Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Preparation and Characterization
2.3. 1 H-NMR Relaxometry
2.4. Soil Rheometry
2.5. Determination of Dynamic Contact Angle Tensiometry (DCAT)
2.6. Determination of SOM Content and Recalcitrance
2.7. Data Analysis
3. Results
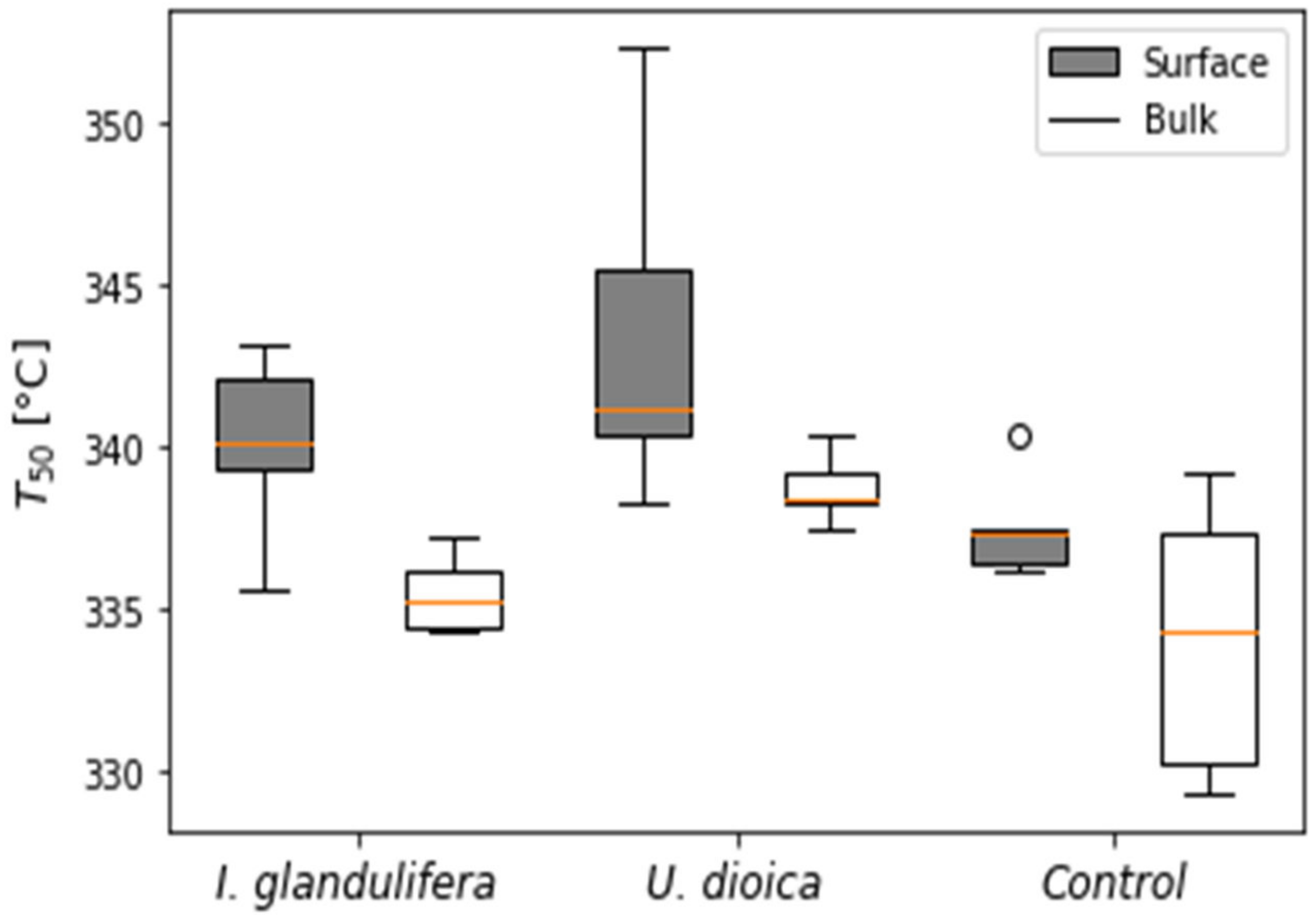
3.1. SOM Thermal Properties
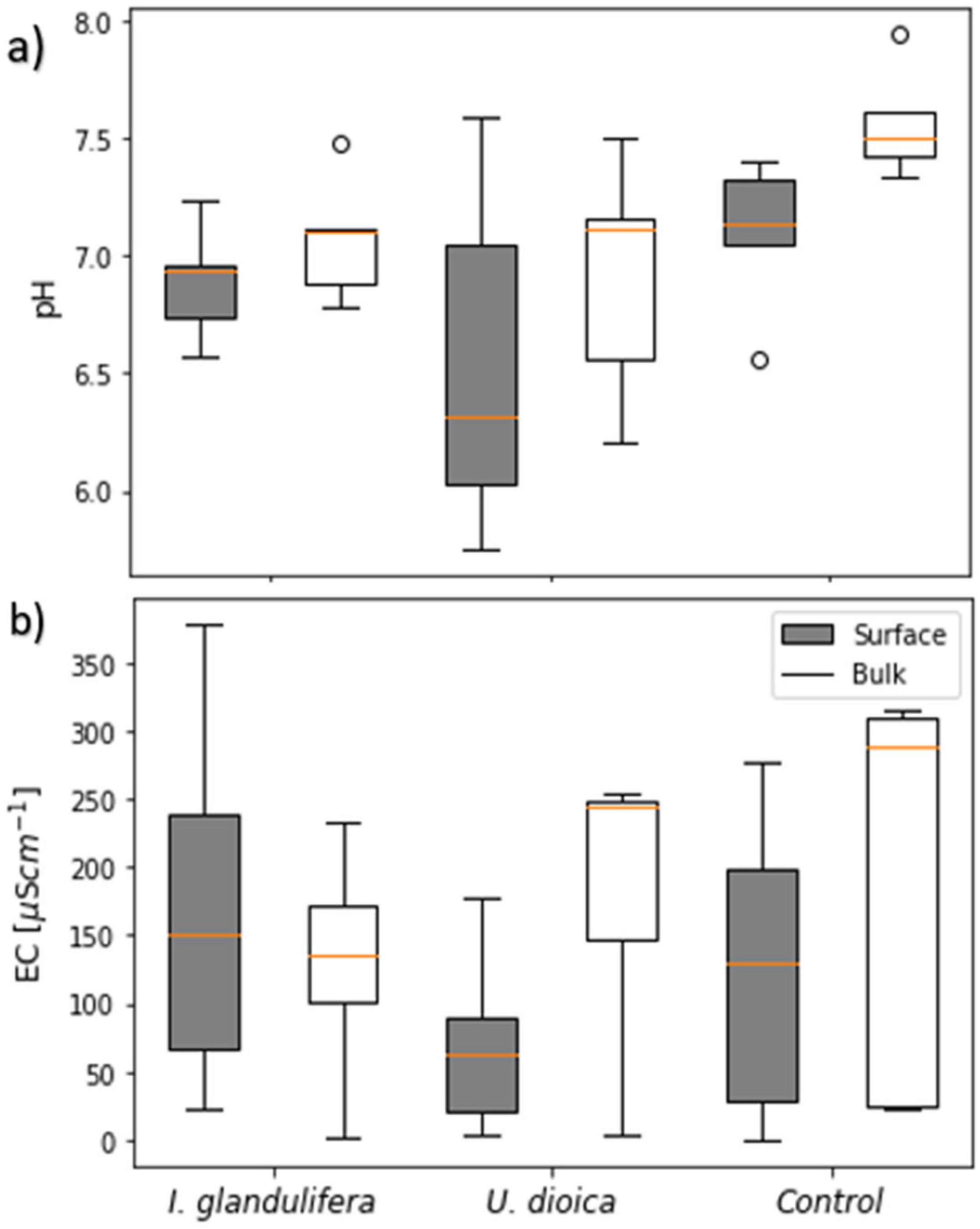
3.2. Soil Physico-Chemical Properties
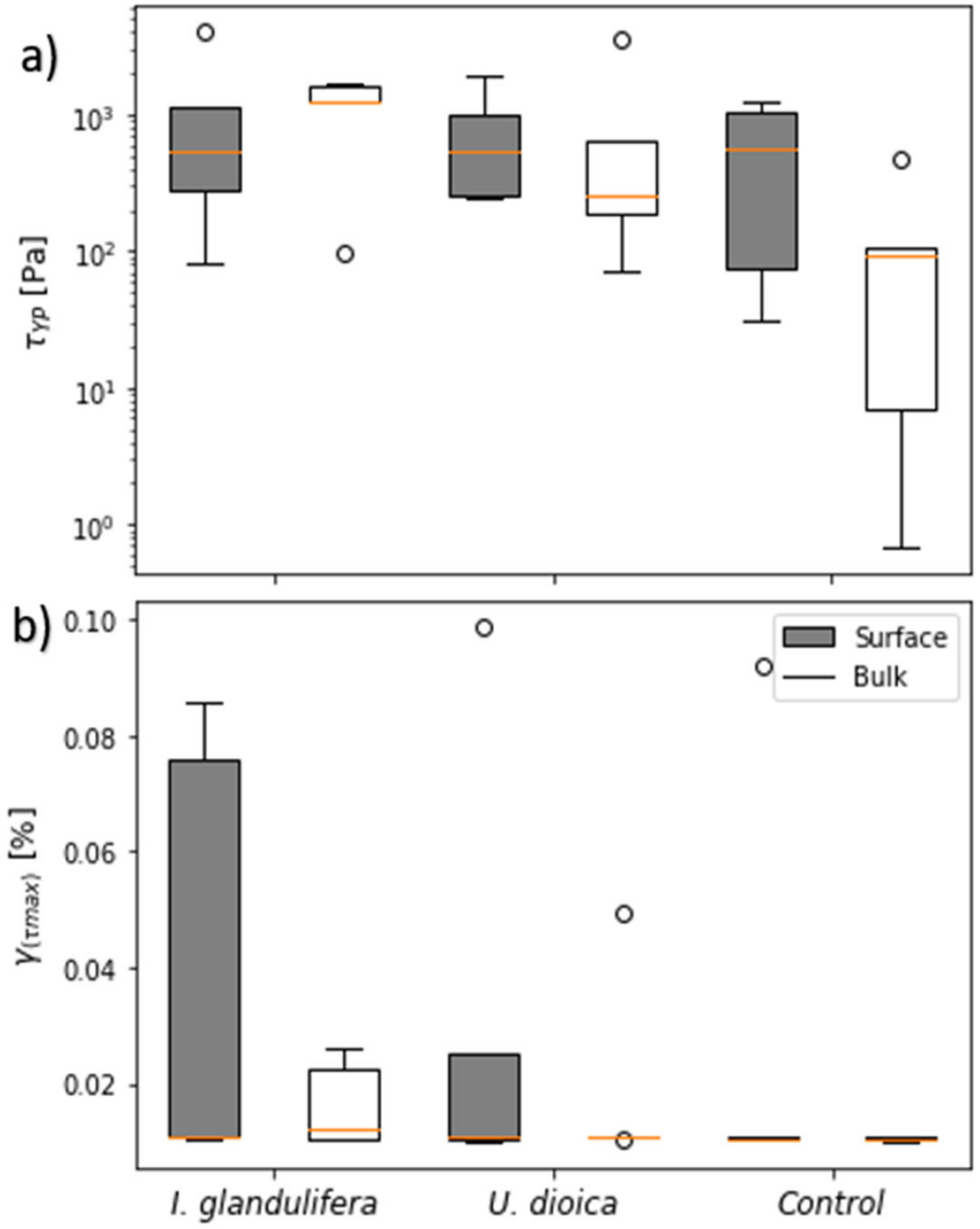
3.3. Soil Microstructural Stability
3.4. Soil Hydraulic Properties and Initial Contact Angle
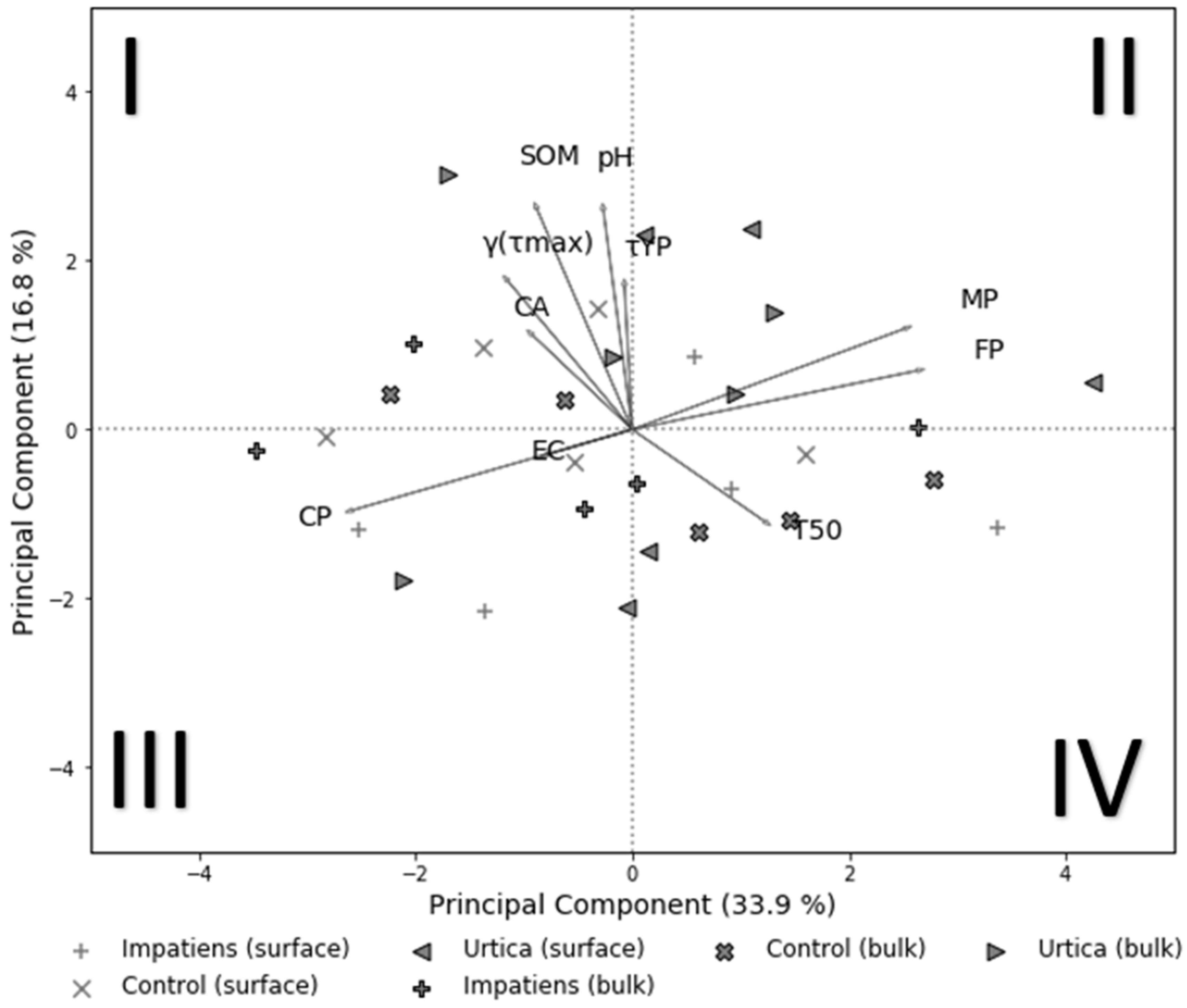
3.5. Relationship between Soil Physico-Chemical Properties
4. Discussion
4.1. SOM Fractions and Thermal Recalcitrance
4.1.1. Depth and Plant Dependent Heterogeneity of SOM
4.1.2. Plant Dependent SOM Quality
4.2. Soil Structural Stability
4.3. Soil Hydraulic Properties
4.4. Soil Physico-Chemical Properties
4.5. Relationships between Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verhoeven, J.T.A.; Arheimer, B.; Yin, C.; Hefting, M. Regional and Global Concerns over Wetlands and Water Quality. Trends Ecol. Evol. 