Rhizosheath: Roles, Formation Processes and Investigation Methods
Abstract
:1. Introduction
2. Rhizosheath
3. Factors Involved in Rhizosheath Formation
3.1. Root Hairs
3.2. Root- and Microbial-Derived Mucilages
3.3. Genetics
4. Benefits and Ecological Functions of Rhizosheaths
5. Methods for Rhizosheath Investigation
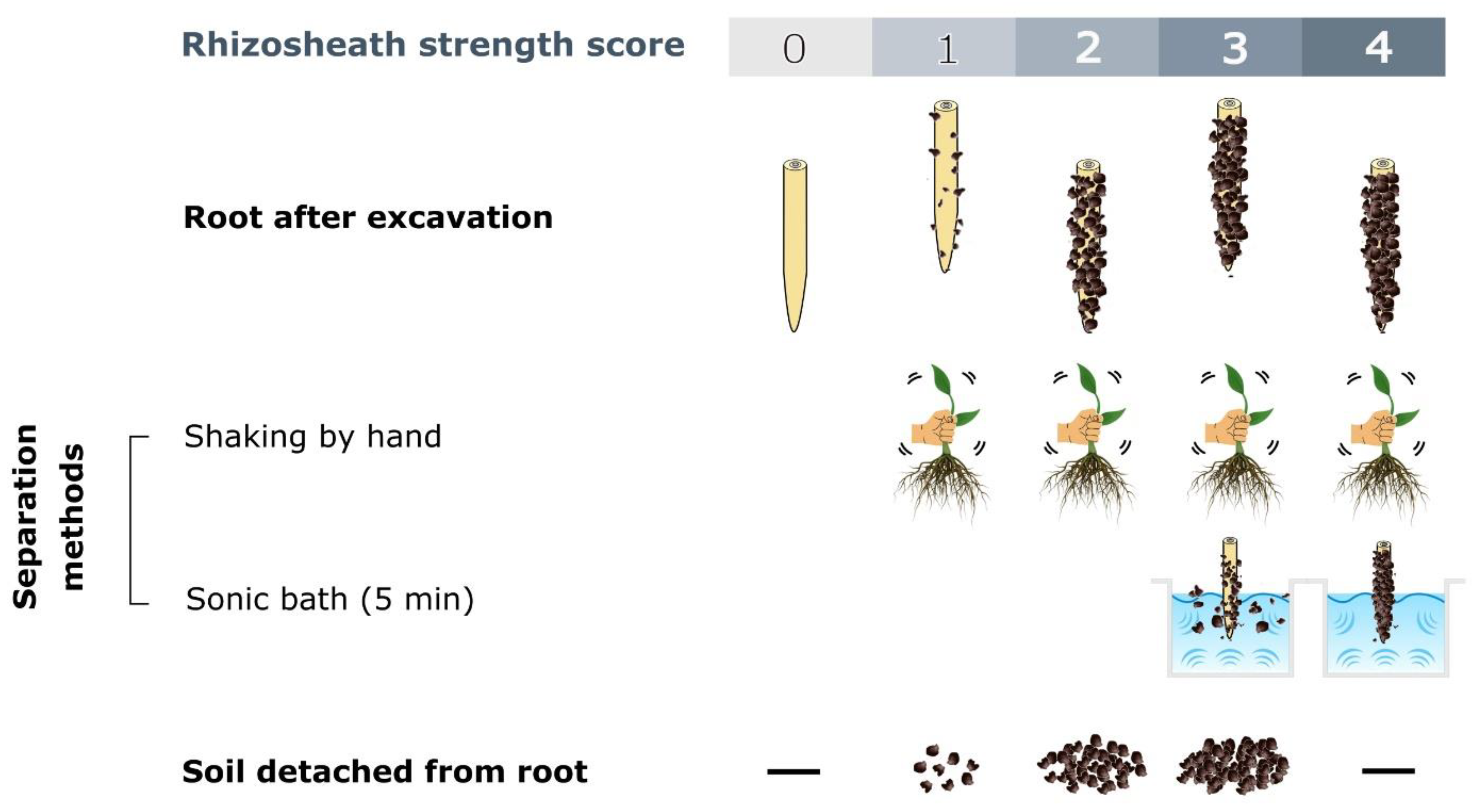
5.1. Rhizosheath Sampling
5.2. Rhizosheath Quantification
5.3. Genetic Studies
5.4. Microbial Investigations
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Hare, W.L.; Cramer, W.; Schaeffer, M.; Battaglini, A.; Jaeger, C.C. Climate Hotspots: Key Vulnerable Regions, Climate Change and Limits to Warming. Reg. Environ. Chang. 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Turco, M.; Palazzi, E.; von Hardenberg, J.; Provenzale, A. Observed Climate Change Hotspots: OBSERVED CLIMATE CHANGE HOTSPOTS. Geophys. Res. Lett. 2015, 42, 3521–3528. [Google Scholar] [CrossRef]
- Islam, S.N.; Winkel, J. Climate Change and Social Inequality; DESA Working Paper 152; Department of Economic & Social Affairs, United Nations: New York, NY, USA, 2017. [Google Scholar]
- FAO. Strategy on Climate Change 2022–2031; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- IPCC. Climate Change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- IPCC. Summary for policymakers. In Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022; Available online: https://report.ipcc.ch/ar6wg2/pdf/IPCC_AR6_WGII_SummaryForPolicymakers.pdf (accessed on 1 April 2022).
- Reynolds, W.D.; Bowman, B.T.; Drury, C.F.; Tan, C.S.; Lu, X. Indicators of Good Soil Physical Quality: Density and Storage Parameters. Geoderma 2002, 110, 131–146. [Google Scholar] [CrossRef]
- Heyder, U.; Schaphoff, S.; Gerten, D.; Lucht, W. Risk of Severe Climate Change Impact on the Terrestrial Biosphere. Environ. Res. Lett. 2011, 6, 034036. [Google Scholar] [CrossRef]
- Farkas, C.; Gelybó, G.; Bakacsi, Z.; Horel, Á.; Hagyó, A.; Dobor, L.; Kása, I.; Tóth, E. Impact of Expected Climate Change on Soil Water Regime under Different Vegetation Conditions. Biologia 2014, 69, 1510–1519. [Google Scholar] [CrossRef]
- Mills, R.T.E.; Gavazov, K.S.; Spiegelberger, T.; Johnson, D.; Buttler, A. Diminished Soil Functions Occur under Simulated Climate Change in a Sup-Alpine Pasture, but Heterotrophic Temperature Sensitivity Indicates Microbial Resilience. Sci. Total Environ. 2014, 473–474, 465–472. [Google Scholar] [CrossRef]
- Mondal, S. Impact of Climate Change on Soil Fertility. In Climate Change and the Microbiome; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 63, pp. 551–569. ISBN 978-3-030-76862-1. [Google Scholar]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Gelybó, G.; Tóth, E.; Farkas, C.; Horel, Á.; Kása, I.; Bakacsi, Z. Potential Impacts of Climate Change on Soil Properties. Agrokem. És Talaj. 2018, 67, 121–141. [Google Scholar] [CrossRef]
