No-Till and Crop Rotation Are Promising Practices to Enhance Soil Health in Cotton-Producing Semiarid Regions: Insights from Citizen Science
Abstract
1. Introduction
2. Materials and Methods
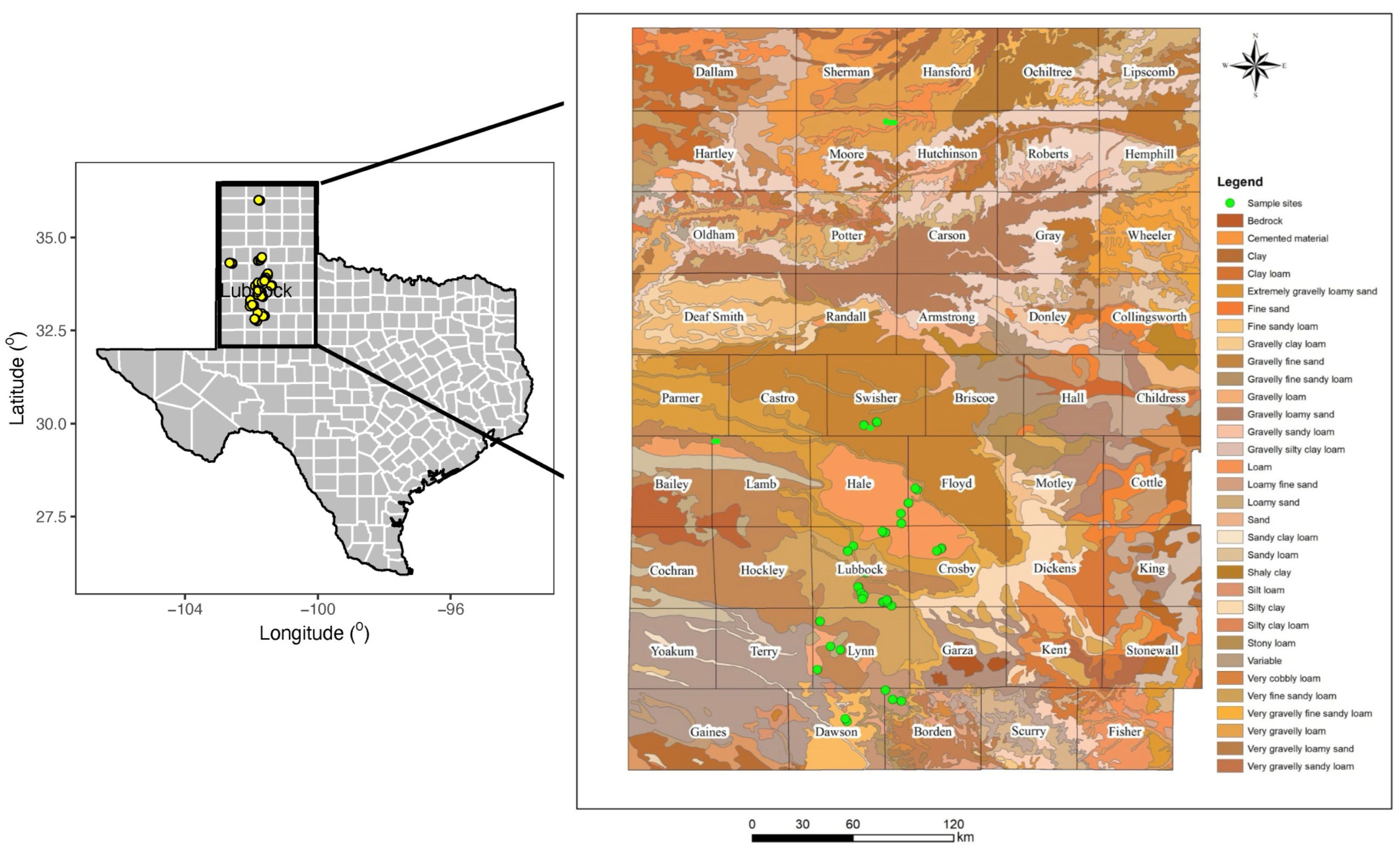
2.1. Study Sites
2.2. Citizen Science Implementation Model and Soil Sample Collection
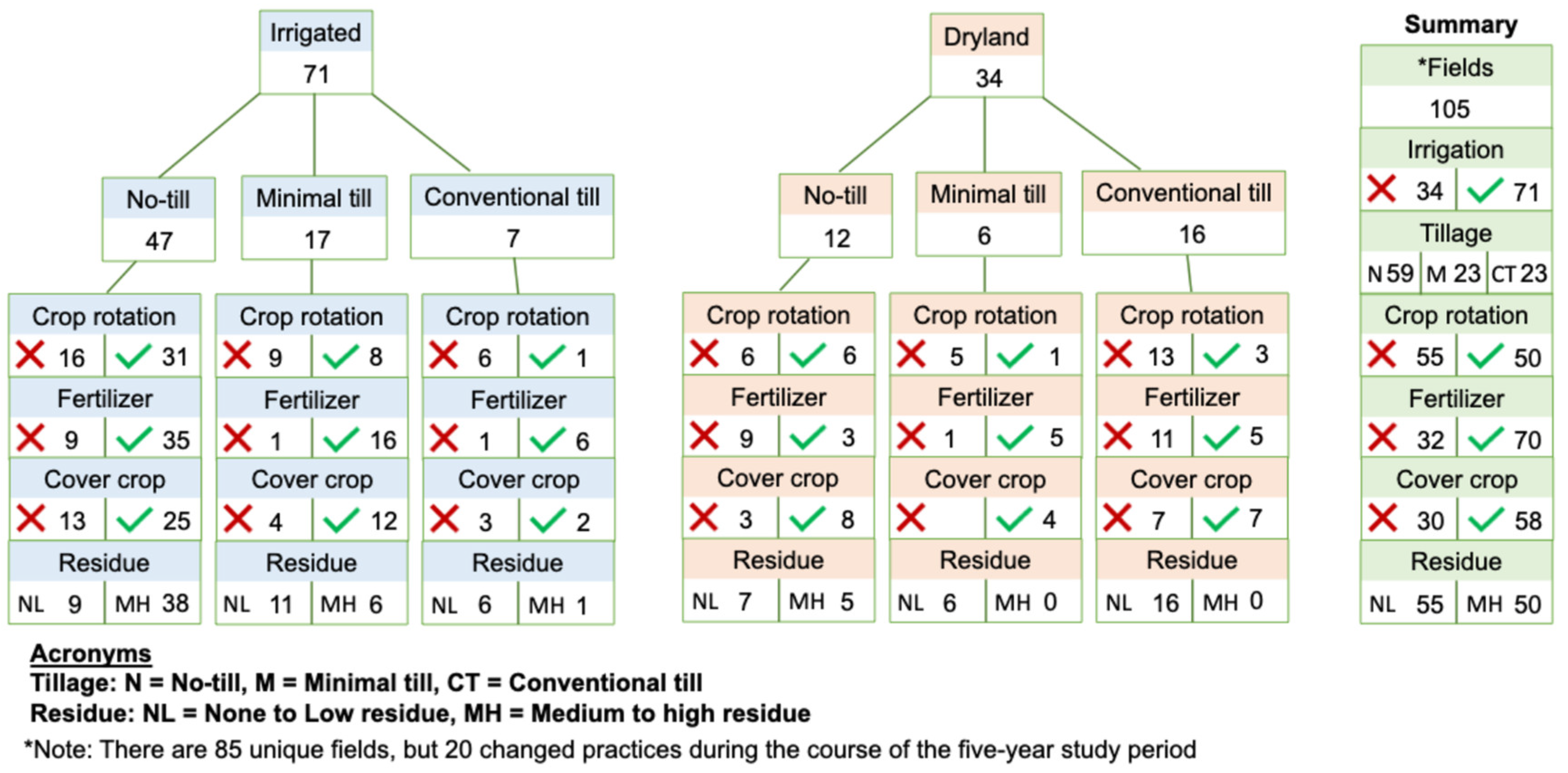
2.3. Management Practices
2.4. Soil Environmental Data
2.5. Laboratory Analysis for Different Soil Health Indicators
2.6. Statistical Analysis
3. Results
4. Discussion
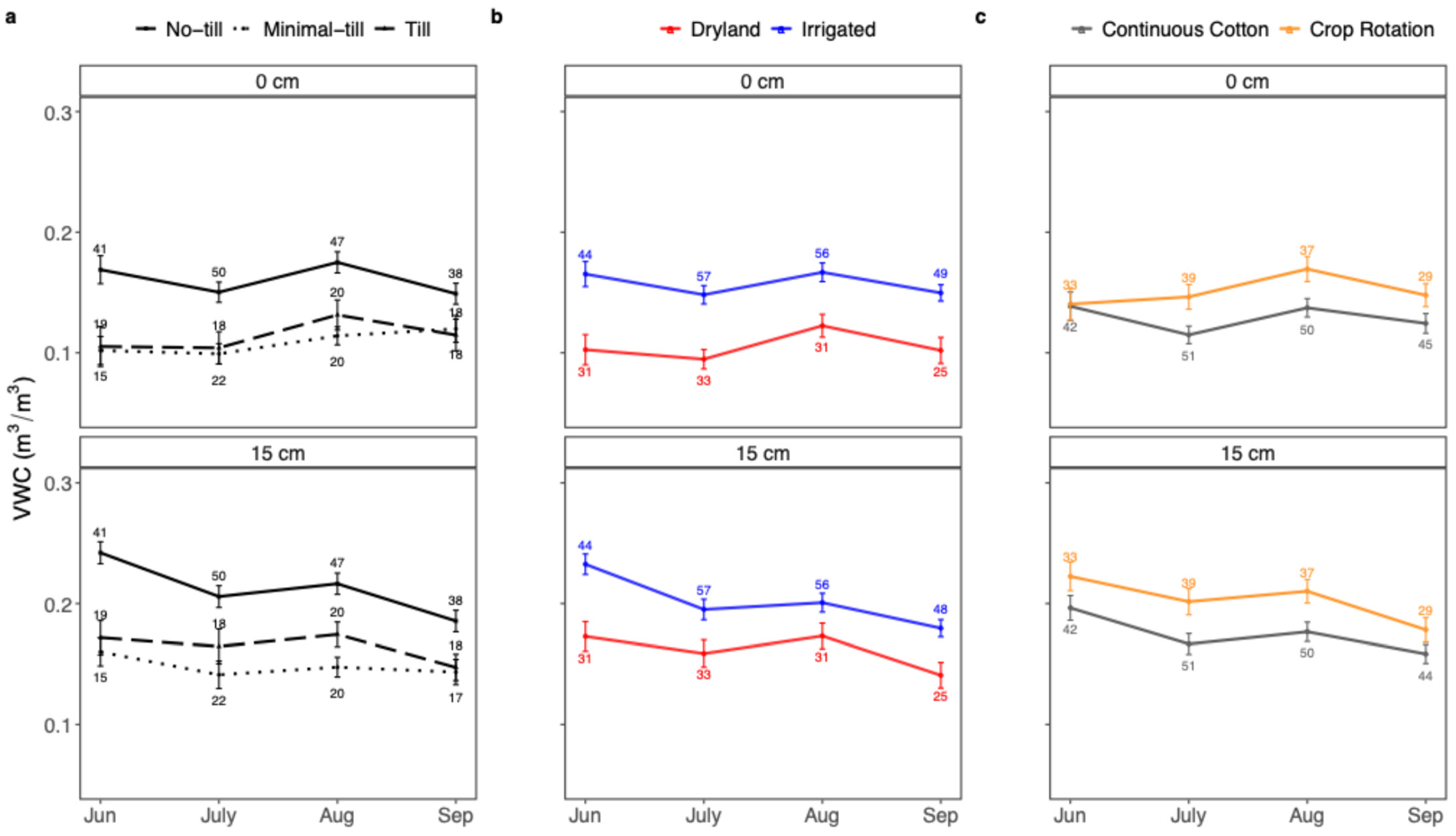
4.1. Effects of Soil Management on Soil Temperature and Moisture
4.2. Effects of Soil Management on Nutrient Availability
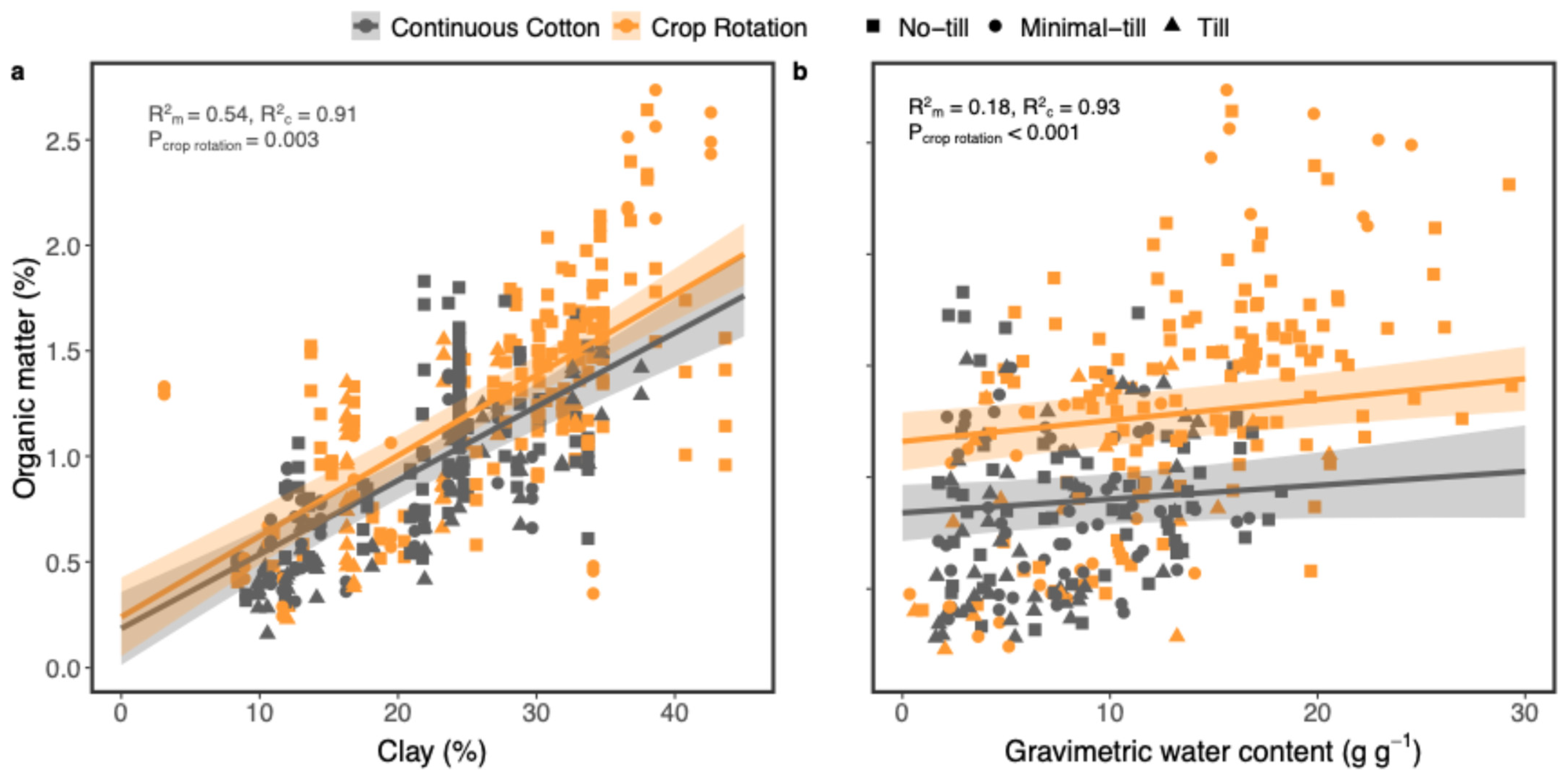
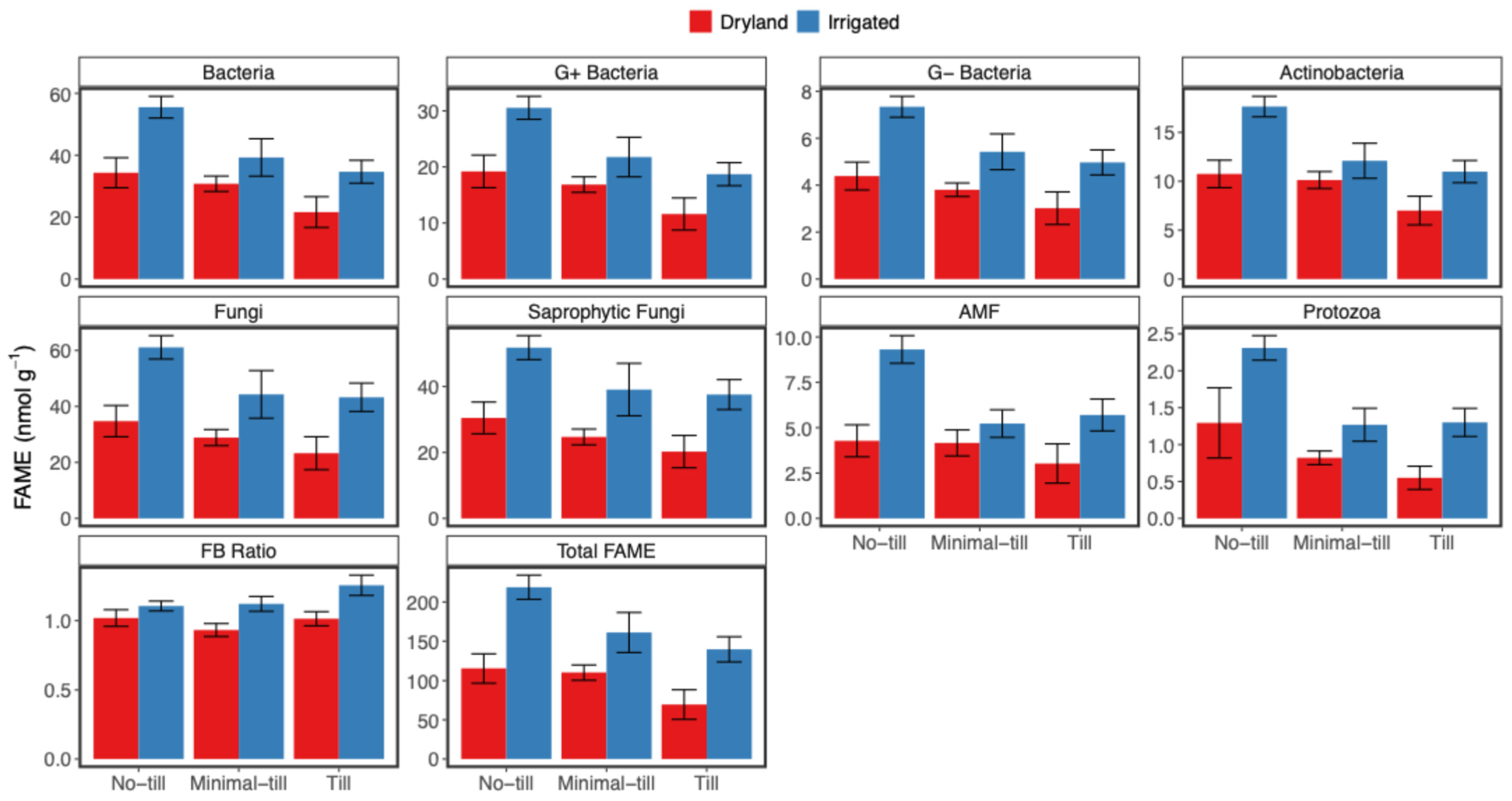
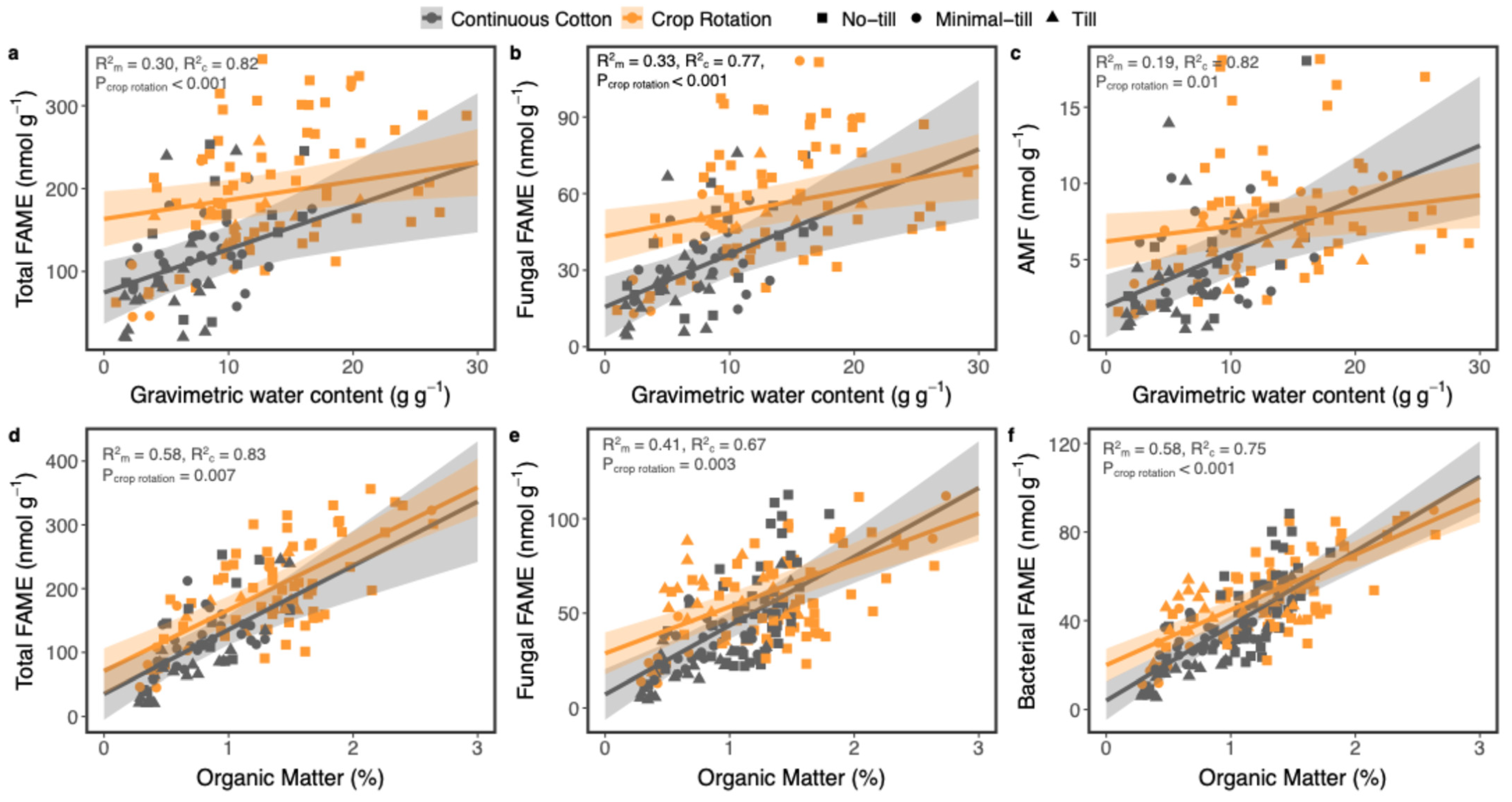
4.3. Effects of Soil Management on Soil Organic Matter and Abundances of Main Microbial Groups
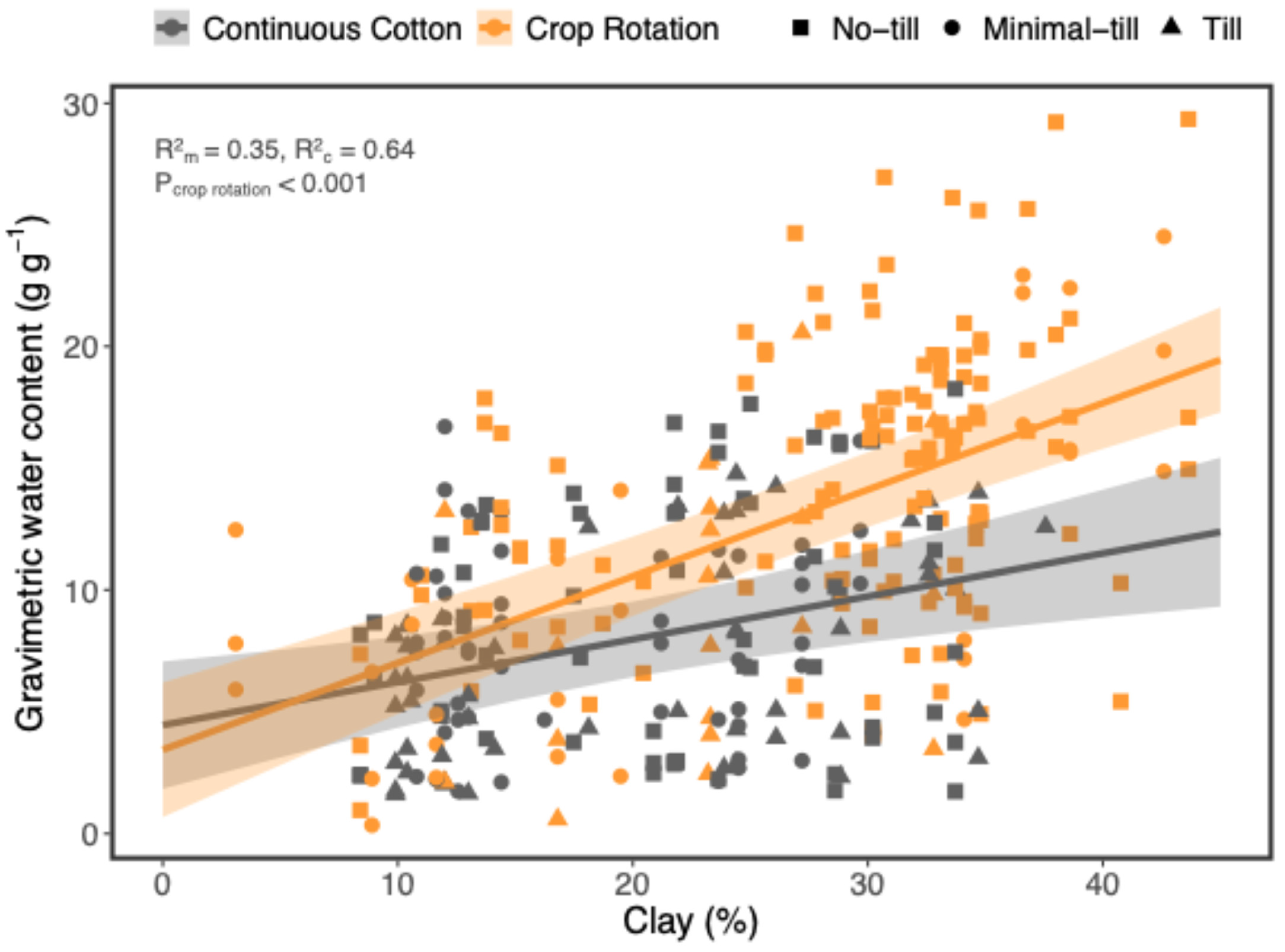
4.4. The Significance of Soil Texture on Soil Health
4.5. Limitation and Future Suggestions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaur, M.K.; Squires, V.R. Climate Variability Impacts on Land Use and Livelihoods in Drylands; Springer International Publishing: New York, NY, USA, 2017; 348p. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Mauget, S.A.; Adhikari, P.; Leiker, G.; Baumhardt, R.L.; Thorp, K.R.; Ale, S. Modeling the effects of management and elevation on West Texas dryland cotton production. Agric. For. Meteorol. 2017, 247, 385–398. [Google Scholar] [CrossRef]
- Jones, J.B. Agronomic Handbook: Management of Crops, Soils, and Their Fertility; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Terrell, B.L.; Johnson, P.N.; Segarra, E. Ogallala aquifer depletion: Economic impact on the Texas high plains. Water Policy 2002, 4, 33–46. [Google Scholar] [CrossRef]
- Fernández Cirelli, A.; Arumí, J.L.; Rivera, D.; Boochs, P.W. Environmental Effects of Irrigation in Arid and Semi-Arid Regions. Chil. J. Agric. Res. 2009, 69, 27–40. [Google Scholar] [CrossRef]
- Basso, B.; Kendall, A.D.; Hyndman, D.W. The future of agriculture over the Ogallala Aquifer: Solutions to grow crops more efficiently with limited water. Earths Future 2013, 1, 39–41. [Google Scholar] [CrossRef]
- Schreefel, L.; Schulte, R.P.O.; De Boer, I.J.M.; Schrijver, A.P.; van Zanten, H.H.E. Regenerative agriculture-the soil is the base. Glob. Food Secur. 2020, 26, 100404. [Google Scholar] [CrossRef]
