Uncovering Hidden Microbial Diversity in Nitrate/Iodide Deposits (NIDs) in the Domeyko District, Atacama Desert, Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Sampling Collection
2.2. Physical, Chemical, and Mineralogical Analysis of NIDs
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Sequence Analysis and Taxonomic Assignation
2.5. Enrichment Cultures of Nitrate/Perchlorate Reducing Bacteria
3. Results and Discussion
3.1. Physical, Chemical, and Mineralogical Characterization of NIDs
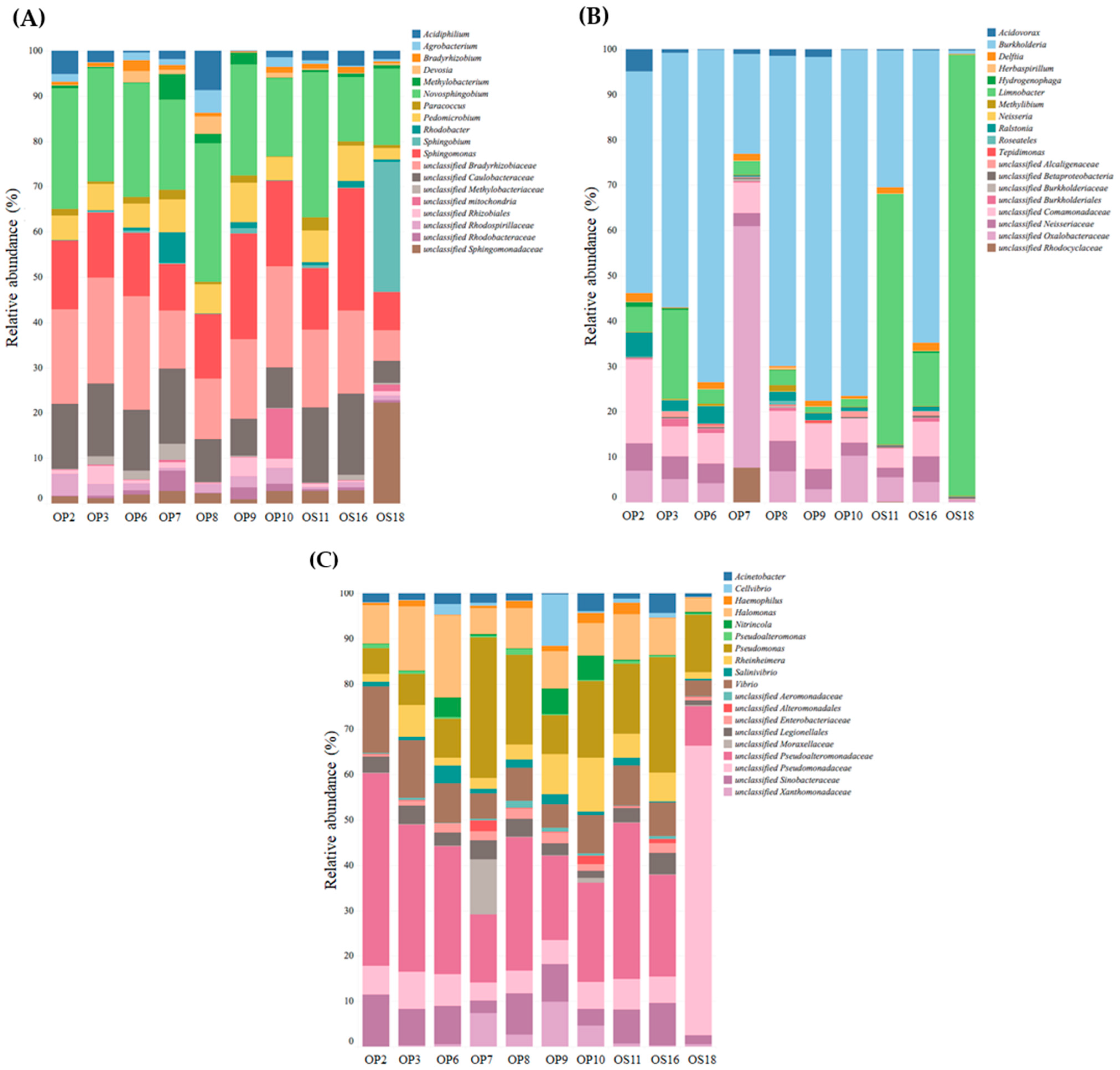
3.2. Genomic Analysis of NID Soils
3.3. An Insight on Nitrate/Perchlorate Reducing Bacteria in NID Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Zerkle, A.L.; Stueeken, E.; Claire, M.W. Nitrates as a Potential N Supply for Microbial Ecosystems in a Hyperarid Mars Analog System. Life 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, G.E. The Chilean Nitrate Deposits. Am. Sci. 1983, 71, 366–374. [Google Scholar]
- Mpodozis, C.; Allmendinger, R.W. Extensional Tectonics, Cretaceous Andes, Northern Chile (27°S). Geol. Soc. Am. Bull. 1993, 105, 1462–1477. [Google Scholar] [CrossRef]
- Maksaev, V. Metallogeny. Geological Evolution and Thermochronology of the Chilcan Andes Between Latitudes 21° and 26° South. and the Origin of Major Porphyry Copper Deposits. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1990; 544p. [Google Scholar]
- Manning, C.V.; Zahnle, K.J.; McKay, C.P. Impact Processing of Nitrogen on Early Mars. Icarus 2009, 199, 273–285. [Google Scholar] [CrossRef]
- Navarro-González, R.; Navarro, K.F.; Coll, P.; McKay, C.P.; Stern, J.C.; Sutter, B.; Archer, P.D.; Buch, A.; Cabane, M.; Conrad, P.G.; et al. Abiotic Input of Fixed Nitrogen by Bolide Impacts to Gale Crater during the Hesperian: Insights from the Mars Science Laboratory. J. Geophys. Res. Planets 2019, 124, 94–113. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; Mulder, A.; De Bruijn, P.; Jetten, M.S.M.; Robertson, L.A.; Kuenen, J.G. Anaerobic Oxidation of Ammonium Is a Biologically Mediated Process. Appl. Environ. Microbiol. 1995, 61, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B. Lithobiontic Life: “Atacama Rocks Are Well and Alive”. Antonie Van Leeuwenhoek Int. J. General. Mol. Microbiol. 2018, 111, 1333–1343. [Google Scholar] [CrossRef]
- Sajjad, W.; Ilahi, N.; Kang, S.; Bahadur, A.; Zada, S.; Iqbal, A. Endolithic Microbes of Rocks, Their Community, Function and Survival Strategies. Int. Biodeterior. Biodegrad. 2022, 169, 105387. [Google Scholar] [CrossRef]