- Patil, A.; Lamnganbi, M. Impact of Climate Change on Soil Health: A Review. Int. J. Chem. Stud. 2018, 6, 2399–2404. [Google Scholar]
- Smith, J.L.; Halvorson, J.J.; Bolton, H. Soil Properties and Microbial Activity across a 500m Elevation Gradient in a Semi-Arid Environment. Soil Biol. Biochem. 2002, 34, 1749–1757. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the Design of Climate Change-Resilient Farming Systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Mayer-Gruner, P.; Uksa, M.; Abdullaeva, Y.; Pilz, S.; Schloter, M.; Lambers, H.; Kandeler, E. The Role of Microbes in the Increase of Organic Phosphorus Availability in the Rhizosheath of Cover Crops. Plant Soil 2022, 476, 353–373. [Google Scholar] [CrossRef]
- Etesami, H. Potential Advantage of Rhizosheath Microbiome, in Contrast to Rhizosphere Microbiome, to Improve Drought Tolerance in Crops. Rhizosphere 2021, 20, 100439. [Google Scholar] [CrossRef]
- Ndour, P.M.S.; Heulin, T.; Achouak, W.; Laplaze, L.; Cournac, L. The Rhizosheath: From Desert Plants Adaptation to Crop Breeding. Plant Soil 2020, 456, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Cao, Y.; Xu, W.; Zhang, Y. Rhizosheath Microbes Induce Root Immune Response under Soil Drying. Plant Signal. Behav. 2021, 16, 1920752. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Firmin, S.; Meglouli, H.; Laruelle, F.; Fontaine, J.; Lounès-Hadj Sahraoui, A.; Coutu, A.; Lambers, H.; Faucon, M. Interactions between Below-ground Traits and Rhizosheath Fungal and Bacterial Communities for Phosphorus Acquisition. Funct. Ecol. 2021, 35, 1603–1619. [Google Scholar] [CrossRef]
- Watt, M.; McCully, M.E.; Canny, M.J. Formation and Stabilization of Rhizosheaths of Zea mays. (Effect of Soil Water Content). Plant Physiol. 1994, 106, 179–186. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Valentine, T.A.; White, P.J.; Young, I.M.; George, T.S. Root Hair Length and Rhizosheath Mass Depend on Soil Porosity, Strength and Water Content in Barley Genotypes. Planta 2014, 239, 643–651. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Xu, F.; Ding, Y.; Gui, Y.; Zhang, J.; Xu, W. Root-Bacteria Associations Boost Rhizosheath Formation in Moderately Dry Soil through Ethylene Responses. Plant Physiol. 2020, 183, 780–792. [Google Scholar] [CrossRef]
- Burak, E.; Quinton, J.N.; Dodd, I.C. Root Hairs Are the Most Important Root Trait for Rhizosheath Formation of Barley (Hordeum Vulgare), Maize (Zea Mays) and Lotus Japonicus (Gifu). Ann. Bot. 2021, 128, 45–57. [Google Scholar] [CrossRef]
- Delhaize, E.; James, R.A.; Ryan, P.R. Aluminium Tolerance of Root Hairs Underlies Genotypic Differences in Rhizosheath Size of Wheat (Triticum aestivum) Grown on Acid Soil. New Phytol. 2012, 195, 609–619. [Google Scholar] [CrossRef]