- Luján, S.R.; Martínez-Mena, M.; Cuéllar, P.M.; de Vente, J. Restoring soil quality of woody agroecosystems in Mediterranean drylands through regenerative agriculture. Agric. Ecosyst. Environ. 2020, 306, 107191. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative Agriculture—A Literature Review on the Practices and Mechanisms Used to Improve Soil Health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Semenov, A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil. Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; Deyn, G.D.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyeper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Colombi, T.; Keller, T. The influence of soil management on soil health: An on-farm study in southern Sweden. Geoderma 2020, 360, 114010. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Guo, R.; Sarwar, M.; Ren, X.; Krstic, D.; Aslam, Z.; Zulifqar, U.; Rauf, A.; Hano, C.; et al. Carbon sequestration to avoid soil degradation: A review on the role of conservation tillage. Plants 2021, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Mikha, M.M.; Rice, C.W. Tillage and Manure Effects on Soil and Aggregate-Associated Carbon and Nitrogen. Soil Sci. Soc. Am. J. 2004, 68, 809–816. [Google Scholar] [CrossRef]
- Thapa, V.R.; Ghimire, R.; Acosta-Martínez, V.; Marsalis, M.A.; Schipanski, M.E. Cover crop biomass and species composition affect soil microbial community structure and enzyme activities in semiarid cropping systems. Appl. Soil Ecol. 2021, 157, 103735. [Google Scholar] [CrossRef]
- Wood, S.A.; Bowman, M. Large-scale farmer-led experiment demonstrates positive impact of cover crops on multiple soil health indicators. Nat. Food 2021, 2, 97–103. [Google Scholar] [CrossRef]
- Romero-Salas, E.A.; Navarro-Noya, Y.E.; Luna-Guido, M.; Verhulst, N.; Crossa, J.; Govaerts, B.; Dendooven, L. Changes in the bacterial community structure in soil under conventional and conservation practices throughout a complete maize (Zea mays L.) crop cycle. Appl. Soil Ecol. 2021, 157, 103733. [Google Scholar] [CrossRef]
- Possinger, A.R.; Byrne, L.B.; Breen, N.E. Effect of buckwheat (Fagopyrum esculentum) on soil-phosphorus availability and organic acids. J. Plant Nutr. Soil Sci. 2013, 176, 16–18. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services: Insights from Studies in Temperate Soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Miner, G.L.; Delgado, J.A.; Ippolito, J.A.; Stewart, C.E. Soil health management practices and crop productivity. Agric. Environ. Lett. 2020, 5, e20023. [Google Scholar] [CrossRef]
