Abstract
Pesticides are extensively used in agriculture to prevent infestation of crops, control plant-associated diseases and pests, and increase crop productivity. With regards to typical agricultural practice, tank mixing of two or more plant protection products or the subsequent applications of herbicides, fungicides, and insecticides are common application strategies to improve pest control. Our study provides evidence that the fungicide mixture consisting of mancozeb, metalaxyl-M, and chlorothalonil, each applied according to their recommended field rates, retarded the degradation of the phenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MPCA) in soil. MCPA dissipation times were between 1.6 and 1.9 days without and 2.5–3.5 days with co-applied fungicides. Furthermore, the proportions of extractable residues, non-extractable residues, mineralization, volatile organic compounds, and MPCA metabolism were altered by the fungicide mixture, i.e., considerably lower amounts of the main transformation product of MCPA, 4-chloro-2-methylphenol, were formed. The effects induced by the fungicides persisted throughout the experiment. Our results demonstrate that the current situation of considering individual active substances in the authorization process for plant protection products could lead to a discrepancy in the exposure assessment for humans and the environment. For specifically these cases, this calls into question whether the legally required level of protection is provided.
1. Introduction
Pesticides are extensively used in agriculture to prevent infestation of crops, control plant-associated diseases and pests, increase crop productivity, and preserve plant products [1]. However, unintended pollution of air, soil, and water by spray drift, volatilization, leaching, and surface runoff [2] poses a threat to human, non-target, and beneficial species. All chemicals, including pesticides, potentially form varying amounts of non-extractable residues (NER) in soil [3,4]. Currently, for pesticides, the state of the art regards total NER as dissipation irrespective of their formation [5,6]. This contradicts the fact that sequestered/entrapped NER can potentially remobilize into the environment [7]. Therefore, the regulatory authority [8] considers including NER type I for REACH chemicals in the persistence assessment.
By tank mixing (TM) or the subsequent applications of herbicides, fungicides, and insecticides [9], pest control can be improved, but environmental contamination increases. In the EU, Regulation (EC) No 1107/2009 [1] partially addresses this concern and differentiates between I) recommended and II) required TM, which are evaluated and declared as such by the authorities. In contrast, untested TM has not been approved [10]. In Germany, the implementation of Regulation (EC) No 1107/2009 is governed by the Plant Protection Act, which also does not provide any regulations on the use of untested TM [11].
There is evidence that pesticide mixtures might affect the dissipation kinetics of other pesticides present in the soil. For example, White et al. [12], Swarcewicz and Gregorczyk [13], and Swarcewicz et al. [14] reported a significantly lower dissipation for the herbicides metolachlor, pendimethalin, and linuron in the presence of the fungicides chlorothalonil, mancozeb, and mancozeb plus thiamethoxam in soil. Overall, these studies highlight the importance of investigating not only the fate of an individual pesticide but also that of the tank mixes/spray series, otherwise leading to discrepancies in the risk assessment. Despite this, only a few studies have examined the effects of such mixtures on the degradation half-life of active substances in soil.
Therefore, our study investigates the effects of the fungicides mancozeb, metalaxyl-M, and chlorothalonil applied as (I) tank mix, (II) spray series, and (III) combination of I + II on the degradation of the phenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) in a sandy loam soil. We solely focused on investigating NER types I and II, as these are considered as being potentially remobilizable residues in persistence assessments. The probability of type II release is much lower than that of type I. This is because type II is considered as being “irreversibly” bound and can be released only in minute amounts and at very slow rates, if at all. Since type III bioNER comprises biomolecules, they are interesting from the scientific point of view but of no environmental concern. Given this fact and that their determination is extremely labor intensive, they were outside the scope of our investigations. A distinctive feature was that realistic fungicide concentrations, as typically found in the environment after appropriate use of the pesticides, were tested. The fungicides mancozeb, metalaxyl-M, and chlorothalonil are applied throughout the season to provide high levels of protection against Phytophthora infestans (Mont.) de Bary, the pathogen responsible for the potato late blight [15]. Moreover, Knillmann et al. [16] evaluated almost 900 PPP spray series applied in 12 different main crops for their environmental risk. Among others, a mixture consisting of MCPA, mancozeb, metalaxyl-M, and chlorothalonil was identified as a typical treatment regimen for potatoes. For these reasons, we selected this mixture for investigation. Metalaxyl-M is a systemic fungicide used to control diseases caused by Phytophthora, e.g., late blight of potatoes and tomatoes [17]. It is often co-applied with mancozeb or chlorothalonil [15,18]. Mancozeb is a contact fungicide used on, e.g., wheats, onions, and potatoes [19]. Chlorothalonil is the second most used fungicide on peanuts, onions, potatoes, and tomatoes [20]. MCPA is one of the most used herbicides for the control of broad-leaf weeds in cereals and grass seed crops [21] and was selected as a model compound, as the biodegradation is well studied [22].
2. Materials and Methods
2.1. Chemicals
[Ring-U-14C] MCPA was purchased from the Institute of Isotopes Co., Ltd. (Izotop, Budapest, Hungary) with a specific radioactivity of 10.349 MBq mg−1, molar activity of 2097 MBq mmol−1, and radiochemical purity of ≥98.74%. Non-labeled MCPA (purity 99.5%) and mancozeb (purity 78.4%) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Metalaxyl-M (PESTANAL®) and chlorothalonil (PESTANAL®) were purchased from Sigma Aldrich (St. Louis, MO, USA). In Supporting Information Table S1, the properties of the test substances are summarized.
2.2. Soil
RefeSol soils are fully characterized, validated, and approved for accreditation studies by the Federal Environment Agency [23]. RefeSol 01-A has a significant area representation and is exemplary for the current soil situation in Germany. It resembles the soils frequently used for laboratory tests (e.g., LUFA 2.2 and lysimeter soil) and therefore is suitable for testing according to the Federal Soil Protection and Contaminated Sites Ordinance. RefeSol 01-A soil was purchased from the Fraunhofer Institute for Molecular Biology and Applied Ecology (Schmallenberg, Germany). The soil was sampled at 51°09′ N, 8°18′ E, Schmallenberg, Germany at a depth of 0–25 cm, and sieved to 2 mm. RefeSol 01-A is a sandy loam soil (74.00% sand, 17.70% silt, and 5.70% clay) with a pHCaCl2 of 5.61, and an organic carbon content of 0.93%. The total nitrogen content is 0.97 g kg−1, maximum water holding capacity (WHCmax) 293 g kg−1 and cation exchange capacity is 11.6 mmolc kg−1.