- James, R.A.; Weligama, C.; Verbyla, K.; Ryan, P.R.; Rebetzke, G.J.; Rattey, A.; Richardson, A.E.; Delhaize, E. Rhizosheaths on Wheat Grown in Acid Soils: Phosphorus Acquisition Efficiency and Genetic Control. J. Exp. Bot. 2016, 67, 3709–3718. [Google Scholar] [CrossRef]
- Brown, L.K.; George, T.S.; Neugebauer, K.; White, P.J. The Rhizosheath—A Potential Trait for Future Agricultural Sustainability Occurs in Orders throughout the Angiosperms. Plant Soil 2017, 418, 115–128. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Yuan, W.; Zhang, Q.; Zhang, J.; Xu, W. Phosphorus Uptake Is Associated with the Rhizosheath Formation of Mature Cluster Roots in White Lupin under Soil Drying and Phosphorus Deficiency. Plant Physiol. Biochem. 2021, 166, 531–539. [Google Scholar] [CrossRef]
- Lynch, J.P. Roots of the Second Green Revolution. Aust. J. Bot. 2007, 55, 493. [Google Scholar] [CrossRef]
- Zhu, A.-M.; Wu, Q.; Liu, H.-L.; Sun, H.-L.; Han, G.-D. Isolation of Rhizosheath and Analysis of Microbial Community Structure around Roots of Stipa Grandis. Sci. Rep. 2022, 12, 2707. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The Holistic Rhizosphere: Integrating Zones, Processes, and Semantics in the Soil Influenced by Roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Siddique, K.H.M.; Simpson, R.J. Unwrapping the Rhizosheath. Plant Soil 2017, 418, 129–139. [Google Scholar] [CrossRef]
- Bergmann, D.; Zehfus, M.; Zierer, L.; Smith, B.; Gabel, M. Grass Rhizosheaths: Associated Bacterial Communities and Potential for Nitrogen Fixation. W. N. Am. Nat. 2009, 69, 105–114. [Google Scholar] [CrossRef]
- George, T.S.; Brown, L.K.; Ramsay, L.; White, P.J.; Newton, A.C.; Bengough, A.G.; Russell, J.; Thomas, W.T.B. Understanding the Genetic Control and Physiological Traits Associated with Rhizosheath Production by Barley (Hordeum Vulgare). New Phytol. 2014, 203, 195–205. [Google Scholar] [CrossRef]
- Karanja, J.K.; Aslam, M.M.; Qian, Z.; Yankey, R.; Dodd, I.C.; Weifeng, X. Abscisic Acid Mediates Drought-Enhanced Rhizosheath Formation in Tomato. Front. Plant Sci. 2021, 12, 658787. [Google Scholar] [CrossRef]
- Marasco, R.; Fusi, M.; Mosqueira, M.; Booth, J.M.; Rossi, F.; Cardinale, M.; Michoud, G.; Rolli, E.; Mugnai, G.; Vergani, L.; et al. Rhizosheath–Root System Changes Exopolysaccharide Content but Stabilizes Bacterial Community across Contrasting Seasons in a Desert Environment. Environ. Microb. 2022, 17, 14. [Google Scholar] [CrossRef]
- Puente, M.E.; Bashan, Y.; Li, C.Y.; Lebsky, V.K. Microbial Populations and Activities in the Rhizoplane of Rock-Weathering Desert Plants. I. Root Colonization and Weathering of Igneous Rocks. Plant Biol. 2004, 6, 629–642. [Google Scholar] [CrossRef]