- Parro, V.; De Diego-Castilla, G.; Moreno-Paz, M.; Blanco, Y.; Cruz-Gil, P.; Rodríguez-Manfredi, J.A.; Fernández-Remolar, D.; Gómez, F.; Gómez, M.J.; Rivas, L.A.; et al. A Microbial Oasis in the Hypersaline Atacama Subsurface Discovered by a Life Detector Chip: Implications for the Search for Life on Mars. Astrobiology 2011, 11, 969–996. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Vilo-Muñoz, C.; Galetović, A.; Dong, Q.; Castelán-Sánchez, H.G.; Pérez-Llano, Y.; Sánchez-Carbente, M.D.R.; Dávila-Ramos, S.; Cortés-López, N.G.; Martínez-ávila, L.; et al. Metagenomics of Atacama Lithobiontic Extremophile Life Unveils Highlights on Fungal Communities, Biogeochemical Cycles and Carbohydrate-Active Enzymes. Microorganisms 2019, 7, 94–113. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Batista-García, R.A. The Atacama Desert: A Biodiversity Hotspot and Not Just a Mineral-Rich Region. Front. Microbiol. 2022, 13, 812842. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetović, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated from Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Warren-Rhodes, K.A.; Rhodes, K.L.; Pointing, S.B.; Ewing, S.A.; Lacap, D.C.; Gómez-Silva, B.; Amundson, R.; Friedmann, E.I.; McKay, C.P. Hypolithic Cyanobacteria, Dry Limit of Photosynthesis, and Microbial Ecology in the Hyperarid Atacama Desert. Microb. Ecol. 2006, 52, 389–398. [Google Scholar] [CrossRef] [PubMed]
- de los Ríos, A.; Valea, S.; Ascaso, C.; Davila, A.; Kastovsky, J.; McKay, C.P.; Gómez-Silva, B.; Ierzchos, J. Comparative Analysis of the Microbial Communities Inhabiting Halite Evaporates of the Atacama Desert. Int. Microbiol. 2010, 13, 79–89. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Rainey, F.A.; Warren-Rhodes, K.A.; Mckay, C.P.; Navarro-González, R. Atacama Desert Soil Microbiology. In Micorbiology Extrem Soils; Springer: Berlin/Heidelberg, Germany, 2008; pp. 117–132. [Google Scholar] [CrossRef]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. Uv-a Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Gelsinger, D.R.; Ma, B.; Wierzchos, J.; Ravel, J.; Davila, A.; Casero, M.C.; DiRuggiero, J. Functional Interactions of Archaea, Bacteria and Viruses in a Hypersaline Endolithic Community. Environ. Microbiol. 2016, 18, 2064–2077. [Google Scholar] [CrossRef]
- Fuentes, B.; Choque, A.; Gómez, F.; Alarcón, J.; Castro-Nallar, E.; Arenas, F.; Contreras, D.; Mörchen, R.; Amelung, W.; Knief, C.; et al. Influence of Physical-Chemical Soil Parameters on Microbiota Composition and Diversity in a Deep Hyperarid Core of the Atacama Desert. Front. Microbiol. 2022, 12, 794743. [Google Scholar] [CrossRef]
- Reutter, K.J.; Scheuber, E.; Wigger, P. Tectonics of the Southern Central Andes: Structure and Evolution of an Active Continental Margin; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994; ISBN 9783642773556. [Google Scholar]
- de Geología y Minería, C.S.N.; Marinovic, N. Carta Oficina Domeyko: Región de Antofagasta; Servicio Nacional de Geología y Minería. 2007. Available online: https://tiendadigital.sernageomin.cl/en/-basic-geology/3137-carta-oficina-domeyko-region-de-antofagasta.html (accessed on 10 September 2023).
