Impact of Land Use Types on Soil Physico-Chemical Properties, Microbial Communities, and Their Fungistatic Effects
Abstract
1. Introduction
- Pathogenic fungi are more sensitive to fungistasis than beneficial ones;
- Fungistasis is greater in soils with a higher content of organic substance and subject to greater organic inputs;
- Fungistasis is greater in soils with higher microbial biomass, activity, and diversity.
2. Materials and Methods
2.1. Study Sites and Soil Collection
2.2. Soil Physical and Chemical Analyses
2.3. Microbiological Analyses
2.4. Assessing Soil Fungistasis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical and Physical Properties
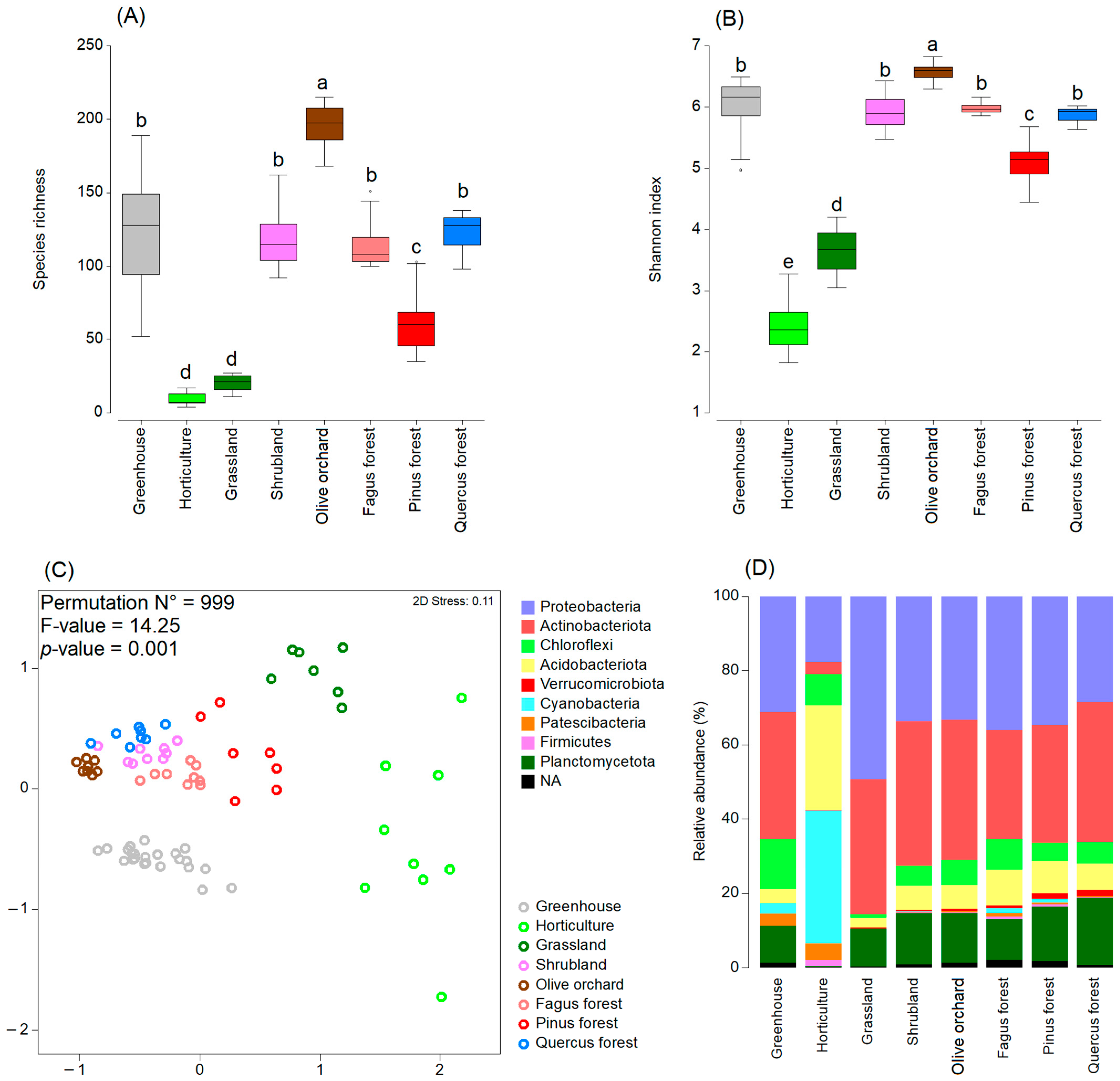
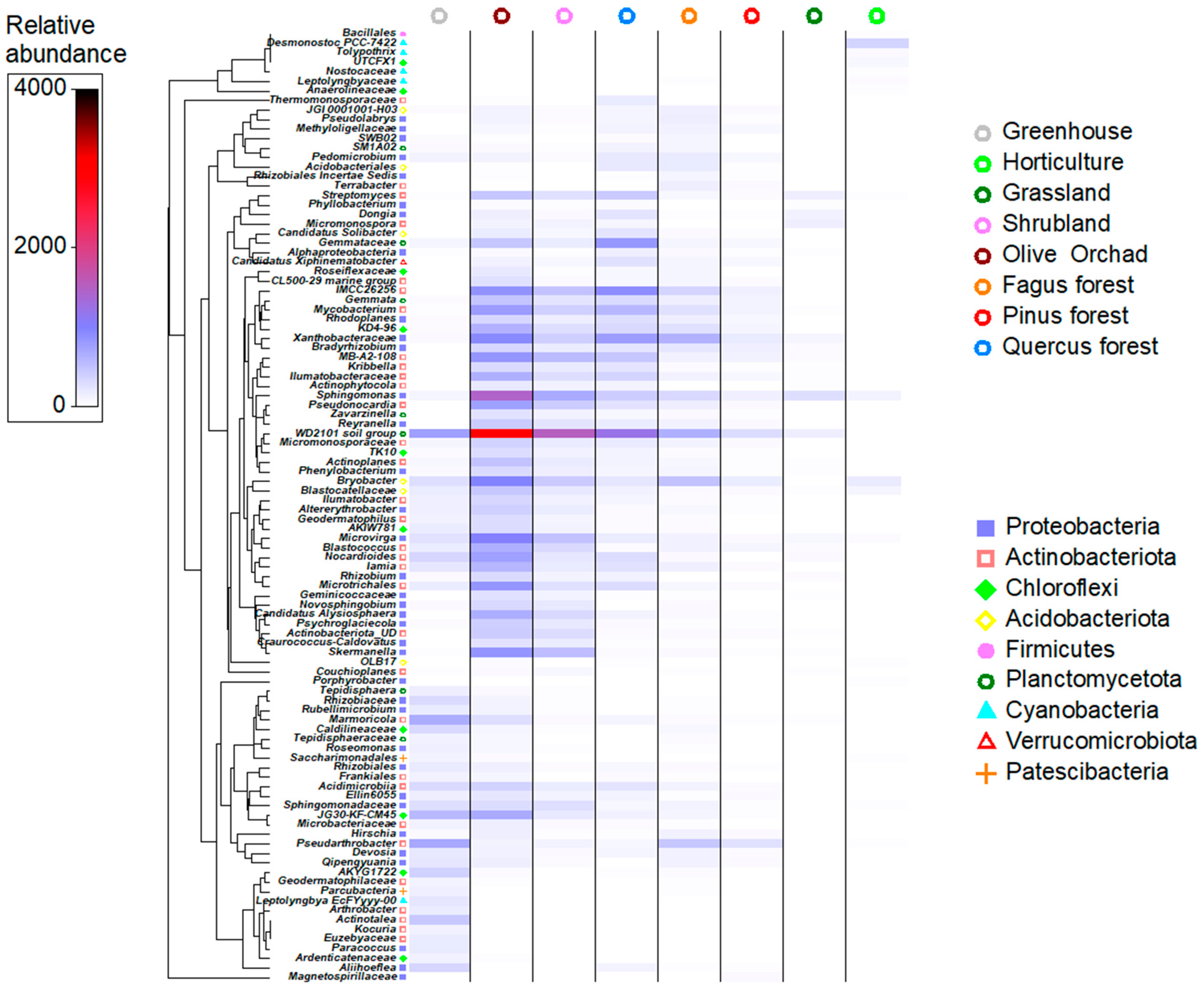
3.2. Soil Microbiome
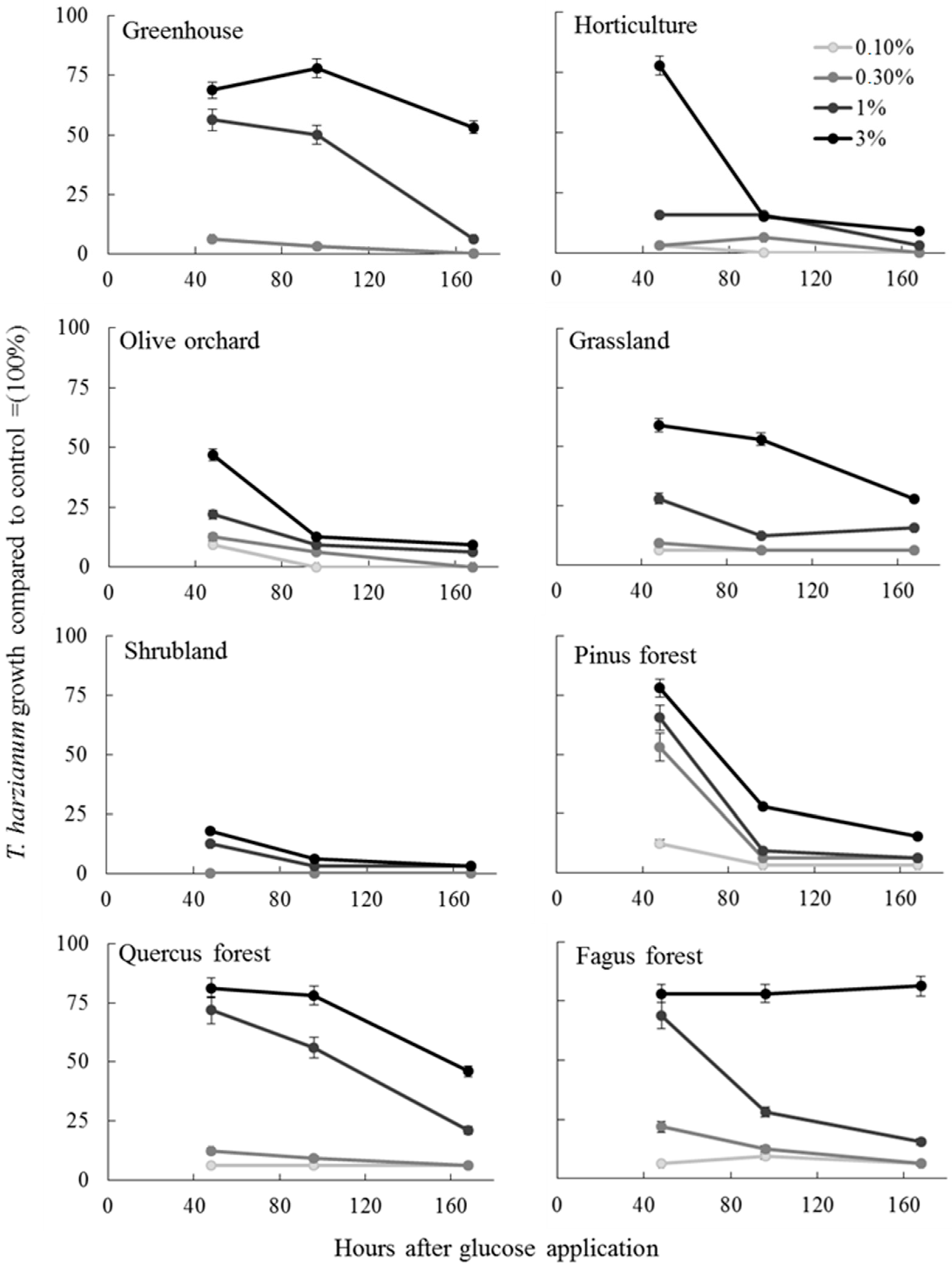