2.3. Studies on Aerobic Degradation of 14C-MCPA
The studies were performed according to OECD 307 to investigate the transformation pathway of a test substance under aerobic conditions [24]. Several studies have shown that the abiotic removal of MCPA is negligible compared to biodegradation [25,26,27]. Specifically, Mierzejewska et al. [28] performed incubation experiments amended with sterile soil extracts plus MCPA. After 24 days of incubation, 12–19% of MCPA was removed from samples with sterile soil extract. In contrast, 99–100% of MCPA was removed from samples with non-sterile soil extract, confirming that MCPA degradation is mainly mediated by biotic transformation processes. For this reason, no sterile incubation experiments were performed. The application rates (g a.s. ha−1) of the pesticides tested in this study were taken from a study conducted by Knillmann et al. [16]. These were converted into soil concentrations, assuming a soil depth of 5 cm and a soil density of 1.5 g cm−3. In this way, the examined treatment concentrations reflect the field-applied rates. One set of triplicate individual samples per scenario and interval was tested. The application schemes of the different treatments are provided in Supporting Information Figure S1. Mancozeb, metalaxyl-M, and MCPA solutions were prepared in ultrapure water [1 mg mL−1]. Chlorothalonil was dissolved in acetonitrile [1.5 mg mL−1]. The total amount of acetonitrile added to the soil corresponded to 0.09% (v/w). For 14C-MCPA, working solutions with 1.000 MBq, 1.667 MBq, and 6.667 MBq mL−1 ultrapure water were prepared. 14C-MCPA was mixed with non-labeled MCPA to achieve a total concentration of 53.3 µg 100 g−1 dry weight soil in each sample of the three fungicide application scenarios. These concentrations had been selected to ensure that the same amount of water was added to the samples and, therefore, the same soil moisture was provided. A total of 100 g dry weight (DW) soil was adjusted to 50% WHCmax by adding 8 mL of tap water. For the spray series, the fungicides were subsequently applied on day 14 (170.7 µg mancozeb plus 10.3 µg metalaxyl-M 100 g−1 DW soil) and day 7 (133.3 µg chlorothalonil 100 g−1 DW soil) prior to 14C-MCPA application (53.3 µg 100 g−1 DW soil) on day 0, while for the tank mix, the same fungicide concentrations plus 14C-MCPA were simultaneously applicated on day 0. The third treatment represents a combination of the spray series and tank mix, with applications on day 14 (mancozeb plus metalaxyl-M), day 7 (chlorothalonil), and day 0 (mancozeb, metalaxyl-M, chlorothalonil, and 14C-MCPA). Every 7 days, the soil water content was checked by gravimetric measurement and readjusted as required. Every 14 days, the soda lime was collected and replaced by fresh soda lime. All approaches were incubated at 18 ± 2 °C in the dark for 0, 7, 14, 28, and 56 days. At given time intervals, the extractable residues, non-extractable residues (NERs), mineralized, and volatile fractions of 14C-MCPA were examined. Additionally, NERs of type I (sequestered residues) and II (covalently bound residues) were determined by silylation and calculation, respectively.
2.4. Distribution of Applided Radioactivity (AR)
A graphical representation of the methodological approach is shown in Supporting Information Figure S2.
Extractable Residues (ERs): The entire soil was sequentially extracted 3 times with 70 mL of aqueous 0.01 M CaCl2, mimicking the ionic strength of the average salt concentration in soils [29], and once with 70 mL of MeOH on an orbital shaker (GFL, Burgwedel, Germany) at 170–180 rpm for 15 min, followed by a centrifugation step at 15,300× g for 15 min (Avanti J-20 XPI, Beckman Coulter, Brea, CA, USA). In the case of the CaCl2 extractions, the supernatants were pooled. Additionally, an exhaustive Soxhlet extraction was performed with 200 mL of MeOH under continuous reflux for 6 h. After each extraction step, the supernatant was collected. Two aliquots were mixed with the LSC cocktail Ultima Gold XR (PerkinElmer, Waltham, MA, USA) and subjected to liquid scintillation counting (LSC) using a Hidex 300 SL (Turku, Finland).
Non-Extractable Residues (NERs): Five aliquots of 0.1–0.2 g of the exhaustively extracted soil were sampled per replicate (in total n = 15) and combusted at 900 °C for 4 min using a biological oxidizer (OX-501; Zinsser Analytic, Frankfurt am Main, Germany). The released 14CO2 was absorbed by the scintillation cocktail Oxysolve C-400 (Zinsser Analytic, Frankfurt am Main, Germany), and the radioactivity was determined by LSC.
Mineralization (MIN): The soda lime (15 g) was dissolved by dropwise addition of 60 mL of 25% HCl under constant stirring. The released 14CO2 was absorbed by the scintillation cocktail Oxysolve C-400, and the radioactivity was determined by LSC.
Volatile Organic Compounds (VOCs): The paraffin-soaked glass wool was extracted with 20 mL of n-hexane for 10 min by an ultrasonic bath (Transsonic T460; Elma, Singen, Germany). The radioactivity in the n-hexane extract was determined by LSC.
2.5. Silylation of Nonextractable 14C-MCPA Residues
The silylation was performed according to Kästner et al. [30], with slight modifications. All solvents were dried over a 0.3 nm molecular sieve and the silylation reaction was kept under an argon atmosphere (99.996 vol % Ar; Westfalen, Münster, Germany). Two aliquots of 6 g of the exhaustively extracted soil were sampled per replicate (in total n = 4) in a Schenk flask. The soil was dried at 105 °C for 30 min prior to the addition of 1.5 g of NaOH micro granules, 30 mL of chloroform and 15 mL of trimethylchlorosilane (TMCS). The suspension was stirred at 300–700 rpm for 3 h. Another 1.5 g of NaOH micro granules and 10 mL of TMCS were added, and the stirring was continued overnight. The following day, the entire suspension was transferred into a centrifuge beaker and centrifuged at 2800× g for 10 min. The supernatant was collected in a Schott bottle. The Schenk flask was rinsed with 20 mL of acetone and the washing solution added to the residues in the centrifuge beaker and shaken at 160 rpm for 2 min. Then, the residues were centrifuged at 2800× g for 10 min and the supernatant collected in the Schott bottle. This step was repeated with another 10 mL of acetone. The total acetone volume was 30 mL. Thereafter, the Schenk flask was rinsed with 20 mL of chloroform and the washing, and extraction steps were performed analogously to the ones with acetone. The total chloroform volume was 30 mL. The radioactivity of the combined supernatant (=NER type I) was determined by LSC. The NER type II fraction was calculated using a mass balance approach (total NER minus NER type I) [3].
2.6. Analysis by Radio—Thin-Layer Chromatography (TLC)
Prior to analysis by thin-layer chromatography (TLC), extracts < 833.33 Bq mL−1 were concentrated by rotary evaporation (Büchi, Flawil, Switzerland). A total of 166.67 Bq each of the CaCl2, MeOH, Soxhlet extracts and 14C-MCPA standard were applied to pre-coated silica gel plates (200 × 200 mm, 0.25 mm, SIL G-25 UV254, Macherey-Nagel, Düren, Germany) in strip form with a width of 1 cm using a TLC spotter Linomat 5 (Camag, Muttenz, Switzerland). The dosing rates were 30 nL s−1 for the CaCl2, and MeOH extracts, and 150 nL s−1 for the Soxhlet extracts. A total of 50 µg of non-labeled 4-chloro-2-methylphenol [4C2MP, stock 1 mg mL−1 MeOH] was used as reference compound. The matching of the radioactive signal with the retention factor (Rf) served as indication that MCPA had undergone aerobic degradation and 4C2MP being formed. The plates were developed in chambers saturated with 2% acetic acid in toluene: ethyl acetate (9:1, v/v; total volume 100 mL). 14C-MCPA was quantified by TLC detector RITA Star (Raytest, Straubenhardt, Germany), and non-labeled 4C2MP was detected by fluorescence quenching at 254 nm under a UV lamp (Camag, Muttenz, Switzerland).
