 27.00 | ±0.11 | 4.98 | −0.16 |
| C50 | 125 | 23.90 | 1.20 | 23.9 | 1.40 | 21.60 | 26.80 | ±0.11 | 5.02 | −0.03 | |
| C75 | 125 | 24.20 | 1.13 | 24.2 | 1.30 | 22.20 | 27.20 | ±0.10 | 4.67 | 0.31 | |
| C100 | 125 | 24.30 | 1.11 | 24.2 | 1.30 | 22.30 | 27.30 | ±0.10 | 4.57 | 0.44 | |
| SWC (v/v) (%) | C0 | 125 | 14.10 | 6.23 | 14.0 | 9.00 | 3.00 | 27.50 | ±0.56 | 44.18 | −0.79 |
| C50 | 125 | 13.70 | 5.84 | 13.5 | 8.50 | 3.50 | 27.50 | ±0.52 | 42.63 | −0.57 | |
| C75 | 125 | 12.20 | 4.84 | 12.5 | 7.50 | 3.00 | 23.00 | ±0.43 | 39.67 | −0.89 | |
| C100 | 125 | 13.60 | 5.63 | 14.0 | 8.50 | 3.00 | 27.00 | ±0.50 | 41.39 | −0.77 |
References
- Canadell, J.G.; Monteiro, P.M.S. Global Carbon and Other Biogeochemical Cycles and Feedbacks. 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter05.pdf (accessed on 30 March 2025).
- Nabuurs, G.-J.; Mrabet, R. Agriculture, Forestry and Other Land Uses (AFOLU). In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Lan, X.; Tans, P.; Thoning, K.W. Trends in Globally-Averaged CO2 Determined From NOAA Global Monitoring Laboratory Measurements. Version Monday, 14 July 2025. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Estimativas Anuais de Emissões de Gases de Efeito Estufa No Brasil. Brasília. 2022. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/sirene/publicacoes/estimativas-anuais-de-emissoes-gee/arquivos/6a-ed-estimativas-anuais.pdf (accessed on 30 March 2025).
- FAO. Recarbonization of Global Soils. Rome. 2019. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9e167594-3d97-4486-b8c9-ed875985dc98/content (accessed on 31 March 2025).
- Brazil’s NDC National Determination to Contribute and Transform. Available online: https://unfccc.int/sites/default/files/2024-11/Brazil_Second%20Nationally%20Determined%20Contribution%20%28NDC%29_November2024.pdf (accessed on 2 August 2025).
- Tóth, E.; Magyar, M.; Cseresnyés, I.; Dencső, M.; Laborczi, A.; Szatmári, G.; Koós, S. Climate-Smart Agricultural Practices—Strategies to Conserve and Increase Soil Carbon in Hungary. Land 2025, 14, 1206. [Google Scholar] [CrossRef]
- Rodríguez, B.C.; Durán-Zuazo, V.H.; Rodríguez, M.S.; García-Tejero, I.F.; Ruiz, B.G.; Tavira, S.C. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Lal, C.R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Yin, L.; Ma, L.; Xu, C.; Chen, H.; Ma, T.; Su, Y.; Zhu, Y.; He, L.; et al. Optimal soil water content and temperature sensitivity differ among heterotrophic and autotrophic respiration from oasis agroecosystems. Geoderma 2022, 425, 116071. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Negassa, W.C.; Guber, A.K.; Rivers, M.L. Protection of soil carbon within macro-aggregates depends on intra-aggregate pore characteristics. Sci. Rep. 2015, 5, 16261. [Google Scholar] [CrossRef]
- Yang, P.; van Elsas, J.D. Mechanisms and ecological implications of the movement of bacteria in soil. Appl. Soil Ecol. 2018, 129, 112–120. [Google Scholar] [CrossRef]
- Vargas-Terminel, M.L.; Flores-Rentería, D.; Sánchez-Mejía, Z.M.; Rojas-Robles, N.E.; Sandoval-Aguilar, M.; Chávez-Vergara, B.; Robles-Morua, A.; Garatuza-Payan, J.; Yépez, E.A. Soil Respiration Is Influenced by Seasonality, Forest Succession and Contrasting Biophysical Controls in a Tropical Dry Forest in Northwestern Mexico. Soil Syst. 2022, 6, 75. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Ferraudo, A.S.; Panosso, A.R.; Bicalho, E.d.S.; Teixeira, D.D.B.; Barbosa, M.d.A.; Tsai, S.M.; Borges, B.M.F.; Cannavan, F.d.S.; de Souza, J.A.M.; et al. Effects of burned and unburned sugarcane harvesting systems on soil CO2 emission and soil physical, chemical, and microbiological attributes. CATENA 2021, 196, 104903. [Google Scholar] [CrossRef]