- Schumacher, B.A.; Shines, K.C.; Burton, J.V.; Papp, M.L. Comparison of three methods for soil homogenization. Soil. Sci. Soc. Am. J. 1990, 54, 1187–1190. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Nomura, Y. A Simple Method to Determine the Water Activity of Ethanol-Containing Samples. Biotechnol. Bioeng. 1999, 62, 242–245. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S RRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High- Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A Flexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Gorley, R.N. Clustering in Non-Parametric Multivariate Analyses. J. Exp. Mar. Biol. Ecol. 2016, 483, 147–155. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Krona-385.Pdf. BMC Bioinform. 2011, 12, 385. [Google Scholar]
- Herman, D.C.; Frankenberger, W.T. Bacterial Reduction of Perchlorate and Nitrate in Water. J. Environ. Qual. 1999, 28, 1018–1024. [Google Scholar] [CrossRef]
- Wierzchos, J.; Casero, M.C.; Artieda, O.; Ascaso, C. Endolithic Microbial Habitats as Refuges for Life in Polyextreme Environment of the Atacama Desert. Curr. Opin. Microbiol. 2018, 43, 124–131. [Google Scholar] [CrossRef]
- Navarro-González, R.; Rainey, F.A.; Molina, P.; Bagaley, D.R.; Hollen, B.J.; De La Rosa, J.; Small, A.M.; Quinn, R.C.; Grunthaner, F.J.; Cáceres, L.; et al. Mars-Like Soils in the Atacama Desert, Chile, and the Dry Limit of Microbial Life. Science 2003, 302, 1018–1021. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Chong, G.; Jensen, A. Neogene Evaporites in Desert Volcanic Environments: Atacama Desert, Northern Chile. Sedimentology 2001, 48, 1411–1431. [Google Scholar] [CrossRef]