2.7. Determination of the Dissipation Time 50 (DT50)
The dissipation time of MCPA was modeled with the software CAKE (Computer Assisted Kinetic Evaluation), version v3.4, by Tessella Ltd., Abingdon, Oxfordshire, UK [31]. The proportions of parent MCPA (% AR) derived from the extractable residues on days 0, 7, and 14 were considered for modeling. A total of 28 and 56 days after MCPA application, MCPA degradation had advanced to the extent that radioactivity was insufficient for TLC analysis; thus, modeling was based on three time points. Four kinetic models, i.e., SFO (single first-order), FOMC (first-order multi-compartment), DFOP (double first-order in parallel), and HS (hockey stick), were tested [5].
2.8. Statistical Data Analysis
The data were visualized using GraphPad Prism version 8.3.0 [32]. Means and standard deviations were calculated using Microsoft Excel 2016 version 16.0 [33]. Data analysis was performed with the program R, version 4.0.5 [34]. A two-tailed t-test [35] was used to identify significant differences between the treatments without and with fungicides. The null hypothesis, “the fungicide application does not alter the degradation of 14C-MCPA”, was rejected when the calculated p-value was <0.05. The alternative hypothesis would be that the fungicide application alters the degradation of 14C-MCPA. The requirements for using the t-test (t.test) are the normal distribution of the data and variance homogeneity for the two independent groups. For this, the Shapiro–Wilk (shapiro.test) [36,37] and the Levene test (leveneTest) [38] were respectively run. If in less than 5% of the tested cases the assumptions were violated, normal distribution and variance homogeneity were still adopted for all t-tests. The sample size was three. The t-tests were not corrected for multiple comparisons, as for every treatment at each time point, an internal and independent control was included in the experimental setup. The statistical analyses of the data are provided in the Supporting Information.
3. Results and Discussion
3.1. Distribution of Applied Radioactivity (AR)
Recoveries were 95.7–106.3% AR, 84.8–95.0% AR, and 89.7–103.9% AR for the combination (SQTM), tank mixes (TM), and spray series (SQ), respectively. The corresponding controls showed a recovery of 90.0–103.5% AR, 83.8–103.5% AR, and 84.1–114.3% AR (Supporting Information Figure S3).
3.1.1. Volatile Organic Compounds (VOCs)
In all treatments, no or negligible quantities of VOC were formed (0.00–0.01% AR). Therefore, low volatility was determined for MCPA and its metabolites, in line with the data of Paszko et al. [39]. However, Comoretto et al. [40] found that during the application, about 0.3% of the applied MPCA was released into the air via spray drift.
3.1.2. Extractable Residues (ERs)
The total extractable residues (ER) of MCPA were obtained by sequential aqueous, organic solvent and exhaustive extractions using 0.01 M CaCl2, MeOH, and Soxhlet conditions, respectively (Figure 1).
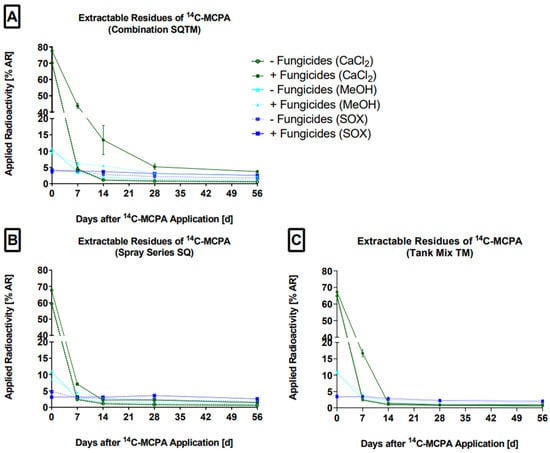
Figure 1.
The extractable residues (% AR) of MCPA in the 0.01 M CaCl2, MeOH, and Soxhlet (SOX) extracts on days 0, 7, 14, 28, and 56 are presented. (A) SQTM−F: control, SQTM+F: combination (B) SQ-F: control, SQ+F: spray series and (C) TM-F: control, TM+F: tank mix. The mean values and standard deviations were derived from triplicates (n = 3).
With 0.01 M CaCl2, most MCPA was extracted (readily desorbable fraction), while with pure MeOH at room temperature (desorbable fraction) and under Soxhlet conditions over 6 h (slowly desorbable fraction), the MCPA proportions were lower. This finding can be explained by the high water solubility of MCPA. In the fungicide treatments, ER was higher compared to the control. This could be shown for each extraction method for all measured time points, except for day 0, where less MCPA was extracted by MeOH and under Soxhlet conditions. Overall, the fungicides exhibited a greater influence on the extractability of MCPA by CaCl2 than by MeOH and Soxhlet conditions. In support of the hypothesis that the fungicides affected MCPA degradation, this result might be explained by the inhibition of the soil microorganisms by the fungicides so that MCPA breakdown was retarded and, accordingly, more of the parent compound remained extractable. Treatment SQTM clearly exhibited the formation of higher ER amounts compared to the treatments SQ and TM. The reason is that SQTM received in total two fungicide applications, and therefore the effects were more pronounced. The rapid decrease in ER from day 0 to 7 in both the controls and treatments suggests that other processes, e.g., sorption to soil matrix, formation of non-extractable residues (NER), or microbial degradation, must have occurred in parallel. Thereafter, the decline in ER continued but was much slower. From day 14, the desorbable and slowly desorbable fractions of MCPA dominated (except for scenario SQTM). These findings of increasing sorption over time are supported by Boivin et al. [41]. They noted that with prolonged soil–herbicide interaction, the ER fraction decreased, and conversely, NER increased. The details are summarized in Supporting Information Figure S4 and Table S2.
3.1.3. Mineralization (MIN)
In all approaches, initially, a linear increase in mineralization was observed (Figure 2).
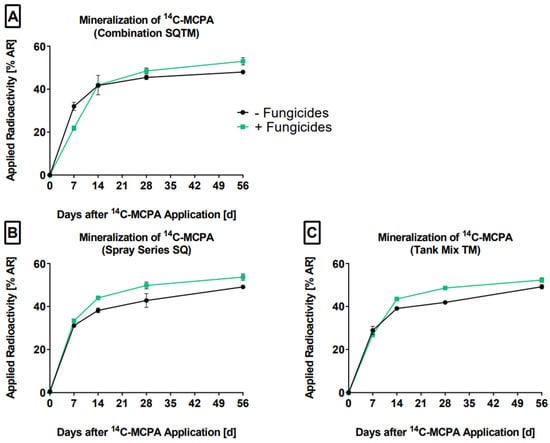
Figure 2.