- Haling, R.E.; Richardson, A.E.; Culvenor, R.A.; Lambers, H.; Simpson, R.J. Root Morphology, Root-Hair Development and Rhizosheath Formation on Perennial Grass Seedlings Is Influenced by Soil Acidity. Plant Soil 2010, 335, 457–468. [Google Scholar] [CrossRef]
- Haling, R.E.; Simpson, R.J.; Culvenor, R.A.; Lambers, H.; Richardson, A.E. Effect of Soil Acidity, Soil Strength and Macropores on Root Growth and Morphology of Perennial Grass Species Differing in Acid-soil Resistance. Plant Cell Environ. 2011, 34, 444–456. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chen, M.-X.; Zhang, Y.; Zhu, F.-Y.; Liu, Y.-G.; Tian, Y.; Fernie, A.R.; Ye, N.; Zhang, J. Comparative Metabolite Profiling of Two Switchgrass Ecotypes Reveals Differences in Drought Stress Responses and Rhizosheath Weight. Planta 2019, 250, 1355–1369. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Fusi, M.; Ramond, J.-B.; Merlino, G.; Booth, J.M.; Maggs-Kölling, G.; Cowan, D.A.; Daffonchio, D. Rhizosheath Microbial Community Assembly of Sympatric Desert Speargrasses Is Independent of the Plant Host. Microbiome 2018, 6, 215. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Dodd, I.C.; Waseem, M.; Weifeng, X. Rhizosheath: An Adaptive Root Trait to Improve Plant Tolerance to Phosphorus and Water Deficits? Plant Cell Environ. 2022, 45, 2861–2874. [Google Scholar] [CrossRef]
- Bailey, C.; Scholes, M. Rhizosheath Occurrence in South African Grasses. S. Afr. J. Bot. 1997, 63, 484–490. [Google Scholar] [CrossRef]
- Smith, R.J.; Hopper, S.D.; Shane, M.W. Sand-Binding Roots in Haemodoraceae: Global Survey and Morphology in a Phylogenetic Context. Plant Soil 2011, 348, 453–470. [Google Scholar] [CrossRef]
- Cheraghi, M.; Mousavi, S.M.; Zarebanadkouki, M. Functions of Rhizosheath on Facilitating the Uptake of Water and Nutrients under Drought Stress: A Review. Plant Soil 2023, 491, 1–25. [Google Scholar] [CrossRef]
- Mo, X.; Wang, M.; Zeng, H.; Wang, J. Rhizosheath: Distinct Features and Environmental Functions. Geoderma 2023, 435, 116500. [Google Scholar] [CrossRef]
- Tahir, M.; Mirza, M.S.; Hameed, S.; Dimitrov, M.R.; Smidt, H. Cultivation-Based and Molecular Assessment of Bacterial Diversity in the Rhizosheath of Wheat under Different Crop Rotations. PLoS ONE 2015, 10, e0130030. [Google Scholar] [CrossRef] [PubMed]
- Basirat, M.; Mousavi, S.M.; Abbaszadeh, S.; Ebrahimi, M.; Zarebanadkouki, M. The Rhizosheath: A Potential Root Trait Helping Plants to Tolerate Drought Stress. Plant Soil 2019, 445, 565–575. [Google Scholar] [CrossRef]
- Bochicchio, R.; Labella, R.; Vitti, A.; Nuzzaci, M.; Logozzo, G.; Amato, M. Root Morphology, Allometric Relations and Rhizosheath of Ancient and Modern Tetraploid Wheats (Triticum Durum Desf.) in Response to Inoculation with Trichoderma Harzianum T-22. Plants 2022, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liao, H.; Zhang, Y.; Yao, M.; Liu, J.; Sun, L.; Zhang, X.; Yang, J.; Wang, K.; Wang, X.; et al. Coordination of Root Auxin with the Fungus Piriformospora Indica and Bacterium Bacillus Cereus Enhances Rice Rhizosheath Formation under Soil Drying. ISME J. 2022, 16, 801–811. [Google Scholar] [CrossRef]