- Krstić, D.; Vujić, S.; Jaćimović, G.; D’Ottavio, P.; Radanović, Z.; Erić, P.; Ćupina, B. The Effect of Cover Crops on Soil Water Balance in Rain-Fed Conditions. J. Atmos. 2018, 9, 492. [Google Scholar] [CrossRef]
- Hao, X.; Abou, N.M.; Steenwerth, K.L.; Nocco, M.A.; Basset, C.; Daccache, A. Are there universal soil responses to cover cropping? A systematic review. Sci. Total Environ. 2023, 861, 160600. [Google Scholar] [CrossRef]
- Burke, J.A.; Lewis, K.L.; Delaune, P.B.; Cobos, C.J.; Keeling, J.W. Soil Water Dynamics and Cotton Production Following Cover Crop Use in a Semi-Arid Ecoregion. Agronomy 2022, 12, 1306. [Google Scholar] [CrossRef]
- Acree, A.; Fultz, L.M.; Lofton, J.; Haggard, B. Soil biochemical and microbial response to wheat and corn stubble residue management in Louisiana. Agrosyst. Geosci. Environ. 2020, 3, e20004. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Yadav, A.K.; Choudhary, V.; Sharma, P.C.; Gathala, M.K.; Jat, M.L.; McDonald, A. Effects of tillage, crop establishment and diversification on soil organic carbon, aggregation, aggregate associated carbon and productivity in cereal systems of semi-arid Northwest India. Soil Till Res. 2019, 190, 128–138. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Khan, W.A.; Wang, G. Conservation Tillage: A Sustainable Approach for Carbon Sequestration and Soil Preservation. A Review. J. Agric. Sustain. Environ. 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Brevik, E.C. The potential impact of climate change on soil properties and processes and corresponding influence on food security. Agriculture 2013, 3, 398–417. [Google Scholar] [CrossRef]
- King, A.E.; Blesh, J. Crop rotations for increased soil carbon: Perenniality as a guiding principle. Ecol. Appl. 2018, 28, 249–261. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Garland, G.; Edlinger, A.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Pescador, D.S.; Herzog, C.; Romdhane, S.; Saghai, A.; Spor, A.; et al. Crop cover is more important than rotational diversity for soil multifunctionality and cereal yields in European cropping systems. Nat. Food 2021, 2, 28–37. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 1–14. [Google Scholar] [CrossRef]