The mineralization (% AR) of MCPA in the absence (-F, black line) and presence (+F, green line) of the fungicides on days 0, 7, 14, 28, and 56 are presented. (A) SQTM-F: control, SQTM+F: combination (B) SQ-F: control, SQ+F: spray series, and (C) TM-F: control, TM+F: tank mix. The mean values and standard deviations were derived from triplicates (n = 3).
After 56 days, a further progression was evident, indicating that a plateau had not been reached. The relatively high extent and rate of 14CO2 formation demonstrated that MCPA was readily degraded. Here, MIN ranged from 29% to 48% AR and 22% to 54% AR for the controls and treatments, respectively. This is in line with Sørensen et al. [42], who reported 48% AR mineralized after 90 days in sandy soil. In our study, the fungicides mancozeb, metalaxyl-M, and chlorothalonil enhanced the mineralization of MCPA throughout the testing period. Two explanations are feasible: (I) Part of the soil fungi was impaired by the fungicides, resulting in the release of nutrients and carbon, which in turn were metabolized by soil bacteria capable of degrading MCPA, and (II) the applicated fungicides were assimilated by the soil bacteria (MCPA degraders) as a nutrient source. Černohlávková et al. [43] reported the stimulation of carbon mineralization after the application of mancozeb at recommended field rates to an arable soil. Similar results were described for metalaxyl-M [44]. In a study by Baćmaga et al. [20], the count of heterotrophic bacteria increased after the application of chlorothalonil at recommended (0.166 mg kg−1), 10-fold and 100-fold field rates to sandy loam and loamy sand soil. This fits with the finding of this study, where carbon mineralization increased in line with the application of the fungicide cyprodinil at recommended, 2-fold, and 4-fold field rates [45]. Bælum et al. [46] showed that bacteria expressing tfdA or tfdA-like genes were able to degrade MCPA. Here, a correlation between the expression of these genes and the mineralization rates was found. Overall, these studies supported the idea that in cases where the fungicides were not toxic to the soil microorganisms, the microbial activity could have been stimulated, and this resulted in an increase in MIN. Of course, this would need to be confirmed in targeted experiments. The observed MIN increase was in line with the higher extractability of MCPA when fungicides were added, with these residues being subsequently mineralized. In the tank mix and the combined tank mix and spray series scenarios, the mineralization rate first decreased on day 7 but subsequently increased. A similar result was described by Baćmaga et al. [20]. They noted that after chlorothalonil application, the fungi counts first decreased and later increased, which was explained by the resistance of some fungi species against chlorothalonil. Another explanation could be the adaptation of the fungi to chlorothalonil and subsequent recovery. We emphasize that the hypotheses presented here do not represent facts supported by data but should be understood as possible explanations for our observed results. Furthermore, these hypotheses were derived on the basis of our data. For future research, it would be interesting to assess the impact of pesticide mixtures on the soil microorganisms, e.g., by investigating the microorganisms’ viability by means of a qPCR approach. In Supporting Information Figure S5 and Table S3, the mineralization results are summarized.
3.1.4. Non-Extractable Residues (NERs)
Under all conditions (SQ, TM, and SQTM), NERs were formed (Figure 3).
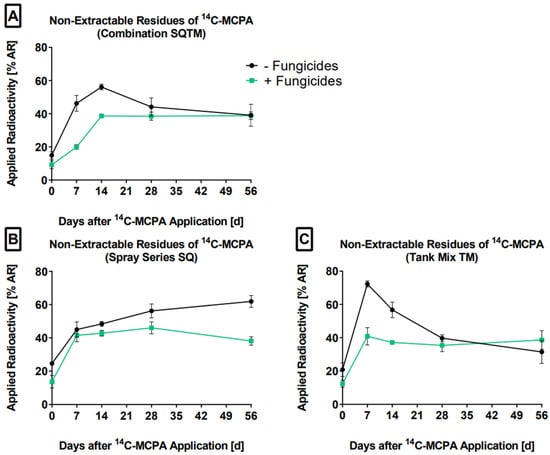
Figure 3.
The non-extractable residues (% AR) of MCPA in the absence (-F, black line) and presence (+F, green line) of the fungicides on days 0, 7, 14, 28, and 56 are presented. (A) SQTM-F: control, SQTM +F: combination (B) SQ-F: control, SQ+F: spray series, and (C) TM-F: control, TM+F: tank mix. The mean values and standard deviations were derived from triplicates (n = 3).
First, all treatments showed a marked NER increase from day 0 to 7. Together with the amount mineralized on day 7, these proportions were roughly equal to the ER that declined during this period. This observation confirms the previous suggestion that the rapid decrease in ER from day 0 to 7 was caused by other processes that occurred in parallel, e.g., mineralization, formation of NER, or sorption to the soil organic matter [7]. The soil organic carbon–water partitioning coefficient of MCPA ranges from 10 to 157 L kg−1, indicating a low sorption affinity to soils [39]. Second, NER continued to increase until day 14 and 28 for SQ and SQTM, respectively. Third, a NER plateau was formed after 7 and 14 days for TM and SQTM, respectively. This might be understood as reaching a steady-state between incorporation (=NER increase) and release/degradation (=NER decrease) processes. Fourth, NER were about 40% AR after 56 days for the fungicide treatments, an amount classified as low to intermediate (30% AR < NER < 50% AR) according to EU registration data [4]. Fifth, we found that with one exception (tank mix scenario, day 56) all fungicide treatments exhibited lower NER amounts compared to the controls and that this effect persisted until the end of the experiment. The covalent binding of MCPA is enhanced by oxidoreductive enzymes [47,48] such as laccase from fungi [49]. It is possible from Figure 3 that the added fungicides inhibit the oxidoreductive enzymes and thus the formation of covalently bound NER [50,51]. As a consequence, extractable residues and mineralization could be enhanced in the MCPA-fungicides mixtures. Overall, NER were reproducible for the fungicide treatments, while for the controls NER fluctuated more noticeably. This may be explained by the heterogeneous distribution of the degrading soil microorganisms, and to a lesser extent by the uneven distribution of MCPA. In Supporting Information Figure S6 and Table S4, the full data are given. Jensen et al. [52] found an inverse correlation between the Freundlich sorption coefficient and the first-order mineralization rate coefficient of MCPA in soil, pointing to competing processes of MIN and NER formation. However, in our study, MIN and NER formation both increased until day 14. Afterward, MIN kept increasing, while the NER amounts remained approximately the same. Talebi and Walker [53] showed that the repeated applications of carbofuran led to an increase in both processes, supporting our observations. These examples highlight that the degradation of a pesticide does not follow a standard pattern.