- Smith, W.L.; Gadd, G.M. Reduction and Precipitation of Chromate by Mixed Culture Sulphate-Reducing Bacterial Biofilms. J. Appl. Microbiol. 2000, 88, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Walvoord, M.A.; Phillips, F.M.; Stonestrom, D.A.; Evans, R.D.; Hartsough, P.C.; Newman, B.D.; Striegl, R.G. A Reservoir of Nitrate Beneath Desert Soils. Science 2003, 302, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- McCalley, C.K.; Sparks, J.P. McCalley Abiotic Gas Formation Drives Nitrogen Loss from a Desert Ecosystem. Science 2009, 326, 837–841. [Google Scholar] [CrossRef]
- Jackson, W.A.; Böhlke, J.K.; Andraski, B.J.; Fahlquist, L.; Bexfield, L.; Eckardt, F.D.; Gates, J.B.; Davila, A.F.; McKay, C.P.; Rao, B.; et al. Global Patterns and Environmental Controls of Perchlorate and Nitrate Co-Occurrence in Arid and Semi-Arid Environments. Geochim. Cosmochim. Acta 2015, 164, 502–522. [Google Scholar] [CrossRef]
- Hallsworth, J.E. Salt Deliquescence Can Support Extraterrestrial Life. Nat. Astron. 2020, 4, 739–740. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Delgado-Baquerizo, M.; An King, P.T.; Benham, M.; Arca, V.; Power, S.A. Ecosystem Type and Resource Quality Are More Important than Global Change Drivers in Regulating Early Stages of Litter Decomposition. Soil. Biol. Biochem. 2019, 129, 144–152. [Google Scholar] [CrossRef]
- Stevenson, A.; Burkhardt, J.; Cockell, C.S.; Cray, J.A.; Dijksterhuis, J.; Fox-Powell, M.; Kee, T.P.; Kminek, G.; Mcgenity, T.J.; Timmis, K.N.; et al. Multiplication of Microbes below 0.690 Water Activity: Implications for Terrestrial and Extraterrestrial Life. Environ. Microbiol. 2015, 17, 257–277. [Google Scholar] [CrossRef]
- Rivera-Valentín, E.G.; Chevrier, V.F.; Soto, A.; Martínez, G. Distribution and Habitability of (Meta)Stable Brines on Present-Day Mars. Nat. Astron. 2020, 4, 756–761. [Google Scholar] [CrossRef]
- Davila, A.F.; Gómez-Silva, B.; de los Rios, A.; Ascaso, C.; Olivares, H.; McKay, C.P.; Wierzchos, J. Facilitation of Endolithic Microbial Survival in the Hyperarid Core of the Atacam Desert by Mineral Deliquescence. J. Geophys. Res. Biogeosci 2008, 113, G1. [Google Scholar] [CrossRef]