- Srour, A.Y.; Ammar, H.A.; Subedi, A.; Pimentel, M.; Cook, R.L.; Bond, J.; Fakhoury, A.M. Microbial Communities Associated With Long-Term Tillage and Fertility Treatments in a Corn-Soybean Cropping System. Front. Microbiol. 2020, 11, 522658. [Google Scholar] [CrossRef]
- Ryan, S.F.; Adamson, N.L.; Aktipis, A.; Andersen, L.K.; Austin, R.; Barnes, L.; Beasley, M.R.; Bedell, K.D.; Briggs, S.; Chapman, B.; et al. The role of citizen science in addressing grand challenges in food and agriculture research. Proc. R. Soc. B 2018, 285, 20181977. [Google Scholar] [CrossRef]
- Kyveryga, P.M. On-Farm Research: Experimental Approaches, Analytical Frameworks, Case Studies, and Impact. Agron. J. 2019, 111, 1–3. [Google Scholar] [CrossRef]
- Pires, C.B.; Krupek, F.S.; Carmona, G.I.; Ortez, O.A.; Thompson, L.; Quinn, D.J.; Reis, A.F.B.; Werle, R.; Kovács, P.; Singh, M.P.; et al. Perspective of US farmers on collaborative on-farm agronomic research. Agron. J. 2024, 116, 1590–1602. [Google Scholar] [CrossRef]
- van de Gevel, J.; van Etten, J.; Deterding, S. Citizen science breathes new life into participatory agricultural research. A review. Agron. Sustain. Dev. 2020, 40, 35. [Google Scholar] [CrossRef]
- Heaton, L.; Fullen, M.; Bhattacharyya, R. Critical Analysis of the van Bemmelen Conversion Factor used to Convert Soil Organic Matter Data to Soil Organic Carbon Data: Comparative Analyses in a UK Loamy Sand Soil. Espaço Aberto 2016, 6, 35–44. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis by Hydrometer: A Simplified Method for Routine Textural Analysis and a Sensitivity Test of Measurement Parameters†. Soil Sci. Soc. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Stursova, M.; Sinsabaugh, R.L.; Collins, S.L. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 2007, 154, 349–359. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, N.C.; Dhungana, N.; Tissue, D.T.; Zak, J.C. Seasonal microbial and nutrient responses during a 5-year reduction in the daily temperature range of soil in a Chihuahuan Desert ecosystem. Oecologia 2016, 180, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schutter, M.E.; Dick, R.P. Comparison of Fatty Acid Methyl Ester (FAME) Methods for Characterizing Microbial Communities. Soil Sci. Soc. Am. J. 2000, 64, 1659–1668. [Google Scholar] [CrossRef]