3.2. Silylation of Nonextractable 14C-MCPA Residues
The silylation was performed for the controls and for the various treatments on day 56 only (Supporting Information Figure S7). After silylation, 11.7% AR, 21.7% AR, and 9.7% AR were released as NER type I for the controls SQTM-F, SQ-F, and TM-F, respectively. A total of 27.4% AR, 40.2% AR, and 21.7% AR remained as covalently bound NER type II for the respective controls. It was remarkable that SQ-F differed from the other controls in that more NER type I as well as NER type II were detected. In this case, this result was considered an outlier due to experimental errors since all other treatments displayed similar values otherwise. For the fungicide treatments, 13.7% AR, 12.0% AR, and 11.8% AR were determined as sequestered NER for SQTM+F, SQ+F, and TM+F, respectively. 25.1% AR, 26.1% AR, and 26.8% AR remained as covalently bound NER for the respective treatments. Overall, by day 56, the formation of NER types I and II was about 1/3 and 2/3 of applied radioactivity, respectively. This was regardless of the fungicide application scenarios. As more of NER type II are present, it is presumed that apart from aging, mechanisms leading to covalent binding, i.e., nucleophile–electrophile interactions, acid–base interactions, and the reaction of functional groups with the soil matrix, proceed more rapidly than for sequestration. Compounds without functional groups could receive the introduction of such a group by microbial or chemical degradation. In contrast, NER type I is formed by the physical entrapment of pesticide residues in micropores and interstitial cavities [54]. It was also noted that after 56 days, the proportions of sequestered NER increased for the treatments SQTM and TM. Here, the chemical structure of the fungicides could provide an explanation. Barriuso et al. [4] reported that reactive groups of pesticides tend to increase NER proportions.
With reference to Regulation (EC) No. 1107/2009 [1], Part I Section 2.5.1.1., an active substance may pose a risk to the environment when the amount of NER exceeds 70% AR and MIN is less than 5% AR after 100 days in laboratory tests. The rationale is that NER may be partly released and that residues thus become bioavailable. In our study, NER amounted to a maximum of 31–62% AR and MIN was 48–54% AR after 56 days. It was also shown that on day 56, about one-third of the total NER was present as type I, which may become slowly released under favorable conditions, such as pH, temperature, or soil organism activity [55]. Studies conducted by Eschenbach et al. [56] and Weiß et al. [57] provided evidence that NER stability was neither affected by biological (e.g., white rot fungi, radical-generating enzymes) nor by mechanical/physical treatments (freezing, thawing, grinding). However, a small amount of NER (<15% AR) was released after chemical stress, i.e., the addition of metal complexing agent EDTA and simulation of acid rain. Furthermore, the mineralization of NER is age-dependent, as reported by Lerch et al. [58]: The addition of fresh soil to young NER resulted in an increase in mineralization, whereas the fresh soil amendment had no effects on the mineralization of aged NER. Thus, the mobilization potential of NER was demonstrated to be low. However, sequestered NER may contain the soil-applied parent compound [59], and therefore, the slow release potential is a critical aspect to consider, at least for persistence assessment. The remaining two-thirds of the total NER were present as NER type II, which are covalently bound to the soil matrix and considered to be of no concern. It has been suggested that remobilization experiments should be run to further characterize the potential risk of NER [3]. These should aim to simulate natural conditions by employing physical (freezing/thawing, wetting/drying), chemical (extraction with water simulating heavy rain events), and biological treatments (addition of compost or ligninolytic fungi). Such studies should be performed for compounds forming high amounts of NER, especially if these are predominantly NER type I. In addition, future research should characterize the NER for the proportion of biogenic NER. These derive from the initial catabolism of MCPA followed by anabolism of the resulting building blocks for the synthesis of amino acids, phospholipids, and nucleic acids.
3.3. Analysis by Radio—Thin-Layer Chromatography (TLC)
On day 0, the proportions of parent MCPA were similar for the controls and treatments. This was true for the tank mix and combination experiment. Unexpectedly, the spray series exhibited less MCPA compared to its respective control. Since the extraction was performed immediately after MCPA application, no differences between the treated and untreated approaches were expected. The difference between SQ-F and SQ+F on day 0 can be explained by the relatively high standard deviation of the radioactivities in the CaCl2 fractions, which likely resulted during sample processing. Therefore, this discrepancy was not linked to the fungicides. On day 7, the impact of the fungicides became obvious with higher amounts of parent MCPA for the treatments compared to the controls. On day 14, the proportions of parent MCPA derived from 14C-ER declined. Still, most MPCA was detected in the treatment SQTM, followed by SQ and TM. The reason that mainly MCPA and less than 1% AR of 4C2MP were present in the CaCl2, MeOH, and SOX extracts was explained by the faster dissipation of its main transformation product 4-chloro-2-methylphenol (4C2MP), with half-lives of 3.55 [60] and 7–60 days [61] reported for 4C2MP and MCPA, respectively. Overall, these results support our findings regarding ER, showing that the co-application of fungicides increased ER likely by inhibiting the MCPA degraders. The details are summarized in Supporting Information Figure S8 and Table S5.
3.4. Determination of the Dissipation Time 50 (DT50)
The DT50 calculation for MCPA was performed by using all four kinetic FOCUS models, i.e., SFO (single first-order), FOMC (first-order multi-compartment), DFOP (double first-order in parallel), and HS (hockey stick). For SQTM-F and SQTM+F, the DFOP model resulted in optimal visual and statistical output. For SQ-F, the use of the SFO model generated suitable visual and statistical results, whereas, for SQ+F, the DFOP model produced superior visual and statistical output. For TM-F, the DFOP model prompted a suitable visual fit, while for TM+F, SFO was selected as the best-fit model. According to OECD 307, major transformation products (≥10% AR) should be identified, and their DT50 also determined. In this study, 4C2MP was formed at levels corresponding to <1.0% AR, and therefore, DT50 (4C2MP) was not determined. For DT50 modeling of MCPA, the proportions of parent MCPA derived from the CaCl2, MeOH, and SOX extracts on days 0, 7, and 14 were used (Supporting Information Figure S9). We found that the co-application of the fungicides mancozeb, metalaxyl-M, and chlorothalonil at recommended field rates resulted in slower dissipation kinetics of MCPA in a sandy loam soil (Table 1). DT50 and DT90 increased 1.6- to 2.0-fold and 1.6- to 3.8-fold, respectively, compared to those in untreated control soils. The reduction in MCPA dissipation was most pronounced for SQTM, followed by SQ and TM. With regard to MCPA mineralization, DT50 increased 1.04 to 1.78-fold in the fungicide treatments compared to the untreated control soils. As mentioned above, the reduction in mineralization was found to be most prominent for SQTM, followed by SQ and TM (Supporting Information Table S10). The DT50 values for NER could not be determined, as none of the models were suitable to describe the kinetics with a reasonable fit. In all settings, MCPA dissipated rapidly without and with fungicides added, with DT50 being between 1.30 and 3.49 days, which is much lower than the 7–41 days reported by Lewis et al. [62]. Our lower DT50 values are supported by other literature, e.g., DT50 between 3.1 and 7.3 days and 7 days were reported for MCPA in a sandy loam soil by Müller and Buser [63] and Thorstensen and Lode [64], respectively. Paszko [65] reported a DT50 of 6.9 days for MCPA in a sandy soil at 25 °C. Therefore, it is likely that the shorter values we measured are typical for more sandy soils with less sorption but that higher values are observed in other soil types. Note that the typically reported values above (i.e., 7–41 days) were summarized from EU dossier lab studies, but the soil types were not given.

Table 1.