- Davila, A.F.; Duport, L.G.; Melchiorri, R.; Jänchen, J.; Valea, S.; De Los Rios, A.; Fairén, A.G.; Möhlmann, D.; McKay, C.P.; Ascaso, C.; et al. Hygroscopic Salts and the Potential for Life on Mars. Astrobiology 2010, 10, 617–628. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Adcock, C.T.; Bechtold, A.; Beck, P.; Benison, K.; Brown, A.; Cardarelli, E.L.; Carman, N.A.; Chide, B.; Christian, J.; et al. An Examination of Soil Crusts on the Floor of Jezero Crater, Mars. J. Geophys. Res. Planets 2023, 128, e2022JE007433. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil. Anal. Part Chem. Methods 1996, 9, 961–1010. [Google Scholar] [CrossRef]
- Zavalaga, C.B.; Hardesty, J.; Mori, G.P.; Chávez-Villavicencio, C.; Tello, A. Current Status of Peruvian Terns Sternula Lorata in Perú: Threats, Conservation and Research Priorities. Bird. Conserv. Int. 2009, 19, 175–186. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Chong, G.; Vega, M. Mineralogía y Evolución de Las Salmueras Madres En El Yacimiento de Nitratos Pedro de Valdivia, Antofagasta, Chile. Rev. Geológica Chile 1998, 25, 3–15. [Google Scholar] [CrossRef]
- Cockell, C.S.; Raven, J.A. Zones of Photosynthetic Potential on Mars and the Early Earth. Icarus 2004, 169, 300–310. [Google Scholar] [CrossRef]
- Zhang, H.; Bruns, M.A.; Logan, B.E. Perchlorate Reduction by a Novel Chemolithoautotrophic, Hydrogen-Oxidizing Bacterium; Environ. Microbiol. 2002, 4, 570–576. [Google Scholar]
- Cheng, Y.; Elrys, A.S.; Merwad, A.R.M.; Zhang, H.; Chen, Z.; Zhang, J.; Cai, Z.; Müller, C. Global Patterns and Drivers of Soil Dissimilatory Nitrate Reduction to Ammonium. Environ. Sci. Technol. 2022, 56, 3791–3800. [Google Scholar] [CrossRef]
- Connon, S.A.; Lester, E.D.; Shafaat, H.S.; Obenhuber, D.C.; Ponce, A. Bacterial Diversity in Hyperarid Atacama Desert Soils. J. Geophys. Res. Biogeosci 2007, 112, 1–9. [Google Scholar] [CrossRef]