- Teodoro, P.E.; Rossi, F.S.; Teodoro, L.P.R.; Santana, D.C.; Ratke, R.F.; de Oliveira, I.C.; Della Silva, J.L.; de Oliveira, J.L.G.; da Silva, N.P.; Baio, F.H.R.; et al. Soil CO2 emissions under different land-use managements in Mato Grosso do Sul, Brazil. J. Clean. Prod. 2024, 434, 139983. [Google Scholar] [CrossRef]
- Holthusen, D.; Haas, C.; Peth, S.; Horn, R. Are standard values the best choice? A critical statement on rheological soil fluid properties viscosity and surface tension. Soil Tillage Res. 2012, 125, 61–71. [Google Scholar] [CrossRef]
- Schneider, W.R.; Doetsch, R.N. Effect of Viscosity on Bacterial Motility. J. Bacteriol. 1974, 117, 696–701. [Google Scholar] [CrossRef]
- Bolton, T.F.; Havenhand, J.N. Physiological versus viscosity-induced effects of water temperature on the swimming and sinking velocity of larvae of the serpulid polychaete Galeolaria caespitosa. Mar. Ecol. Prog. Ser. 1997, 159, 209–218. [Google Scholar] [CrossRef]
- Larsen, P.S.; Madsen, C.V.; Riisgård, H.U. Effect of temperature and viscosity on swimming velocity of the copepod Acartia tonsa, brine shrimp Artemia salina and rotifer Brachionus plicatilis. Aquat. Biol. 2008, 4, 47–54. [Google Scholar] [CrossRef][Green Version]
- Sohn, M.H.; Lim, S.; Seo, K.W.; Lee, S.J. Effect of ambient medium viscosity on the motility and flagella motion of Prorocentrum minimum (Dinophyceae). J. Plankton Res. 2013, 35, 1294–1304. [Google Scholar] [CrossRef]
- Mirbagheri, S.A.; Fu, H.C. Helicobacter pylori Couples Motility and Diffusion to Actively Create a Heterogeneous Complex Medium in Gastric Mucus. Phys. Rev. Lett. 2016, 116, 198101. [Google Scholar] [CrossRef]
- Joiner, K.L.; Baljon, A.; Barr, J.; Rohwer, F.; Luque, A. Impact of bacteria motility in the encounter rates with bacteriophage in mucus. Sci. Rep. 2019, 9, 16427. [Google Scholar] [CrossRef] [PubMed]
- La Scala, N., Jr.; Martinez, A.S.; Spokas, K.A.; Gonçalves, D.R.P.; Etto, R.M. Should alterations in water viscosity be addressed in soil carbon models? arXiv 2022. [Google Scholar] [CrossRef]
- Adhikari, R.; Mingtarja, H.; Freischmidt, G.; Bristow, K.L.; Casey, P.S.; Johnston, P.; Sangwan, P. Effect of viscosity modifiers on soil wicking and physico-mechanical properties of a polyurethane based sprayable biodegradable polymer membrane. Agric. Water Manag. 2019, 222, 346–353. [Google Scholar] [CrossRef]
- Castanho, G.M.; Regitano, J.B.; Tornisielo, V.L.; Abdalla, A.L. Sorption and mobility of polyethylene glycol (PEG 4000) in tropical soils. Toxicol. Environ. Chem. 2009, 91, 1263–1271. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Asayesh, Z.M.; Driver, J.; Vahdati, K. Stomatal features and desiccation responses of Persian walnut leaf as caused by in vitro stimuli aimed at stomatal closure. Trees Struct. Funct. 2020, 34, 1219–1232. [Google Scholar] [CrossRef]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the pore size of cell walls of living plant cells. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Rolim, G.d.S.; de Camargo, M.B.P.; Lania, D.G.; de Moraes, J.F.L. Climatic classification of Köppen and Thornthwaite sistems and their applicability in the determination of agroclimatic zonning for the state of São Paulo, Brazil. Bragantia 2007, 66, 711–720. [Google Scholar] [CrossRef]
- Santos, G.A.d.A.; Moitinho, M.R.; Silva, B.d.O.; Xavier, C.V.; Teixeira, D.D.B.; Corá, J.E.; Júnior, N.L.S. Effects of long-term no-tillage systems with different succession cropping strategies on the variation of soil CO2 emission. Sci. Total. Environ. 2019, 686, 413–424. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Available online: https://wrb.isric.org/files/WRB_fourth_edition_2022-12-18_errata_correction_2024-09-24.pdf (accessed on 30 July 2025).