Dissipation kinetics of MCPA for the controls (−F) and fungicide treatment scenarios (+F): combination (SQTM), spray series (SQ), and tank mix (TM). SFO: single first-order; FOMC: first-order multi-compartment; DFOP: double first-order in parallel; HS: hockey stick. The DT50, DT90, coefficient of determination (r2), and efficiency were calculated with CAKE version 3.4. The mean values and standard deviations were derived from triplicates (n = 3).
3.5. Statistical Data Analysis
The two-tailed t-test (α = 0.05) was performed to assess whether the visually observed differences for ER, MIN, and NER in the controls and fungicide treatments were statistically significant. Significance was confirmed for the extractable residues in the treatment SQTM. Moreover, the k-values of the treatments were compared with those of their respective controls. Here, DT50 differed significantly between SQ+F and SQ-F. This was evident because the 90% confidence intervals of SQ+F and SQ-F were not overlapping. Nevertheless, slower degradation kinetics were still noticed for the other treatments when compared to their respective controls. Here, the deceleration of DT50 of MCPA was up to 55%, 85%, and 86% for the tank mix, spray series, and combination, respectively. The details are summarized in Supporting Information Tables S6–S9.
3.6. Limitations of the Study
Our research focused on an arable soil, which has a significant area representation in Germany. However, investigating the degradation pathway of MCPA in different agricultural and grassland soils will contribute to a better understanding, as greater variability in soil properties (e.g., pH, texture: loamy, silty, clayey) can be tested. Of particular interest is how different levels of soil organic carbon will affect the fate of a pesticide, including biogenic NER formation.
4. Conclusions
Taking into consideration that up to now, only single active substances and commercialized products are examined for their environmental fate and effects as part of the approval procedure for plant protection products, this study investigated the impact of the fungicide mixture mancozeb, metalaxyl-M, and chlorothalonil on MCPA degradation in soil. This is relevant since it is the rule rather than the exception that multiple pesticides are applied, either in tank mixes prepared by the farmers or as an application series during the agricultural season. Importantly, our data provide evidence that the co-application of the fungicides at recommended field rates, either as a tank mix, spray series, or a combination of both, can lead to reduced dissipation rates of MCPA. Dissipation times 50 and 90 increased 1.6- to 2.0-fold and 1.6- to 3.8-fold, respectively, compared to those in untreated control soils. This decrease in the dissipation rates was further supported by the chromatographic analyses, showing that 4C2MP, the main transformation product of MCPA, was formed in considerably lower amounts when the fungicides were co-applied. This work is consistent with the few other studies that report pesticide mixtures affect the degradation of other pesticides present in the soil. Importantly, we show that this is the case even at realistic fungicide application levels. Therefore, at least exemplarily, the degradation of plant protection products should be tested not only as isolated substances but also in the presence of fungicide by-products, which are used as part of typical agricultural practice. Environmental implications for MCPA degradation are unlikely since, for this compound, the degradation occurs rapidly and the longer half-lives in the presence of fungicides are still quite low. Therefore, it is unlikely that it will, for example, leach into groundwater. However, for pesticides with longer half-lives, the combination with fungicides may decrease degradation rates further so that persistence triggers in the authorization process may be surpassed. In addition, increased levels remaining in the soil could, of course, impact adjacent environmental compartments such as groundwater. However, whether this is the case is hard to generalize since this depends on the pesticide properties. These are issues that need to be addressed in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems6040094/s1, Figure S1: Application schemes of the different treatments; Figure S2: Flowchart illustrating the sample processing and analysis; Figure S3: Distribution of applied radioactivity (AR) on days 0, 7, 14, 28 and 56; Figure S4: The extractable residues (dpm) of MCPA in the 0.01 M CaCl2, MeOH, and Soxhlet (SOX) extracts after 1, 168, 336, 672, and 1344 h; Figure S5: The mineralization (dpm) of MCPA in absence and presence of the fungicides after 1, 168, 336, 672, and 1344 h; Figure S6: The non-extractable residues (dpm) of MCPA in absence and presence of the fungicides after 1, 168, 336, 672, and 1344 h; Figure S7: Silylation of the controls and treatments on day 56; Figure S8: Proportions of parent MCPA (% AR) in the CaCl2, MeOH, and SOX extracts in absence and presence of the fungicides on days 0, 7, and 14 for the different application scenarios as investigated by thin layer chromatography (TLC); Figure S9: Dissipation kinetics of MCPA in soil; Table S1: Overview of the test substances and their physicochemical properties [16,62,66]; Table S2: Extractable residues of 14C-MCPA (% AR) in soil after 0, 7, 14, 28, and 56 days of incubation obtained by extractions with 0.01 M CaCl2, MeOH, and Soxhlet; Table S3: Mineralization of 14C-MCPA (% AR) in soil after 0, 7, 14, 28, and 56 days of incubation; Table S4: Non-extractable residues of 14C-MCPA (% AR) in soil after 0, 7, 14, 28, and 56 days of incubation; Table S5: Proportions of parent MCPA (% AR) in the CaCl2, MeOH, and SOX extracts in absence and presence of the fungicides on days 0, 7, and 14 for the different application scenarios as investigated by thin layer chromatography (TLC); Table S6: Results of the two-sample t-test for the extractable residues (ER) on days 0, 7, 14, 28, and 56; Table S7: Results of the two-sample t-test for the non-extractable residues (NER) on days 0, 7, 14, 28, and 56; Table S8: Results of the two-sample t-test for the mineralization (MIN) on days 0, 7, 14, 28, and 56; Table S9: Statistics of the dissipation kinetics of MCPA for the controls and fungicide treatment scenarios; Table S10: One-phase exponential association of MCPA mineralization for the controls and fungicide treatment scenarios.
Author Contributions
K.T.N.: conceptualization, investigation, methodology, validation, project administration, formal analysis, data curation, visualization, writing—original draft, writing—review and editing, and funding acquisition. K.E.C.S.: conceptualization and writing—review and editing. R.O.: data curation and writing—review and editing. C.W.: writing—review and editing. J.T.v.D.: resources and writing—review and editing. A.S.: conceptualization, resources, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
Funding by my alma mater, RWTH Aachen University, is gratefully acknowledged.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- European Parliament. Regulation (EC) No 1107/2009. EUR. Available online: http://data.europa.eu/eli/reg/2009/1107/2021-03-27 (accessed on 4 April 2022).
- Cycoń, M.; Piotrowska-Seget, Z. Transformations of pesticides in soil environment—A Review. Pestycydy 2006, 3–4, 45–56. Available online: https://www.researchgate.net/publication/248393911_Transformations_of_pesticides_in_soil_environment_-_a_review (accessed on 22 March 2022).
- Schäffer, A.; Kästner, M.; Trapp, S. A unified approach for including non-extractable residues (NER) of chemicals and pesticides in the assessment of persistence. Environ. Sci. Eur. 2018, 30, 51. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, E.; Benoit, P.; Dubus, I.G. Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- FOrum for the Co- ordination of pesticide models and their USe. Guidance Document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU registration. Report of the FOCUS Work Group on Degradation Kinetics 2006, EC Document Reference SANCO/10058/2005 Version 2.0. p. 434. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 22 March 2022).