- Costello, E.K.; Halloy, S.R.P.; Reed, S.C.; Sowell, P.; Schmidt, S.K. Fumarole-Supported Islands of Biodiversity within a Hyperarid, High-Elevation Landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 2009, 75, 735–747. [Google Scholar] [CrossRef]
- Campos, V.L.; Escalante, G.; Yañez, J.; Zaror, C.A.; Mondaca, M.A. Isolation of Arsenite-Oxidizing Bacteria from a Natural Biofilm Associated to Volcanic Rocks of Atacama Desert, Chile. J. Basic. Microbiol. 2009, 49, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Comparative Genomic Analysis Reveals Habitat-Specific Genes and Regulatory Hubs within the Genus Novosphingobium Roshan. MSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mormile, M.R.; Romine, M.F.; Garcia, M.T.; Ventosa, A.; Bailey, T.J.; Peyton, B.M. Halomonas campisalis sp.nov., a Denitrifying, Moderately Haloalkaliphilic Bacterium. Syst. Appl. Microbiol. 1999, 22, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Huang, J.; Guo, X.; Zhang, Y.; Liu, D.; Wu, R.; He, H.; Wang, J. Diversity of the Microbial Community and Cultivable Protease-Producing Bacteria in the Sediments of the Bohai Sea, Yellow Sea and South China Sea. PLoS ONE 2019, 14, e0215328. [Google Scholar] [CrossRef]
- Thomas, T.; Elain, A.; Bazire, A.; Bruzaud, S. Complete Genome Sequence of the Halophilic PHA-Producing Bacterium Halomonas sp. SF2003: Insights into Its Biotechnological Potential. World J. Microbiol. Biotechnol. 2019, 35, 50. [Google Scholar] [CrossRef]
- Nozawa-Inoue, M.; Scow, K.M.; Rolston, D.E. Reduction of Perchlorate and Nitrate by Microbial Communities in Vadose Soil. Appl. Environ. Microbiol. 2005, 71, 3928–3934. [Google Scholar] [CrossRef]

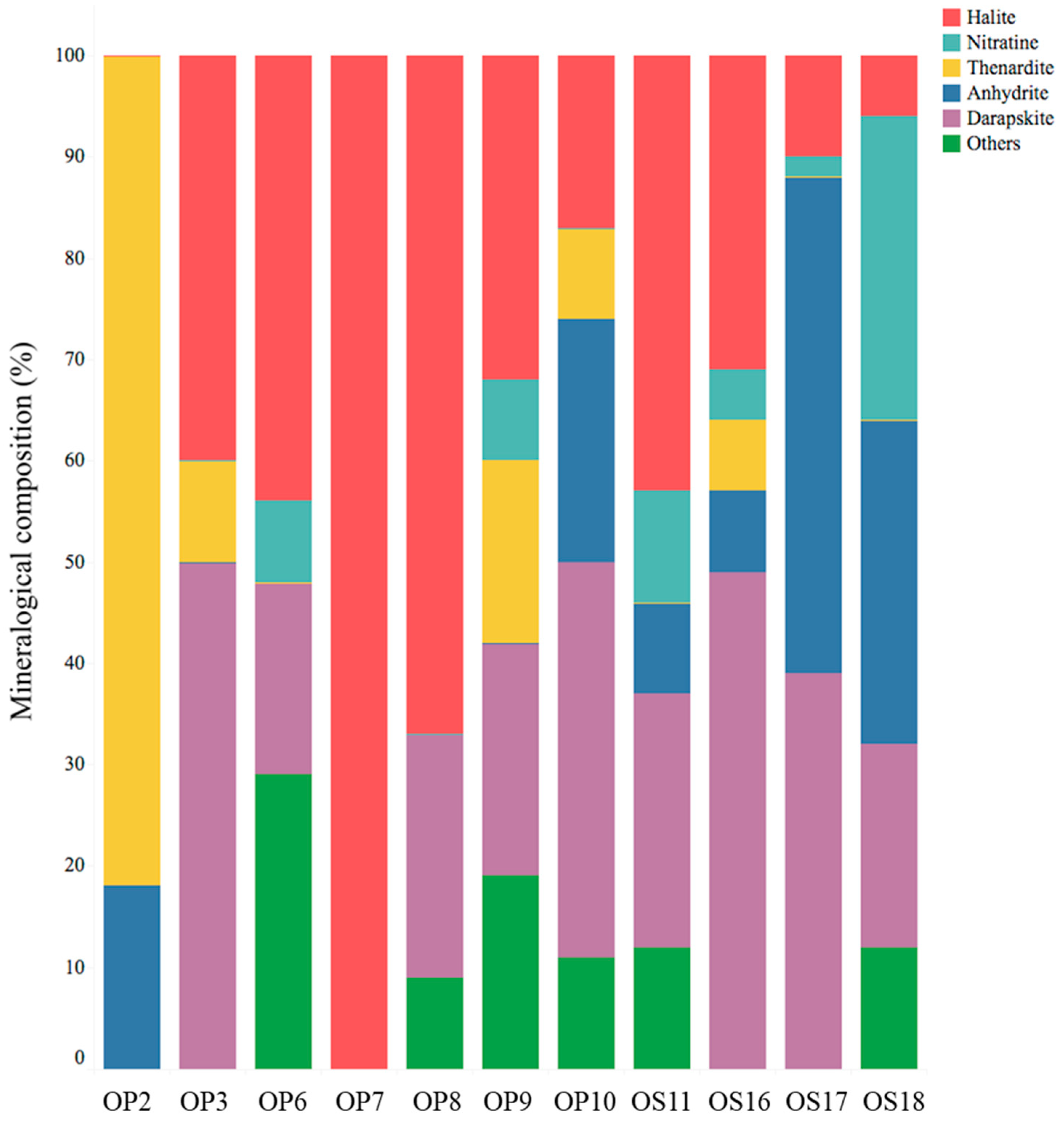
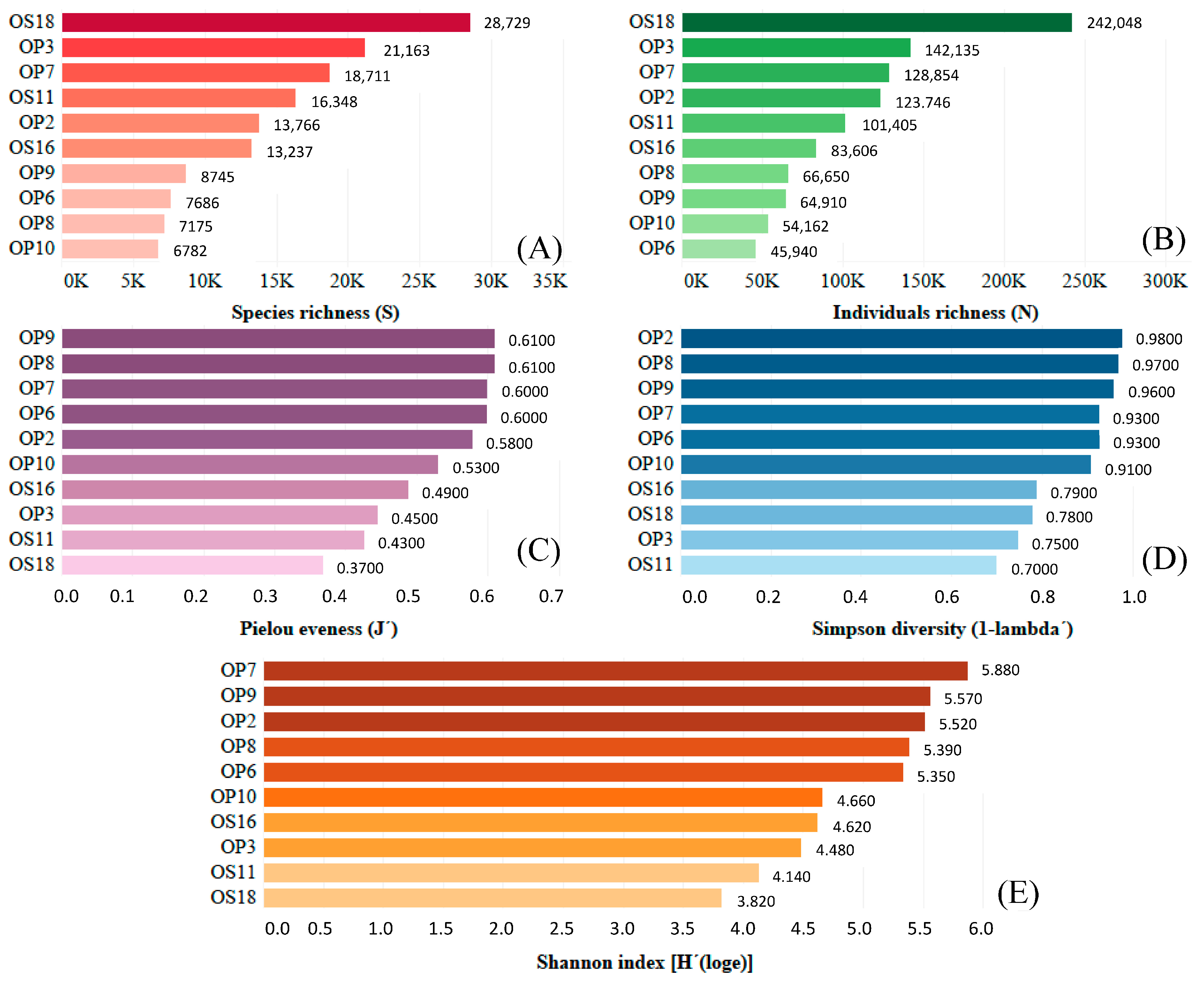
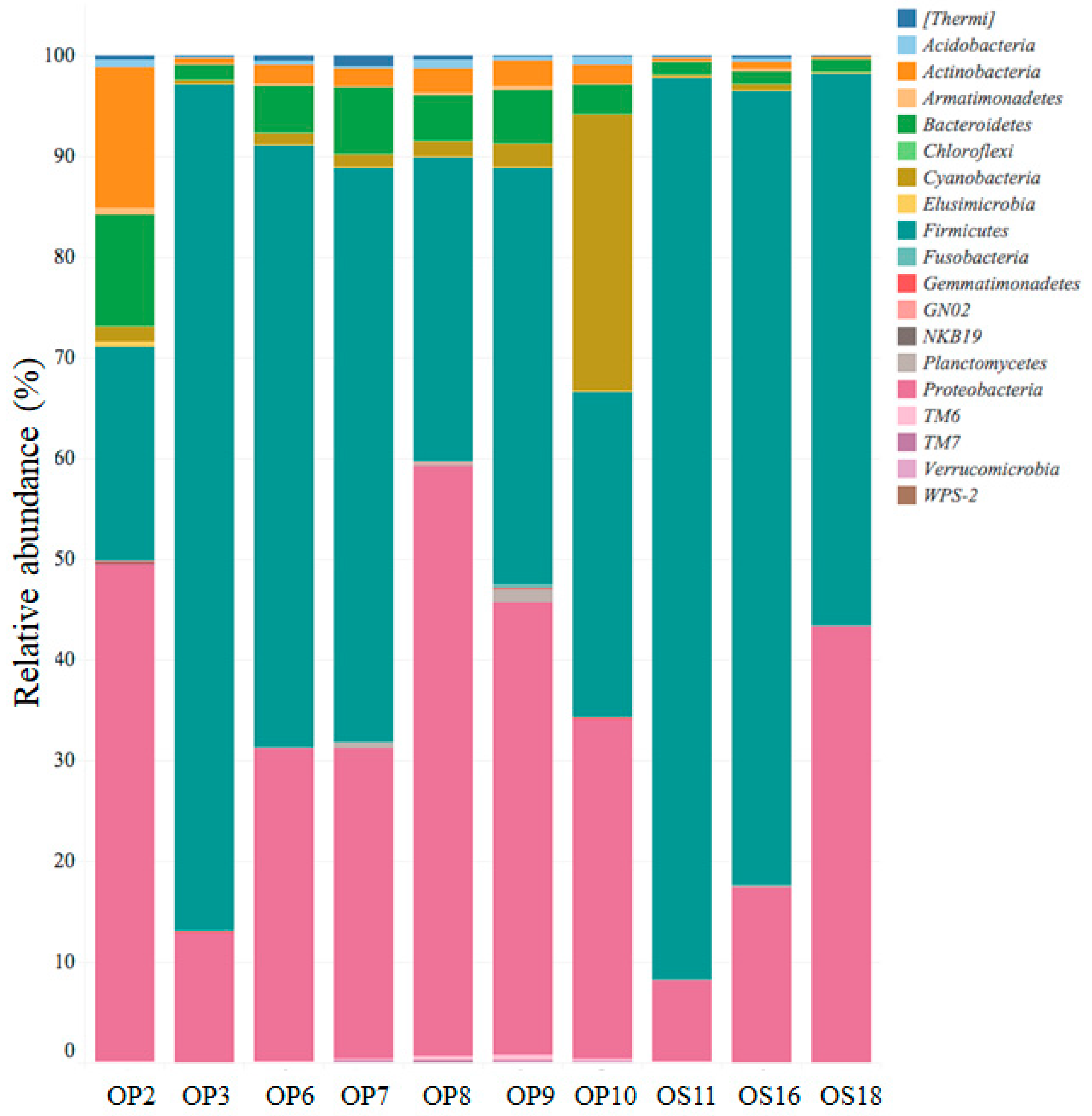
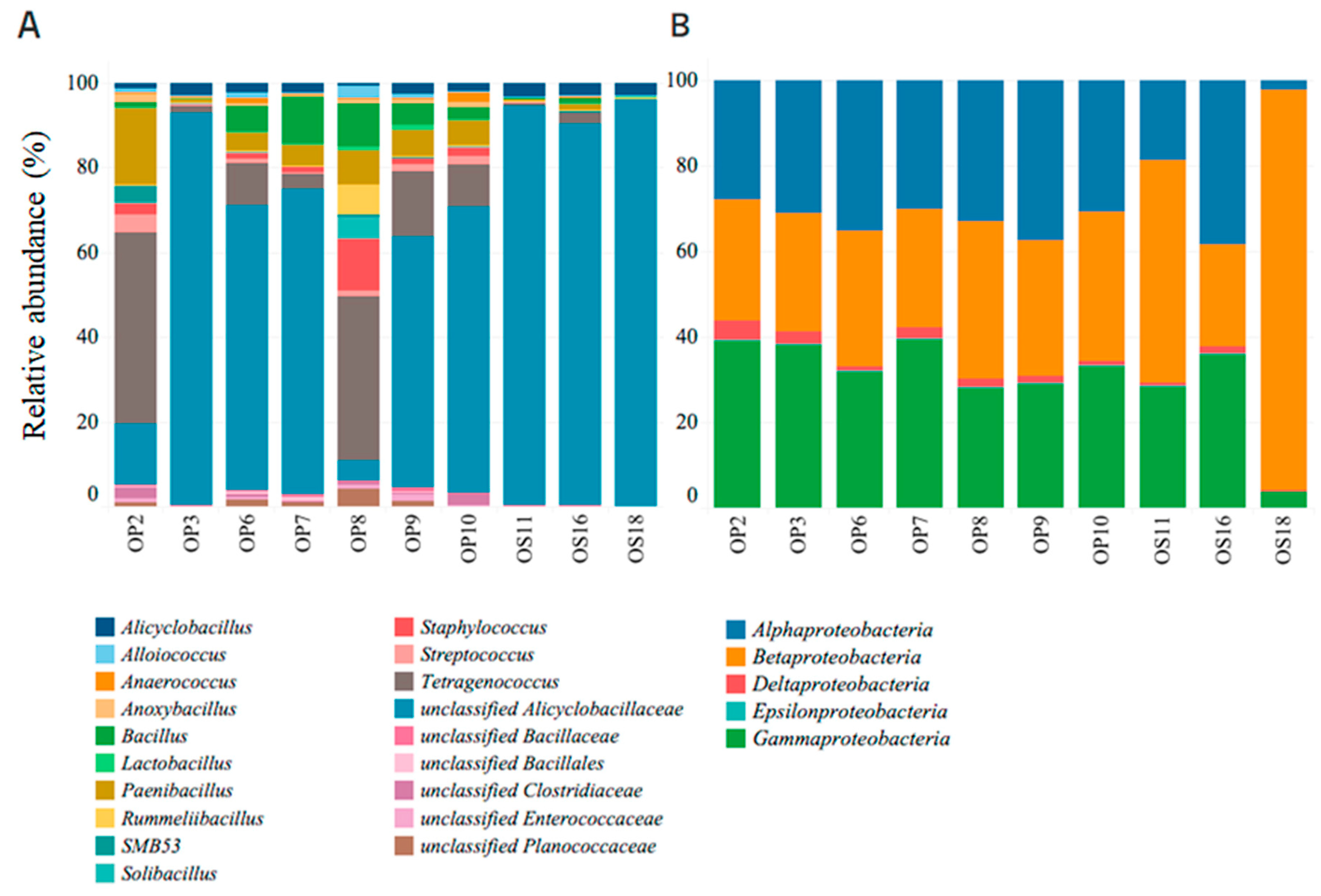
| Name | Site | Samples | Temp. (°C) | pH | Salinity (%) | Conductivity (mS/cm) | Aw | OC (%) | Dw (%) | Humidity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| OP1 | OP | Salts–thenardite mixture (surface) | 26.5 | 7.98 | 3.4 | 15.53 | 0.503 | 0.0737 | 0.002 | 0.0418 |