3.3. Soil Fungistasis
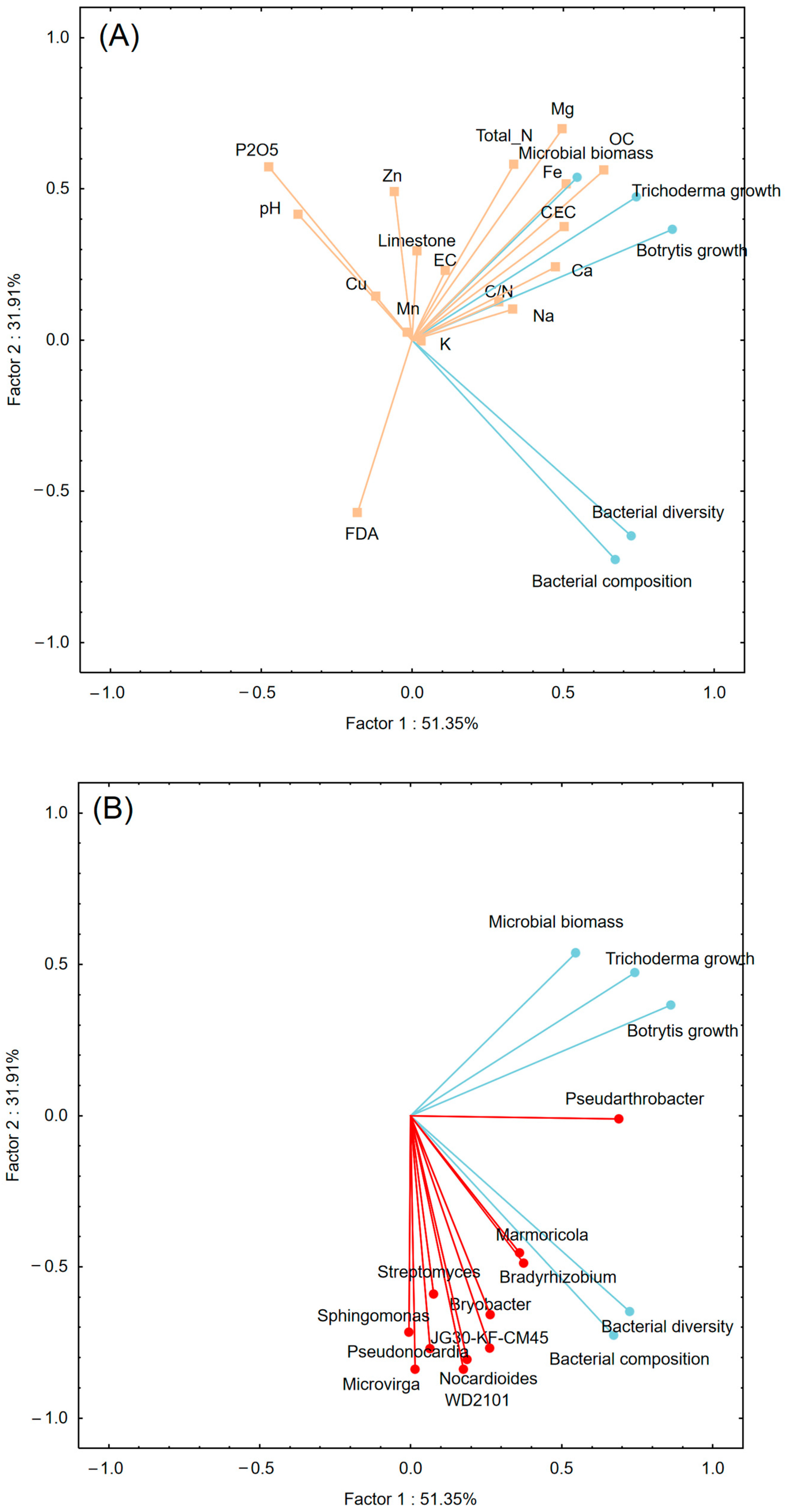
3.4. Linking Soil Fungistasis to Chemical and Microbiological Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, A.G.; Ford, E.J. Soil fungistasis—A reappraisal. Annu. Rev. Phytopathol. 1972, 10, 327–346. [Google Scholar] [CrossRef]
- Lockwood, J.L. Soil Fungistasis. Annu. Rev. Phytopathol. 1964, 2, 341–362. [Google Scholar] [CrossRef]
- Legrand, F.; Chen, W.; Cobo-Díaz, J.F.; Picot, A.; Floch, G.L. Co-occurrence analysis reveal that biotic and abiotic factors influence soil fungistasis against Fusarium graminearum. FEMS Microbiol. Ecol. 2019, 95, fiz056. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Silby, M.W.; Raaijmakers, J.M.; Levy, S.B.; Boer, W.D. Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competitors. ISMEJ 2011, 5, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Gaglione, S.A.; Incerti, G.; Zoina, A. Biochemical quality of organic amendments affects soil fungistasis. Appl. Soil Ecol. 2013, 72, 135–142. [Google Scholar] [CrossRef]
- Romine, M.; Baker, R. Soil fungistasis: Evidence for an inhibitory factor. Phytopathology 1973, 63, 756–759. [Google Scholar] [CrossRef]
- Alabouvette, C. Fusarium wilt suppressive soils: An example of disease-suppressive soils. Australas. Plant Pathol. 1999, 28, 57–64. [Google Scholar] [CrossRef]
- Ellis, R.J.; Timms-Wilson, T.M.; Bailey, M.J. Identification of conserved traits in fluorescent pseudomonads with antifungal activity. Environ. Microbiol. 2000, 2, 274–284. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M. Microbiota management for effective disease suppression: A systematic comparison between soil and mammals gut. Sustainability 2021, 13, 7608. [Google Scholar] [CrossRef]
- de Boer, W.; Verheggen, P.; Klein Gunnewiek, P.J.A.; Kowalchuk, G.A.; van Veen, J.A. Microbial community composition affects soil fungistasis. Appl. Environ. Microbiol. 2003, 69, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18, GB4002. [Google Scholar] [CrossRef]
- de Boer, W.; Li, X.G.; Meisner, A.; Garbeva, P. Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol. Ecol. 2019, 95, fiz105. [Google Scholar] [CrossRef] [PubMed]
- Liebman, J.A.; Epstein, L. Partial characterization of volatile fungistatic compound (s) from soil. Phytopathology 1994, 84, 442–446. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The suppressive effects of biochar on above-and belowground plant pathogens and pests: A review. Plants 2022, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Bloem, J.; Hopkins, D.W.; Benedetti, A. Microbiological Methods for Assessing Soil Quality; CABI: Wallingford, UK, 2005. [Google Scholar]
- Sparks, D.; Page, A.; Helmke, P.; Loeppert, R. Methods of Soil Analysis. Part 3; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis. Part 3; SSSA Book Ser. 5; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Ministero Politiche Agricole e Forestali (Italy). Metodi Ufficiali di Analisi Chimica del Suolo. Decreto Ministeriale del 13/09/1999; Gazzetta Ufficiale della Repubblica Italiana, n. 248, 21/10/1999 Supplemento Ordinario n. 185. Available online: http://www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf (accessed on 12 February 2018). (In Italian).