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Pinotti, C.R.; Hirai, W.Y.; de Moraes, M.L.T.; Montanari, R.; Filho, M.C.M.T.; Milori, D.M.B.P.; Júnior, N.L.S.; Panosso, A.R. CO2 emission and its relation to soil temperature, moisture, and O2 absorption in the reforested areas of Cerrado biome, Central Brazil. Plant Soil 2019, 444, 193–211. [Google Scholar] [CrossRef]
- De Lucena, W.B.; Vicentini, M.E.; Santos, G.A.D.A.; Silva, B.D.O.; Da Costa, D.V.M.; Canteral, K.F.F.; Román, J.A.N.; Rolim, G.D.S.; Panosso, A.R.; La Scala, N., Jr. Temporal variability of the CO2 emission and the O2 influx in a tropical soil in contrasting coverage conditions. J. S. Am. Earth Sci. 2023, 121, 104120. [Google Scholar] [CrossRef]
- Jassal, R.S.; Black, T.A.; Nesic, Z.; Gaumont-Guay, D. Using automated non-steady-state chamber systems for making continuous long-term measurements of soil CO2 efflux in forest ecosystems. Agric. For. Meteorol. 2012, 161, 57–65. [Google Scholar] [CrossRef]
- Giacomo, G.; Angelo, F.; Fabio, B.; Stefano, B.; Riccardo, M. Measurements of soil carbon dioxide emissions from two maize agroecosystems at harvest under different tillage conditions. Sci. World J. 2014, 2014, 141345. [Google Scholar] [CrossRef]
- Smagin, A.V.; Dolgikhb, A.V.; Karelin, D.V. Experimental studies and physically substantiated model of carbon dioxide emission from the exposed cultural layer of Velikii Novgorod. Eurasian J. Soil Sci. 2016, 49, 450–456. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Teixeira, D.d.B.; Montanari, R.; Bolonhezi, A.C.; Teixeira, E.B.; Moitinho, M.R.; Panosso, A.R.; Spokas, K.A.; La Scala, N., Jr. Ratio of CO2 and O2 as index for categorising soil biological activity in sugarcane areas under contrasting straw management regimes. Soil Res. 2018, 56, 373–381. [Google Scholar] [CrossRef]
- Whittaker, E.T.; Robinson, G. The trapezoidal and parabolic rules. In The Calculus of Observations: A Treatise on Numerical Mathematics, 4th ed.; Blackie and Son limited Ltd.: London, UK; Glasgow, UK, 1944; pp. 156–158. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 April 2025).
- Faimon, J.; Lang, M. What actually controls the minute to hour changes in soil carbon dioxide concentrations? Geoderma 2018, 323, 52–64. [Google Scholar] [CrossRef]
- Yan, Z.; Bond-Lamberty, B.; Todd-Brown, K.E.; Bailey, V.L.; Li, S.; Liu, C.; Liu, C. A moisture function of soil heterotrophic respiration that incorporates microscale processes. Nat. Commun. 2018, 9, 2562. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, R.; Verma, P.; Raghubanshi, A.S. Spatio-temporal variability in soil CO2 efflux and regulatory physicochemical parameters from the tropical urban natural and anthropogenic land use classes. J. Environ. Manag. 2021, 295, 113141. [Google Scholar] [CrossRef]
- Xavier, C.V.; Moitinho, M.R.; Teixeira, D.D.B.; Santos, G.A.d.A.; Corá, J.E.; La Scala, N., Jr. Crop rotation and sequence effects on temporal variation of CO2 emissions after long-term no-till application. Sci. Total. Environ. 2020, 709, 136107. [Google Scholar] [CrossRef]