2006, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Bundschuh, M.; Gergs, R.; Brühl, C.A.; Diehl, D.; Entling, M.H.; Fahse, L.; Frör, O.; Jungkunst, H.F.; Lorke, A.; et al. Review on Environmental Alterations Propagating from Aquatic to Terrestrial Ecosystems. Sci. Total Environ. 2015, 538, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Kisielius, V.; Hama, J.R.; Skrbic, N.; Hansen, H.C.B.; Strobel, B.W.; Rasmussen, L.H. The Invasive Butterbur Contaminates Stream and Seepage Water in Groundwater Wells with Toxic Pyrrolizidine Alkaloids. Sci. Rep. 2020, 10, 19784. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Perrins, J.M. Impatiens glandulifera Royle (Impatiens Roylei Walp.). J. Ecol. 1993, 81, 367–382. [Google Scholar] [CrossRef]
- Lee, M.R.; Flory, S.L.; Phillips, R.P. Positive Feedbacks to Growth of an Invasive Grass through Alteration of Nitrogen Cycling. Oecologia 2012, 170, 457–465. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource Competition in Plant Invasions: Emerging Patterns and Research Needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Greenwood, P.; Kuhn, N.J. Does the Invasive Plant, Impatiens glandulifera, Promote Soil Erosion along the Riparian Zone? An Investigation on a Small Watercourse in Northwest Switzerland. J. Soils Sediments 2014, 14, 637–650. [Google Scholar] [CrossRef]