- Workneh, F.; Van Bruggen, A.H.C.; Drinkwater, L.E.; Shennan, C. Variables associated with corky root and Phytophthora root rot of tomatoes in organic and conventional farms. Phytopathology 1993, 83, 581–589. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; De Caro, C.; Coretti, L.; Chiariotti, L.; Gilbert, J.A.; et al. Specific Signatures of the Gut Microbiota and Increased Levels of Butyrate in Children Treated With Fermented Cow’s Milk Containing Heat-Killed Lactobacillus Paracasei Cba L74. Appl. Environ. Microbiol. 2017, 83, e01206-17. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.B.; Papavizas, G.C. Survival of root-infecting fungi in soil. X. Sensitivity of propagules of Thielaviopsis basicola to soil fungistasis in natural. Phytopathology 1969, 59, 135–138. [Google Scholar]
- Steiner, G.W.; Lockwood, J.L. Soil fungistasis: Mechanism in sterilized, reinoculated soil. Phytopathology 1970, 60, 89–91. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Kalkhove, S.I.; Lugones, L.G.; Wösten, H.A.; Bakker, P.A. Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environ. Microbiol. Rep. 2012, 4, 227–233. [Google Scholar] [CrossRef]
- Bonanomi, G.; Gaglione, S.A.; Cesarano, G.; Sarker, T.C.; Pascale, M.; Scala, F.; Zoina, A. Frequent applications of organic matter to agricultural soil increase fungistasis. Pedosphere 2017, 27, 86–95. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Gokul, A.; Mabaso, J.; Henema, N.; Otomo, L.; Bakare, O.O.; Klein, A.; Daniel, A.I.; Omolola, A.; Niekerk, L.A.; Nkomo, M.; et al. Sustainable agriculture through the enhancement of microbial biocontrol agents: Current challenges and new perspectives. Appl. Sci. 2023, 13, 6507. [Google Scholar] [CrossRef]
- Bharti, L.; Yadav, K.; Kumar Chaubey, A. Trichoderma spp.: Approach for bio-control agent. In Challenges in Plant Disease Detection and Recent Advancements; IntechOpen Limited: London, UK, 2024. [Google Scholar]
- Zandyavari, N.; Sulaiman, M.A.; Hassanzadeh, N. Molecular characterization and biocontrol potential of Trichoderma spp. against Fusarium oxysporum f. sp. dianthi in carnation. Egypt. J. Biol. Pest Control. 2024, 34, 1. [Google Scholar] [CrossRef]
- Sipilä, T.P.; Yrjälä, K.; Alakukku, L.; Palojärvi, A. Cross-site soil microbial communities under tillage regimes: Fungistasis and microbial biomarkers. Appl. Environ. Microbiol. 2012, 78, 8191–8201. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.J. Impact of tree litter decomposition on soil biochemical properties obtained from a temperate secondary forest in Northeast China. J. Soils Sediments 2015, 15, 13–23. [Google Scholar] [CrossRef]
- Yoder, D.L.; Lockwood, J.L. Fungal spore germination on natural and sterile soil. Microbiology 1973, 74, 107–117. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; Lyu, A.; Zhang, J.; Wu, M.; Yang, L.; Chen, W.; Li, G. Competitive saprophytic ability of the hypovirulent isolate QT5-19 of Botrytis cinerea and its importance in biocontrol of necrotrophic fungal pathogens. Biol. Control. 2020, 142, 104182. [Google Scholar] [CrossRef]