- Verslues, P.E.; Ober, E.S.; Sharp, R.E. Root Growth and Oxygen Relations at Low Water Potentials. Impact of Oxygen Availability in Polyethylene Glycol Solutions. Plant Physiol. 1998, 116, 1403–1412. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Salazar, A.; Sulman, B.N.; Dukes, J.S. Microbial dormancy promotes microbial biomass and respiration across pulses of drying-wetting stress. Soil Biol. Biochem. 2018, 116, 237–244. [Google Scholar] [CrossRef]
- Tavares, D.S.; Fernandes, T.E.K.; Rita, Y.L.; Rocha, D.C.; Sant’Anna-Santos, B.F.; Gomes, M.P. Germinative metabolism and seedling growth of cowpea (Vigna unguiculata) under salt and osmotic stress. S. Afr. J. Bot. 2021, 139, 399–408. [Google Scholar] [CrossRef]

| Variable | Variable Quadratic Regression = b0 + b1 C + b2 C2 | RMSE | Minimum | ||||
|---|---|---|---|---|---|---|---|
| b0 | b1 | b2 | R2 | X | Y | ||
| FCO2 (1) | 5.3958 ± 0.2217 (p = 0.026) | −0.0433 ± 0.0101 (p = 0.14) | 0.0004 ± 0.0001 (p = 0.17) | 0.95 | 0.2681 | 54.13 | 4.22 |
| FO2 (2) | 0.7882 ± 0.0190 (p = 0.015) | −0.0067 ± 0.0008 (p = 0.08) | 0.0001 ± 0.000008 (p = 0.06) | 0.99 | 0.0296 | 33.50 | 0.68 |
| Ts (3) | 24.0873 ± 0.023 (p = 0.003) | −0.0069 ± 0.0061 (p = 0.46) | 0.0001 ± 0.0001 (p = 0.36) | 0.79 | 0.0709 | 38.33 | 23.96 |
| SWC (4) | 14.2027 ± 1.0725 (p = 0.05) | −0.0356 ± 0.0491 (p = 0.60) | 0.0003 ± 0.0005 (p = 0.68) | 0.44 | 0.5746 | 59.50 | 13.14 |
| TFCO2 (5) | 3.199 ± 0.1562 (p = 0.0311) | −0.0256 ± 0.0072 (p = 0.1733) | 0.0002 ± 0.0001 (p = 0.20) | 0.93 | 0.1145 | 64.00 | 2.38 |
| TFO2 (6) | 39.225 ± 2.3491 (p = 0.0381) | −0.4133 ± 0.1075 (p = 0.1620) | 0.0049 ± 0.0011 (p = 0.14) | 0.96 | 1.1836 | 42.17 | 30.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Nicolella, A.I.; Canteral, K.F.F.; De Lucena, W.B.; Vicentini, M.E.; Panosso, A.R.; Spokas, K.; de Souza Rolim, G.; da Silva, T.R.G.; La Scala, N., Jr. Soil Solution Viscosity Reduces CO2 Emissions in Tropical Soils: Implications for Climate Change Mitigation. Soil Syst. 2025, 9, 101. https://doi.org/10.3390/soilsystems9030101
Santos-Nicolella AI, Canteral KFF, De Lucena WB, Vicentini ME, Panosso AR, Spokas K, de Souza Rolim G, da Silva TRG, La Scala N Jr. Soil Solution Viscosity Reduces CO2 Emissions in Tropical Soils: Implications for Climate Change Mitigation. Soil Systems. 2025; 9(3):101. https://doi.org/10.3390/soilsystems9030101
Chicago/Turabian StyleSantos-Nicolella, Arianis Ibeth, Kleve Freddy Ferreira Canteral, Wanderson Benerval De Lucena, Maria Elisa Vicentini, Alan Rodrigo Panosso, Kurt Spokas, Glauco de Souza Rolim, Thaís Rayane Gomes da Silva, and Newton La Scala, Jr. 2025. "Soil Solution Viscosity Reduces CO2 Emissions in Tropical Soils: Implications for Climate Change Mitigation" Soil Systems 9, no. 3: 101. https://doi.org/10.3390/soilsystems9030101
APA StyleSantos-Nicolella, A. I., Canteral, K. F. F., De Lucena, W. B., Vicentini, M. E., Panosso, A. R., Spokas, K., de Souza Rolim, G., da Silva, T. R. G., & La Scala, N., Jr. (2025). Soil Solution Viscosity Reduces CO2 Emissions in Tropical Soils: Implications for Climate Change Mitigation. Soil Systems, 9(3), 101. https://doi.org/10.3390/soilsystems9030101