- European Food Safety Authority. Guidance document for evaluating laboratory and field dissipation studies to obtain DEGT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA J. 2014. [Google Scholar] [CrossRef]
- Kästner, M.; Nowak, K.M.; Miltner, A.; Trapp, S.; Schäffer, A. Classification and modelling of nonextractable residue (NER) formation of xenobiotics in soil—A synthesis. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2107–2171. [Google Scholar] [CrossRef]
- European Chemicals Agency. Options to Address Non-Extractable Residues in Regulatory Persistence Assessment. 2019. Available online: https://echa.europa.eu/documents/10162/13632/bg_note_addressing_non-extractable_residues.pdf/e88d4fc6-a125-efb4-8278-d58b31a5d342 (accessed on 22 March 2022).
- Fogg, P.; Boxall, A.B.; Walker, A. Degradation of pesticides in Biobeds: the effect of concentration and pesticide mixtures. J. Agric. Food Chem. 2003, 51, 5344–5349. [Google Scholar] [CrossRef]
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Tankmischungen im Zulassungsverfahren für Pflanzenschutzmittel. 2015. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/Tankmischungen.pdf?__blob=publicationFile&v=3 (accessed on 22 March 2022).
- Pflanzenschutzgesetz. Gesetz zum Schutz der Kulturpflanzen (Pflanzenschutzgesetz—PflSchG) in der Fassung der Bekanntmachung vom 06. Februar 2012 (BGBl. I S. 148, 1281), geändert durch Artikel 4 Absatz 84 des Gesetzes vom 18. Juli 2016 (BGBl. I S. 1666). PflSchG—Gesetz zum Schutz der Kulturpflanzen. Available online: https://www.gesetze-im-internet.de/pflschg_2012/BJNR014810012.html (accessed on 22 March 2022).
- White, P.M.; Potter, T.L.; Culbreath, A.K. Fungicide dissipation and impact on metolachlor aerobic soil degradation and soil microbial dynamics. Sci. Total Environ. 2010, 408, 1393–1402. [Google Scholar] [CrossRef]
- Swarcewicz, M.K.; Gregorczyk, A. The effects of pesticide mixtures on degradation of Pendimethalin in soils. Environ. Monit. Assess. 2011, 184, 3077–3084. [Google Scholar] [CrossRef]
- Swarcewicz, M.; Gregorczyk, A.; Sobczak, J. Comparison of Linuron degradation in the presence of pesticide mixtures in soil under laboratory conditions. Environ. Monit. Assess. 2013, 185, 8109–8114. [Google Scholar] [CrossRef]
- Platt, H.W. Effects of metalaxyl, mancozeb, and chlorothalonil on blight, yield, and tuber rot of potato. Can. J. Plant Pathol. 1983, 5, 38–42. [Google Scholar] [CrossRef]
- Knillmann, S.; Liess, M.; Scholz-Starke, B.; Daniels, B.; Ottermanns, R.; Schäffer, A.; Sybertz, A.; Roß-Nickoll, M. Environmental risks of pesticides between Forecast and Reality: How Reliable are Results of the Environmental risk Assessment for Individual Products in the Light of Agricultural Practice (Tank Mixtures, Spray Series)? 2021. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/5750/publikationen/2021-05-26_texte_82-2021_combitox_pesticides.pdf (accessed on 22 March 2022).
- Sukul, P.; Spiteller, M. Metalaxyl: Persistence, degradation, metabolism, and analytical methods. Rev. Environ. Contam. Toxicol. 2000, 164, 1–26. [Google Scholar] [PubMed]
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb: Past, Present, and Future. Plant Dis. 2010, 94, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z.; Kozdrój, J. Responses of indigenous microorganisms to a fungicidal mixture of mancozeb and dimethomorph added to sandy soils. Int. Biodeterior. Biodegrad. 2010, 64, 316–323. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. 2018. Available online: http://pesticidetruths.com/wp-content/uploads/2013/06/Health-Canada-MCPA-2008-05-22-Re-Evaluation-Decision-Document-RVD2008-20.pdf (accessed on 11 July 2022).
- CrespÍn, M.A.; Gallego, M.; Valcárcel, M.; González, J.L. Study of the degradation of the herbicides 2,4-D and MCPA at different depths in contaminated agricultural soil. Environ. Sci. Technol. 2001, 35, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- RefeSol. Available online: https://www.refesol.de (accessed on 22 March 2022).
- OECD Test no. 307. Aerobic and anaerobic transformation in soil. OECD Guidelines for the Testing of Chemicals; Section 3; OECD Publishing: Paris, France, 2002. [CrossRef]
- González, A.J.; Fortunato, M.S.; Gallego, A.; Korol, S.E. Simultaneous Biodegradation and Detoxification of the Herbicides 2,4-Dichlorophenoxyacetic Acid and 4-Chloro-2-Methylphenoxyacetic Acid in a Continuous Biofilm Reactor. Water Air Soil Pollut. 2017, 228, 300. [Google Scholar] [CrossRef]
- Matamoros, V.; Franco, J. Assessing the use of sand, peat soil, and pine bark for the attenuation of polar pesticides from agricultural run-off: A bench-scale column experiment. Env. Sci. Pollut. Res. 2018, 25, 20640–20647. [Google Scholar] [CrossRef]
- Morton, P.A.; Fennell, C.; Cassidy, R.; Doody, D.; Fenton, O.; Mellander, P.E.; Jordan, P. A review of the pesticide MCPA in the land-water environment and emerging research needs. WIREs Water 2019, 7, e1402. [Google Scholar] [CrossRef]
- Mierzejewska, E.; Baran, A.; Tankiewicz, M.; Urbaniak, M. Removal and Ecotoxicity of 2,4-D and MCPA in Microbial Cultures Enriched with Structurally-Similar Plant Secondary Metabolites. Water 2019, 11, 1451. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 m calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Kästner, M.; Trapp, S.; Schäffer, A. Consultancy Services to Support ECHA in Improving the Interpretation of Non-Extractable Residues (NER) in Degradation Assessment 2018. Available online: https://echa.europa.eu/documents/10162/13630/echa_discussion_paper_en.pdf/4185cf64-8333-fad2-8ddb-85c09a560f7c (accessed on 22 March 2022).
- Tessella Ltd. CAKE 3.4: Computer Assisted Kinetic Evaluation. 2022. Available online: https://www.tessella.com/showcase/computer-assisted-kinetic-evaluation (accessed on 22 March 2022).
- GraphPad Prism. GraphPad Software, La Jolla, CA, USA. 2022. Available online: https://www.graphpad.com (accessed on 22 March 2022).
- Microsoft Excel 2016, Microsoft Corporation: Redmond, WA, USA, 2016.
- R. R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org./ (accessed on 31 January 2022).