- Majou, D. Effects of carbon dioxide on germination of Clostridium botulinum spores. Int. J. Food Microbiol. 2024, 427, 110958. [Google Scholar] [CrossRef] [PubMed]
- Lijiahong, G.; Yalun, F.; Xiaohua, P.; Zhengkun, Y.; Mengke, Z.; Zhiyu, S.; Ning, G.; Shuangchen, C.; Junliang, C.; Bing, B.; et al. Biocontrol potential of Trichoderma harzianum against Botrytis cinerea in tomato plants. Biol. Control 2022, 174, 105019. [Google Scholar]
- Weinmann, M.; Bradáčová, K.; Nikolic, M. Relationship between mineral nutrition, plant diseases, and pests. In Marschner’s Mineral Nutrition of Higher Plants, 4th ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2023; Chapter 10; pp. 445–476. [Google Scholar]


| Ecosystem | Coordinates | Altitude (m) | Mean Annual Temperature (°C) | Mean Annual Rainfall (mm) | Pesticides Application | Organic Inputs and Fertilizers Application | Tillage Regime | Cover Vegetation |
|---|---|---|---|---|---|---|---|---|
| Greenhouse | 40°26′53.59″ N 14°59′20.44″ E | 8 | 19.6 | - | 8 to 16 times application per year of copper, synthetic fungicides and insecticides. Soil fumigation with Metham sodium | Crop residues (1–2 t ha−1 year−1), pelletized manure 0.5 t ha−1 year−1 180–250 kg ha−1 year−1 of NPK | Soil milling 4–8 times a year | Lettuce (Lactuca sativa), rocket (Eruca sativa) |
| Open-field horticulture | 40°26′49.45″ N 14°59′50.70″ E | 10 | 1050 | 8 to 16 times application per year of copper, synthetic fungicide and insecticides. Soil fumigation with Metham sodium | Crop residues (1–2 t ha−1 year−1), pelletized manure 0.5 t ha−1 year−1 180–250 kg ha−1 year−1 of NPK | Soil milling 6–8 times a year | Lettuce (Lactuca sativa), tomato (Solanum lycopersicon), zucchini (Cucurbita pepo), cabbage (Brassica oleracea) | |
| Grassland | 40°19′37.81″ N 15°07′22.52″ E | 170 | 14.9 | 1328 | - | Mowed grasses (2–3 t ha−1 year−1). No mineral fertilizers | Mowed twice a year | Perennial bunchgrass dominated by Ampelodes mauritanics |
| Shrubland | 40°01′33.37″ N 15°16′11.37″ E | 105 | 17.6 | 789 | - | Natural, mixed litterfall (1 t ha−1 year−1). No mineral fertilizers | - | Woody shrubs: Euphorbia dendroides, Juniperus phoenicea, Myrtus communis, Pistacia lentiscus, Rosmarinus officinalis, |
| Olive orchard | 40°19′42.67″ N 15° 7′25.51″ E | 198 | 14.8 | 1328 | Two applications per year of copper | Mowed mixed grasses (1–2 t ha−1 year−1). 40–60 kg ha−1 year−1 of NPK | Mowed twice a year, | Olea europea |
| Pinus forest | 40°25′12.25″ N 14°59′15.43″ E | 17 | 17.6 | 987 | - | Natural, mixed litterfall (2.5 t ha−1 year−1). No mineral fertilizers | - | Pinus pinea |
| Quercus forest | 40°24′24.40″ N 15°05′59.16″E | 285 | 14.3 | 929 | - | Natural, mixed litterfall (2 t ha−1 year−1). No mineral fertilizers | - | Dominated by Quercus ilex and Quercus pubescens |
| Fagus forest | 40°24′25.19″ N 15°09′24.20″ E | 1210 | 10.6 | 1480 | - | Natural, mixed litterfall (3 t ha−1 year−1). No mineral fertilizers | - | Dominated by Fagus sylvatica |
| Parameter | Greenhouse | Open-Field Horticulture | Grassland | Shrubland | Olive Orchard | Pinus Forest | Quercus Forest | Fagus Forest |
|---|---|---|---|---|---|---|---|---|