| OP2 | OP | Salts–thenardite mixture (deep) | 26.4 | 8.65 | 3.2 | 15.63 | 0.529 | 0.0224 | 0.033 | 0.0143 |
| OP3 | OP | White caliche | 26.6 | 8.54 | 3.4 | 21.49 | 0.505 | 0.0191 | 0.010 | 0.4106 |
| OP4 | OP | Sample with chromate | 23.9 | 6.26 | 3.0 | 15.92 | 0.617 | 0.0225 | 0.385 | Bdl * |
| OP5 | OP | Massive light greenish-yellow mineral | 26.7 | 8.04 | 3.0 | 15.56 | 0.590 | 0.0747 | 0.022 | Bdl |
| OP6 | OP | Moistened salt inside Socavon | 25.9 | 7.76 | 3.2 | 18.87 | 0.501 | 0.0498 | 0.013 | 0.3030 |
| OP7 | OP | Fibrous halite | 26.3 | 6.39 | 3.8 | 23.52 | 0.511 | 0.0242 | 0.007 | 0.3290 |
| OP8 | OP | Salts from the deep zone | 26.1 | 6.08 | 3.0 | 17.37 | 0.511 | 0.0959 | 0.008 | 0.6444 |
| OP9 | OP | Sample nitrous of porous texture | 23.4 | 8.43 | 3.0 | 17.04 | 0.542 | 0.0345 | 0.015 | 0.3431 |
| OP10 | OP | Yellow-green salts | 26.1 | 8.48 | 3.4 | 13.40 | 0.469 | 0.0774 | 0.012 | 0.8349 |
| OS11 | OS | Anhydrite (cast) pink salts | 26.4 | 7.58 | 3.0 | 16.50 | 0.496 | 0.0418 | 0.016 | 0.3997 |
| OS12 | OS | Mixed mineral | 25.0 | 9.06 | 1.4 | 6.00 | 0.521 | 0.3406 | 0.401 | 0.5071 |
| OS14 | OS | Mineral redeposited nitratine (yellow base) | 25.2 | 9.03 | 2.4 | 17.45 | 0.527 | 0.0413 | 0.006 | 0.1057 |
| OS16 | OS | Guano nitrate | 25.6 | 4.68 | 2.0 | 8.96 | 0.487 | 3.6568 | 0.012 | 0.8319 |
| OS17 | OS | Guano nitrate | 25.6 | 8.92 | 3.0 | 15.49 | 0.487 | 0.0709 | 0.010 | 1.3600 |
| OS18 | OS | Cement-type sample | 23.0 | 8.97 | 2.0 | 10.70 | 0.555 | 0.1593 | 0.021 | 0.7898 |
| Samples | Site | Nitrate (mg/kg) | Chloride (mg/kg) | Sulfate (mg/kg) | Perchlorate (mg/kg) |
|---|---|---|---|---|---|
| OP1 | OP | 22.8 | 2.4 | 28.5 | bdl * |
| OP2 | OP | 22.3 | 2.5 | 63.9 | bdl |
| OP3 | OP | 18.0 | 2.3 | 12.9 | bdl |
| OP4 | OP | 23.3 | 3.1 | 32.2 | bdl |