- Li, C.; Cano, A.; Acosta-Martinez, V.; Veum, K.S.; Moore-Kucera, J. A comparison between fatty acid methyl ester profiling methods (PLFA and EL-FAME) as soil health indicators. Soil Sci. Soc. Am. J. 2020, 84, 1153–1169. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Olsson, P.A.; Thingstrup, I.; Jakobsen, I.; Baê Aê Th, E. Estimation of the biomass of arbuscular mycorrhizal fungi in a linseed feld. Soil Biol. Biochem. 1999, 31, 1879–1887. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for STATISTICAL Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 9 October 2024).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Powlson, D.S.; Prookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratio of microbial biomass carbon to total organic carbon in arable soils. Aust. J. Soil Res. 1989, 30, 195–207. [Google Scholar] [CrossRef]
- Calcagno, V. glmulti: Model Selection and Multimodel Inference Made Easy. R Package Version 1.0.8. 2020. Available online: https://CRAN.R-project.org/package=glmulti (accessed on 9 October 2024).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.8.9. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 9 October 2024).
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 9 October 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 9 October 2024)ISBN 978-3-319-24277-4.
- Gibbons, L.V. Regenerative—The New Sustainable? Sustainability 2020, 12, 5483. [Google Scholar] [CrossRef]
- Blevins, R.L.; Cook, D.; Phillips, S.H.; Phillips, R.E. Influence of No-tillage on Soil Moisture. Agron. J. 1971, 63, 593–596. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Guo, Z.; Li, J.; Tian, C.; Hua, D.; Shi, C.; Wang, H.; Han, J.; Xu, Y. Effects of Conservation Tillage on Soil Physicochemical Properties and Crop Yield in an Arid Loess Plateau, China. Sci. Rep. 2020, 10, 4716. [Google Scholar] [CrossRef]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Till Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Lascano, R.J.; Baumhardt, R.L.; Hicks, S.K.; Heilman, J.L. Soil and Plant Water Evaporation from Strip-Tilled Cotton: Measurement and Simulation. Agron. J. 1994, 86, 987–994. [Google Scholar] [CrossRef]
- Alexander, L. Climate science: Extreme heat rooted in dry soils. Nat. Geosci. 2011, 4, 12–13. [Google Scholar] [CrossRef]