- Lei, Z.; Ding, Y.; Xu, W.; Zhang, Y. Microbial Community Structure in Rice Rhizosheaths under Drought Stress. J. Plant Ecol. 2023, 16, rtad012. [Google Scholar] [CrossRef]
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Ackah, F.K.; Amoah, K.K.; Nyarko, M.A.; Andoh, D.A. Quantifying Variations in Rhizosheath and Root System Phenotypes of Landraces and Improved Varieties of Juvenile Maize. Rhizosphere 2017, 3, 29–39. [Google Scholar] [CrossRef]
- de la Fuente Cantó, C.; Diouf, M.N.; Ndour, P.M.S.; Debieu, M.; Grondin, A.; Passot, S.; Champion, A.; Barrachina, C.; Pratlong, M.; Gantet, P.; et al. Genetic Control of Rhizosheath Formation in Pearl Millet. Sci. Rep. 2022, 12, 9205. [Google Scholar] [CrossRef]
- Adu, M.O.; Zigah, N.; Yawson, D.O.; Amoah, K.K.; Afutu, E.; Atiah, K.; Darkwa, A.A.; Asare, P.A. Plasticity of Root Hair and Rhizosheath Traits and Their Relationship to Phosphorus Uptake in Sorghum. Plant Direct 2023, 7, e521. [Google Scholar] [CrossRef] [PubMed]
- Duell, R.W.; Peacock, G.R. Rhizosheaths on Mesophytic Grasses. Crop Sci. 1985, 25, 880–883. [Google Scholar] [CrossRef]
- Bibikova, T.; Gilroy, S. Root Hair Development. J. Plant Growth Regul. 2002, 21, 383–415. [Google Scholar] [CrossRef]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root Hairs. Arab. Book 2014, 12, e0172. [Google Scholar] [CrossRef]
- McCully, M.E. ROOTS IN SOIL: Unearthing the Complexities of Roots and Their Rhizospheres. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef]
- Liu, T.; Ye, N.; Song, T.; Cao, Y.; Gao, B.; Zhang, D.; Zhu, F.; Chen, M.; Zhang, Y.; Xu, W.; et al. Rhizosheath Formation and Involvement in Foxtail Millet (Setaria italica) Root Growth under Drought Stress. J. Integr. Plant Biol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Morel, J.L.; Habib, L.; Plantureux, S.; Guckert, A. Influence of Maize Root Mucilage on Soil Aggregate Stability. Plant Soil 1991, 136, 111–119. [Google Scholar] [CrossRef]
- Czarnes, S.; Hallett, P.D.; Bengough, A.G.; Young, I.M. Root- and Microbial-Derived Mucilages Affect Soil Structure and Water Transport: Mucilages, Soil Structure and Sorptivity. Eur. J. Soil Sci. 2000, 51, 435–443. [Google Scholar] [CrossRef]
- Galloway, A.F.; Akhtar, J.; Marcus, S.E.; Fletcher, N.; Field, K.; Knox, P. Cereal Root Exudates Contain Highly Structurally Complex Polysaccharides with Soil-binding Properties. Plant J. 2020, 103, 1666–1678. [Google Scholar] [CrossRef]