| OP5 | OP | 30.8 | 2.5 | 25.0 | bdl |
| OP6 | OP | 106.2 | 708 | 127.2 | bdl |
| OP7 | OP | 129.9 | 1.2 | 142.3 | bdl |
| OP8 | OP | 83.3 | 628 | 163 | bdl |
| OP9 | OP | 107.9 | 462 | 203.8 | bdl |
| OP10 | OP | 106.1 | 295 | 207.1 | bdl |
| OS11 | OS | 205.9 | 568 | 258.3 | bdl |
| OS12 | OS | Bdl | 380 | 188.4 | bdl |
| OS14 | OS | 562.7 | 465 | 68.6 | bdl |
| OS16 | OS | 67.4 | 143 | 147.6 | bdl |
| OS17 | OS | 156.4 | 226 | 296.5 | bdl |
| OS18 | OS | 133.2 | 101 | 198 | bdl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, M.; Avendaño, P.; Encalada, O.; Salazar-Ardiles, C.; Andrade, D.C.; Gómez-Silva, B.; Contreras, D.; Toro, N.; Arias, D.; Escudero, L.V. Uncovering Hidden Microbial Diversity in Nitrate/Iodide Deposits (NIDs) in the Domeyko District, Atacama Desert, Chile. Soil Syst. 2024, 8, 46. https://doi.org/10.3390/soilsystems8020046
Cortés M, Avendaño P, Encalada O, Salazar-Ardiles C, Andrade DC, Gómez-Silva B, Contreras D, Toro N, Arias D, Escudero LV. Uncovering Hidden Microbial Diversity in Nitrate/Iodide Deposits (NIDs) in the Domeyko District, Atacama Desert, Chile. Soil Systems. 2024; 8(2):46. https://doi.org/10.3390/soilsystems8020046
Chicago/Turabian StyleCortés, Mayra, Priscilla Avendaño, Olga Encalada, Camila Salazar-Ardiles, David C. Andrade, Benito Gómez-Silva, Daniel Contreras, Norman Toro, Dayana Arias, and Lorena V. Escudero. 2024. "Uncovering Hidden Microbial Diversity in Nitrate/Iodide Deposits (NIDs) in the Domeyko District, Atacama Desert, Chile" Soil Systems 8, no. 2: 46. https://doi.org/10.3390/soilsystems8020046
APA StyleCortés, M., Avendaño, P., Encalada, O., Salazar-Ardiles, C., Andrade, D. C., Gómez-Silva, B., Contreras, D., Toro, N., Arias, D., & Escudero, L. V. (2024). Uncovering Hidden Microbial Diversity in Nitrate/Iodide Deposits (NIDs) in the Domeyko District, Atacama Desert, Chile. Soil Systems, 8(2), 46. https://doi.org/10.3390/soilsystems8020046