- Yu, T.; Mahe, L.; Li, Y.; Wei, X.; Deng, X.; Zhang, D. Benefits of Crop Rotation on Climate Resilience and Its Prospects in China. Agronomy 2022, 12, 436. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, X.; Ding, W.; Song, X.; Li, S.; Wu, X. Positive effects of crop rotation on soil aggregation and associated organic carbon are mainly controlled by climate and initial soil carbon content: A meta-analysis. Agric. Ecosyst. Environ. 2023, 355, 108600. [Google Scholar] [CrossRef]
- Fang, S.; Yan, X.; Liao, H. 3D reconstruction and dynamic modeling of root architecture in situ and its application to crop phosphorus research. Plant J. 2009, 60, 1096–1108. [Google Scholar] [CrossRef]
- Picone, L.; Zamuner, E.; Berardo, A.; Marino, M. Phosphorus transformations as affected by sampling date, fertilizer rate, and phosphorus uptake in soil under pasture. Nutr. Cycl. Agroecosyst. 2003, 67, 225–232. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Magallon-Servin, P.; Lopez, B.R.; Nannipieri, P. Biological activities affect the dynamic of P in dryland soils. Biol. Fertil. Soils 2021, 58, 105–119. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action, Biological Processes in Soil Phosphorus Cycling; Bunemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin, Germany, 2011; pp. 215–241. [Google Scholar]
- Xomphoutheb, T.; Jiao, S.; Guo, X.; Mabagala, F.S.; Sui, B.; Wang, H.; Zhao, L.; Zhao, X. The effect of tillage systems on phosphorus distribution and forms in rhizosphere and non-rhizosphere soil under maize (Zea mays L.) in Northeast China. Sci. Rep. 2020, 10, 6574. [Google Scholar] [CrossRef]
- Carpenter-Boggs, L.; Stahl, P.D.; Lindstrom, M.J.; Schumacher, T.E. Soil microbial properties under permanent grass, conventional tillage, and no-till management in South Dakota. Soil Till Res. 2003, 71, 15–23. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Gill, T.E.; Kennedy, A.C. Enzyme activities and microbial community structure in semiarid agricultural soils. Biol. Fertil. Soils 2003, 38, 216–227. [Google Scholar] [CrossRef]
- Lu, X.; Liao, Y. Effect of tillage practices on net carbon flux and economic parameters from farmland on the Loess Plateau in China. J. Clean. Prod. 2017, 162, 1617–1624. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R.; Wang, Z.H.; Chhabra, B.S. Tillage, nitrogen and crop residue effects on crop yield, nutrient uptake, soil quality, and greenhouse gas emissions. Soil Till Res. 2006, 90, 171–183. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M.; Matocha, J.E. Tillage impacts on microbial biomass and soil carbon and nitrogen dynamics of corn and cotton rotations. Appl. Soil. Ecol. 2005, 29, 85–92. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Zolti, A.; Shaltiel-Harpaz, L.; Argaman, E.; Rabinovich, R.; Green, S.J.; Minz, D. Effects of tillage practices on soil microbiome and agricultural parameters. Sci. Total Environ. 2020, 705, 135791. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkom, K.S. Impact of No-tillage and conventional tillage systems on soil microbial communities. Appl. Environ. Soil Sci. 2012, 2012, 548620. [Google Scholar] [CrossRef]
- Stegarescu, G.; Escuer-Gatius, J.; Soosaar, K.; Kauer, K.; Tõnutare, T.; Astover, A.; Reintam, E. Effect of Crop Residue Decomposition on Soil Aggregate Stability. Agriculture 2020, 10, 527. [Google Scholar] [CrossRef]
- Núñez, A.; Cotrufo, M.F.; Schipanski, M. Irrigation effects on the formation of soil organic matter from aboveground plant litter inputs in semiarid agricultural systems. Geoderma 2022, 416, 115804. [Google Scholar] [CrossRef]
- Calderon, F.J.; Nielsen, D.; Acosta-Mart’inez, V.; Vigil, M.F.; Lyon, D. Cover Crop and Irrigation Effects on Soil Microbial Communities and Enzymes in Semiarid Agroecosystems of the Central Great Plains of North America. Pedosphere 2016, 26, 192–205. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Griffin, T.S.; Olanya, O.M.; Halloran, J.M.; He, Z. Effects of different potato cropping system approaches and water management on soilborne diseases and soil microbial communities. Phytopathology 2011, 101, 58–67. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Z.; Chen, C.; Jia, Z. Soil microbial community structure and diversity are largely influenced by soil pH and nutrient quality in 78-year-old tree plantations. Biogeosciences 2017, 14, 2101–2111. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rilling, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Ding, G.; Liu, X.; Herbert, S.; Novak, J.; Amarasiriwardena, D.; Xing, B. Effect of cover crop management on soil organic matter. Geoderma 2006, 130, 229–239. [Google Scholar] [CrossRef]