- Di Marsico, A.; Scrano, L.; Labella, R.; Lanzotti, V.; Rossi, R.; Cox, L.; Perniola, M.; Amato, M. Mucilage from fruits/seeds of chia (Salvia hispanica L.) improves soil aggregate stability. Plant Soil 2018, 425, 57–69. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Kroener, E.; Holz, M.; Zarebanadkouki, M.; Carminati, A. Mucilage Exudation Facilitates Root Water Uptake in Dry Soils. Functional Plant Biol. 2014, 41, 1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Zhou, Q.; Hu, J.; Lei, Y.; Liu, W. Specific Rhizobacteria Responsible in the Rhizosheath System of Kengyilia Hirsuta. Front. Plant Sci. 2022, 12, 785971. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Brown, L.K.; Raffan, A.C.; George, T.S.; Bengough, A.G.; Roose, T.; Sinclair, I.; Koebernick, N.; Cooper, L.; Hackett, C.A.; et al. Plant Exudates May Stabilize or Weaken Soil Depending on Species, Origin and Time: Effect of Plant Exudates on Rhizosphere Formation. Eur. J. Soil Sci. 2017, 68, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Kroener, E.; Holz, M.; Zarebanadkouki, M.; Ahmed, M.; Carminati, A. Effects of Mucilage on Rhizosphere Hydraulic Functions Depend on Soil Particle Size. Vadose Zone J. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- McCully, M.E. Water Efflux from the Surface of Field-Grown Grass Roots. Observations by Cryo-Scanning Electron Microscopy. Physiol. Plant. 1995, 95, 217–224. [Google Scholar] [CrossRef]
- Ahmadi, K.; Zarebanadkouki, M.; Ahmed, M.A.; Ferrarini, A.; Kuzyakov, Y.; Kostka, S.J.; Carminati, A. Rhizosphere Engineering: Innovative Improvement of Root Environment. Rhizosphere 2017, 3, 176–184. [Google Scholar] [CrossRef]
- Moreno-Espíndola, I.P.; Rivera-Becerril, F.; de Jesús Ferrara-Guerrero, M.; De León-González, F. Role of Root-Hairs and Hyphae in Adhesion of Sand Particles. Soil Biol. Biochem. 2007, 39, 2520–2526. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic Mechanisms of Abiotic Stress Tolerance That Translate to Crop Yield Stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Galloway, A.F.; Knox, P.; Krause, K. Sticky Mucilages and Exudates of Plants: Putative Microenvironmental Design Elements with Biotechnological Value. New Phytol. 2020, 225, 1461–1469. [Google Scholar] [CrossRef]
- Young, I.M. Variation in Moisture Contents between Bulk Soil and the Rhizosheath of Wheat (Triticum aestivum L. Cv. Wembley). New Phytol. 1995, 130, 135–139. [Google Scholar] [CrossRef]
- North, G.B.; Nobel, P.S. Drought-induced changes in soil contact and hydraulic conductivity for roots of Opuntia ficus-indica with and without rhizosheaths. Plant Soil 1997, 191, 249–258. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Kroener, E.; Benard, P.; Zarebanadkouki, M.; Kaestner, A.; Carminati, A. Drying of Mucilage Causes Water Repellency in the Rhizosphere of Maize: Measurements and Modelling. Plant Soil 2016, 407, 161–171. [Google Scholar] [CrossRef]