| Sand (g/kg) | 959 a | 516 c | 840 a | 602 b | 670 b | 942 a | 930 a | 860 a |
| Loam (g/kg) | 17 e | 309 a | 158 c | 237 b | 163 d | 35 e | 66 e | 117 d |
| Clay (g/kg) | 24 b | 175 a | 2 b | 161 a | 167 a | 23 b | 4 b | 23 b |
| pH | 7.06 b | 7.82 a | 5.79 c | 7.16 b | 6.96 | 8.01 a | 7.19 b | 7.32 b |
| Electrical conductivity (dS/m) | 0.213 b | 0.263 a | 0.103 d | 0.131 d | 0.124 d | 0.126 d | 0.274 a | 0.161 c |
| Limestone (g/kg) | 6.79 d | 47.7 c | 5.91 d | 5.78 d | 4.07 d | 224 a | 31.8 c | 91.9 b |
| Organic carbon (g/kg) | 14.4 d | 13.3 d | 64.5 ab | 26.3 c | 13.7 d | 30 c | 41.6 b | 76.1 a |
| Total nitrogen (g/kg) | 1.68 d | 1.96 d | 9.56 a | 3.07 c | 2.01 d | 1.64 d | 4.85 b | 5.92 ab |
| C/N ratio | 8.6 c | 6.8 d | 6.7 d | 8.6 c | 6.9 d | 18.3 a | 8.6 c | 12.9 b |
| Phosphoric anhydride (mg/kg) | 107.1 b | 272.2 a | 15.3 d | 30.8 d | 19.3 d | 28.3 d | 76.4 c | 61.8 c |
| Cation exchange capacity (meq/100 g) | 14.4 c | 20.1 c | 40.3 a | 37.7 ab | 16.8 c | 25.1 b | 36.1 ab | 51.0 a |
| Calcium (meq/100 g) | 10.1 d | 13.5 d | 28.2 ab | 32.1 a | 14.2 d | 18.6 c | 26.2 b | 38.5 a |
| Magnesium (meq/100 g) | 1.83 d | 3.23 c | 1.71 d | 3.12 c | 1.46 d | 5.11 b | 5.05 b | 9.51 a |
| Sodium (meq/100 g) | 0.14 c | 0.09 d | 0.13 c | 0.34 b | 0.03 d | 0.06 d | 0.67 a | 0.24 b |
| Potassium (meq/100 g) | 1.37 b | 1.93 a | 0.62 c | 2.05 a | 0.63 c | 0.13 d | 2.73 a | 0.96 c |
| Iron (mg/kg) | 13.2 b | 4.87 c | 59.7 a | 6.98 c | 7.57 c | 7.62 c | 15.8 b | 17.9 b |
| Copper (mg/kg) | 12.8 a | 11.1 a | 1.01 c | 1.08 c | 1.11 c | 0.29 c | 4.44 b | 0.94 c |
| Zinc (mg/kg) | 10.9 b | 2.98 c | 1.91 c | 1.22 c | 1.01 c | 1.94 c | 19.3 a | 8.16 b |
| Manganese (mg/kg) | 4.11 c | 15.7 a | 4.44 c | 9.39 b | 14.4 a | 15.7 a | 7.43 b | 16.3 a |
| Fluorescein diacetate | 0.41 bc | 0.21 d | 0.58 ab | 0.77 a | 0.53 b | 0.39 c | 0.68 a | 0.13 d |
| Microbial biomass (mg C 100 g−1) | 6.36 e | 12.11 d | 58.7 b | 47.6 bc | 7.38 d | 12.71 d | 33.26 c | 146.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, G.; Idbella, M.; Gaglione, S.; Abd-ElGawad, A.M.; Bonanomi, G. Impact of Land Use Types on Soil Physico-Chemical Properties, Microbial Communities, and Their Fungistatic Effects. Soil Syst. 2024, 8, 131. https://doi.org/10.3390/soilsystems8040131
Iacomino G, Idbella M, Gaglione S, Abd-ElGawad AM, Bonanomi G. Impact of Land Use Types on Soil Physico-Chemical Properties, Microbial Communities, and Their Fungistatic Effects. Soil Systems. 2024; 8(4):131. https://doi.org/10.3390/soilsystems8040131
Chicago/Turabian StyleIacomino, Giuseppina, Mohamed Idbella, Salvatore Gaglione, Ahmed M. Abd-ElGawad, and Giuliano Bonanomi. 2024. "Impact of Land Use Types on Soil Physico-Chemical Properties, Microbial Communities, and Their Fungistatic Effects" Soil Systems 8, no. 4: 131. https://doi.org/10.3390/soilsystems8040131
APA StyleIacomino, G., Idbella, M., Gaglione, S., Abd-ElGawad, A. M., & Bonanomi, G. (2024). Impact of Land Use Types on Soil Physico-Chemical Properties, Microbial Communities, and Their Fungistatic Effects. Soil Systems, 8(4), 131. https://doi.org/10.3390/soilsystems8040131