- Xu, M.; Fralick, D.; Zheng, J.Z.; Wang, B.; Tu, X.M.; Feng, C. The Differences and Similarities Between Two-Sample T-Test and Paired T-Test. Shanghai Arch. Psychiatry 2017, 29, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mishra, P.; Pandey, C.M.; Singh, U.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of levene’s test of equality of variances on statistical theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Paszko, T.; Muszyński, P.; Materska, M.; Bojanowska, M.; Kostecka, M.; Jackowska, I. Adsorption and degradation of phenoxyalkanoic acid herbicides in soils: A Review. Environ. Toxicol. Chem. 2016, 35, 271–286. [Google Scholar] [CrossRef]
- Comoretto, L.; Arfib, B.; Talva, R.; Chauvelon, P.; Pichaud, M.; Chiron, S.; Höhener, P. Runoff of pesticides from rice fields in the Ile de Camargue (Rhône River Delta, France): Field Study and Modeling. Environ. Pollut. 2008, 151, 486–493. [Google Scholar] [CrossRef]
- Boivin, A.; Amellal, S.; Schiavon, M.; van Genuchten, M.T. 2,4-dichlorophenoxyacetic acid (2,4-D) sorption and degradation dynamics in three agricultural soils. Environ. Pollut. 2005, 138, 92–99. [Google Scholar] [CrossRef]
- Sørensen, S.R.; Schultz, A.; Jacobsen, O.S.; Aamand, J. Sorption, desorption and mineralisation of the herbicides glyphosate and MCPA in samples from two Danish soil and subsurface profiles. Environ. Pollut. 2006, 141, 184–194. [Google Scholar] [CrossRef]
- Černohlávková, J.; Jarkovský, J.; Hofman, J. Effects of fungicides Mancozeb and dinocap on carbon and nitrogen mineralization in soils. Ecotoxicol. Environ. Saf. 2009, 72, 80–85. [Google Scholar] [CrossRef]
- Monkiedje, A.; Spiteller, M. Degradation of metalaxyl and mefenoxam and effects on the microbiological properties of tropical and temperate soils. Int. J. Environ. Res. Public Health 2005, 2, 272–285. [Google Scholar] [CrossRef]
- Koçak, B. Changes in Soil Carbon Mineralization under the Effects of Fungicide Cyprodinil. In Proceedings of the Conference: IV. International Eurasian Agriculture and Natural Sciences Congress, Online, 30–31 October 2020; 2020. Available online: https://www.researchgate.net/publication/347357941_Changes_in_Soil_Carbon_Mineralization_under_the_Effects_of_Fungicide_Cyprodinil (accessed on 22 March 2022).
- Bælum, J.; Henriksen, T.; Hansen, H.C.; Jacobsen, C.S. Degradation of 4-chloro-2-methylphenoxyacetic acid in top- and subsoil is quantitatively linked to the class III tfda gene. Appl. Environ. Microbiol. 2006, 72, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Bollag, J.-M.; Myers, C.J.; Minard, R.D. Biological and chemical interactions of pesticides with soil organic matter. Sci. Total Environ. 1992, 123–124, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, P.G.; Bortiatynski, J.M.; Minard, R.D.; Dec, J.; Bollag, J.M. Use of high-resolution carbon-13 NMR to examine the enzymatic covalent binding of carbon-13-labeled 2,4-Dichlorophenol to humic substances. Environ. Sci. Technol. 1993, 27, 2098–2103. [Google Scholar] [CrossRef]
- Bollag, J.M.; Shuttleworth, K.L.; Anderson, D.H. Laccase-mediated detoxification of phenolic compounds. Appl. Environ. Microbiol. 1988, 54, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.; Brock, A.L.; Nowak, K.; Kästner, M. Prediction of the formation of biogenic nonextractable residues during degradation of environmental chemicals from biomass yields. Environ. Sci. Technol. 2017, 52, 663–672. [Google Scholar] [CrossRef]
- Trapp, S.; Brock, A.L.; Kästner, M.; Schäffer, A.; Hennecke, D. Critical evaluation of the microbial turnover to biomass approach for the estimation of biogenic non-extractable residues (NER). Environ. Sci. Eur. 2022, 34, 15. [Google Scholar] [CrossRef]
- Jensen, P.H.; Hansen, H.C.; Rasmussen, J.; Jacobsen, O.S. Sorption-controlled degradation kinetics of MCPA in soil. Environ. Sci. Technol. 2004, 38, 6662–6668. [Google Scholar] [CrossRef]
- Talebi, K.; Walker, C.H. A comparative study of carbofuran metabolism in treated and untreated soils. Pestic. Sci. 1993, 39, 65–69. [Google Scholar] [CrossRef]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound pesticide residues in soils: A Review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef]
- Katayama, A.; Bhula, R.; Burns, G.R.; Carazo, E.; Felsot, A.; Hamilton, D.; Harris, C.; Kim, Y.-H.; Kleter, G.; Koedel, W.; et al. Bioavailability of xenobiotics in the soil environment. Rev. Environ. Contam. Toxicol. 2009, 203, 1–86. [Google Scholar] [CrossRef]
- Eschenbach, A.; Wienberg, R.; Mahro, B. Fate and stability of nonextractable residues of [14c]pah in contaminated soils under environmental stress conditions. Environ. Sci. Technol. 1998, 32, 2585–2590. [Google Scholar] [CrossRef]
- Weiß, M.; Geyer, R.; Günther, T.; Kaestner, M. Fate and stability of 14C-labeled 2,4,6-trinitrotoluene in contaminated soil following microbial bioremediation processes. Environ. Toxicol. Chem. 2004, 23, 2049. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Dignac, M.-F.; Nunan, N.; Barriuso, E.; Mariotti, A. Ageing processes and soil microbial community effects on the biodegradation of soil 13C-2,4-D nonextractable residues. Environ. Pollut. 2009, 157, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, S.; Zhao, Y.; Wang, L.; Ma, Y.; Schäffer, A.; Ji, R. Fate of Bisphenol S (BPS) and characterization of non-extractable residues in soil: Insights into persistence of BPS. Environ. Int. 2020, 143, 105908. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Comptox Chemicals Dashboard. 2022. Available online: https://comptox.epa.gov/dashboard/chemical/details/DTXSID5022510 (accessed on 23 March 2022).
- Hornsby, A.G.; Wauchope, R.D.; Herner, A.E. Pesticide Properties in the Environment; Springer: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Müller, M.D.; Buser, H.R. Conversion Reactions of Various Phenoxyalkanoic Acid Herbicides in Soil. 1. Enantiomerization and Enantioselective Degradation of the Chiral 2-Phenoxypropionic Acid Herbicides. Environ. Sci. Technol. 1997, 31, 1953–1959. [Google Scholar] [CrossRef]
- Thorstensen, C.W.; Lode, O. Laboratory Degradation Studies of Bentazone, Dichlorprop, MCPA, and Propiconazole in Norwegian Soils. J. Environ. Qual. 2001, 30, 947–953. [Google Scholar] [CrossRef]
- Paszko, T. Degradation of MCPA in Soil Horizons of Polish Agricultural Soils. Pol. J. Environ. Stud. 2009, 18, 1083–1091. [Google Scholar]
- Tadeo, J.L. Analysis of Pesticides in Food and Environmental Samples, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).