- Marasco, R.; Fusi, M.; Ramond, J.-B.; Van Goethem, M.W.; Seferji, K.; Maggs-Kölling, G.; Cowan, D.A.; Daffonchio, D. The Plant Rhizosheath–Root Niche Is an Edaphic “Mini-Oasis” in Hyperarid Deserts with Enhanced Microbial Competition. ISME Commun. 2022, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.P.C.; Trautmann, S.; Buegger, F.; Felde, V.J.M.N.L.; Pausch, J.; Müller, C.W.; Kögel-Knabner, I. Role of Root Hair Elongation in Rhizosheath Aggregation and in the Carbon Flow into the Soil. Biol. Fertil. Soils 2023, 59, 351–361. [Google Scholar] [CrossRef]
- Pathan, S.I.; Ceccherini, M.T.; Sunseri, F.; Lupini, A. Rhizosphere as Hotspot for Plant-Soil-Microbe Interaction. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R.S., Pathan, S.I., Ceccherini, M.T., Eds.; Springer: Singapore, 2020; pp. 17–43. ISBN 9789811372636. [Google Scholar]
- Ndour, P.M.S.; Gueye, M.; Barakat, M.; Ortet, P.; Bertrand-Huleux, M.; Pablo, A.-L.; Dezette, D.; Chapuis-Lardy, L.; Assigbetsé, K.; Kane, N.A.; et al. Pearl Millet Genetic Traits Shape Rhizobacterial Diversity and Modulate Rhizosphere Aggregation. Front. Plant Sci. 2017, 8, 1288. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Rathjen, T.M.; Cavanagh, C.R. The Genetics of Rhizosheath Size in a Multiparent Mapping Population of Wheat. J. Exp. Bot. 2015, 66, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Pueyo, J.J.; Pang, J.; Yang, J.; Chen, W.; Chen, H.; Waseem, M.; Li, Y.; Zhang, J.; Xu, W. Root Acid Phosphatases and Rhizobacteria Synergistically Enhance White Lupin and Rice Phosphorus Acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]
- Streit, W.R.; Schmitz, R.A. Metagenomics—The Key to the Uncultured Microbes. Curr. Opin. Microbiol. 2004, 7, 492–498. [Google Scholar] [CrossRef]
- Baldrian, P. The Known and the Unknown in Soil Microbial Ecology. FEMS Microbiol. Ecol. 2019, 95, fiz005. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The Unseen Rhizosphere Root–Soil–Microbe Interactions for Crop Production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addesso, R.; Sofo, A.; Amato, M. Rhizosheath: Roles, Formation Processes and Investigation Methods. Soil Syst. 2023, 7, 106. https://doi.org/10.3390/soilsystems7040106
Addesso R, Sofo A, Amato M. Rhizosheath: Roles, Formation Processes and Investigation Methods. Soil Systems. 2023; 7(4):106. https://doi.org/10.3390/soilsystems7040106
Chicago/Turabian StyleAddesso, Rosangela, Adriano Sofo, and Mariana Amato. 2023. "Rhizosheath: Roles, Formation Processes and Investigation Methods" Soil Systems 7, no. 4: 106. https://doi.org/10.3390/soilsystems7040106
APA StyleAddesso, R., Sofo, A., & Amato, M. (2023). Rhizosheath: Roles, Formation Processes and Investigation Methods. Soil Systems, 7(4), 106. https://doi.org/10.3390/soilsystems7040106