- Strickland, M.S.; Thomason, W.E.; Avera, B.; Franklin, J.; Minick, K.; Yamada, S.; Badgley, B.D. Short-Term Effects of Cover Crops on Soil Microbial Characteristics and Biogeochemical Processes across Actively Managed Farms. Agrosyst. Geosci. Environ. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Naugle, D.E.; Allred, B.W.; Jones, M.O.; Twidwell, D.; Maestas, J.D. Coproducing Science to Inform Working Lands: The Next Frontier in Nature Conservation. Bioscience 2020, 70, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, Y.; Jiang, W.; Pan, F.; Wang, M.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The effects of crop rotation combinations on the soil quality of old apple orchard. Hortic. Plant J. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Dold, C.; Büyükcangaz, H.; Rondinelli, W.; Prueger, J.H.; Sauer, T.J.; Hatfield, J.L. Long-term carbon uptake of agro-ecosystems in the Midwest. Agric. For. Meteorol. 2017, 232, 128–140. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Town, J.R.; Gregorich, E.G.; Drury, C.F.; Lemke, R.L.; Phillips, L.A.; Helgason, B.L. Diverse crop rotations influence the bacterial and fungal communities in root, rhizosphere and soil and impact soil microbial processes. Appl. Soil Ecol. 2022, 169, 104241. [Google Scholar] [CrossRef]
- Aylmore, L.A.G.; Quirk, J.P. The micropore size distributions of clay mineral systems. J. Soil Sci. 1967, 18, 1–17. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hailu, T.A.; Devkota, P.; Osoko, T.O.; Singh, R.K.; Zak, J.C.; van Gestel, N. No-Till and Crop Rotation Are Promising Practices to Enhance Soil Health in Cotton-Producing Semiarid Regions: Insights from Citizen Science. Soil Syst. 2024, 8, 108. https://doi.org/10.3390/soilsystems8040108
Hailu TA, Devkota P, Osoko TO, Singh RK, Zak JC, van Gestel N. No-Till and Crop Rotation Are Promising Practices to Enhance Soil Health in Cotton-Producing Semiarid Regions: Insights from Citizen Science. Soil Systems. 2024; 8(4):108. https://doi.org/10.3390/soilsystems8040108
Chicago/Turabian StyleHailu, Tirhas A., Pawan Devkota, Taiwo O. Osoko, Rakesh K. Singh, John C. Zak, and Natasja van Gestel. 2024. "No-Till and Crop Rotation Are Promising Practices to Enhance Soil Health in Cotton-Producing Semiarid Regions: Insights from Citizen Science" Soil Systems 8, no. 4: 108. https://doi.org/10.3390/soilsystems8040108
APA StyleHailu, T. A., Devkota, P., Osoko, T. O., Singh, R. K., Zak, J. C., & van Gestel, N. (2024). No-Till and Crop Rotation Are Promising Practices to Enhance Soil Health in Cotton-Producing Semiarid Regions: Insights from Citizen Science. Soil Systems, 8(4), 108. https://doi.org/10.3390/soilsystems8040108