- Ruckli, R.; Hesse, K.; Glauser, G.; Rusterholz, H.-P.; Baur, B. Inhibitory Potential of Naphthoquinones Leached from Leaves and Exuded from Roots of the Invasive Plant Impatiens glandulifera. J. Chem. Ecol. 2014, 40, 371–378. [Google Scholar] [CrossRef]
- Chittka, L.; Schürkens, S. Successful Invasion of a Floral Market. Nature 2001, 411, 653. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What Is the Impact of Impatiens glandulifera on Species Diversity of Invaded Riparian Vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Ennos, A.R.; Crook, M.J.; Grimshaw, C. A Comparative Study of the Anchorage Systems of Himalayan Balsam Impatiens glandulifera and Mature Sunflower Helianthus annuus. J. Exp. Bot. 1993, 44, 133–146. [Google Scholar] [CrossRef]
- Gruntman, M.; Pehl, A.K.; Joshi, S.; Tielbörger, K. Competitive Dominance of the Invasive Plant Impatiens glandulifera: Using Competitive Effect and Response with a Vigorous Neighbour. Biol. Invasions 2014, 16, 141–151. [Google Scholar] [CrossRef]
- Taylor, K. Biological Flora of the British Isles: Urtica dioica L. J. Ecol. 2009, 97, 1436–1458. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Andrews, M.; Maule, H.G.; Hodge, S.; Cherrill, A.; Raven, J.A. Seed Dormancy, Nitrogen Nutrition and Shade Acclimation of Impatiens glandulifera: Implications for Successful Invasion of Deciduous Woodland. Plant Ecol. Divers. 2009, 2, 145–153. [Google Scholar] [CrossRef][Green Version]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of Alien Invasive Plants on Soil Nutrients Are Correlated with Initial Site Conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Ruckli, R.; Rusterholz, H.-P.; Baur, B. Invasion of Impatiens glandulifera Affects Terrestrial Gastropods by Altering Microclimate. Acta Oecologica 2013, 47, 16–23. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Majewska, M.L.; Stanek, M.; Nobis, M.; Zubek, S. Differential Influence of Four Invasive Plant Species on Soil Physicochemical Properties in a Pot Experiment. J. Soils Sediments 2018, 18, 1409–1423. [Google Scholar] [CrossRef]
- Pysek, P.; Prach, K. Plant Invasions and the Role of Riparian Habitats: A Comparison of Four Species Alien to Central Europe. J. Biogeogr. 1993, 20, 413–420. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G.; Kourtev, P.; Huang, W. Changes in Soil Functions Following Invasions of Exotic Understory Plants in Deciduous Forests. Ecol. Appl. 2001, 11, 1287–1300. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zukswert, J.M. Invasive Plant Species and Litter Decomposition: Time to Challenge Assumptions. New Phytol. 2016, 209, 5–7. [Google Scholar] [CrossRef]
- Markgraf, W.; Horn, R.; Peth, S. An Approach to Rheometry in Soil Mechanics—Structural Changes in Bentonite, Clayey and Silty Soils. Soil Tillage Res. 2006, 91, 1–14. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhao, Y.; Horn, R. Soil Wettability as Affected by Soil Characteristics and Land Use. Pedosphere 2010, 20, 43–54. [Google Scholar] [CrossRef]
- Doerr, S.H. Soil Hydrophobicity in Wet Mediterranean Pine and Eucalyptus Forests, Águeda Basin, North-Central Portugal. Ph.D. Thesis, University of Wales, Swansea, UK, 1997. [Google Scholar]
- Meyer, M.; Buchmann, C.; Schaumann, G.E. Determination of Quantitative Pore-Size Distribution of Soils with 1H NMR Relaxometry. Eur. J. Soil Sci. 2018, 69, 393–406. [Google Scholar] [CrossRef]
- Barros, N.; Fernandez, I.; Byrne, K.A.; Jovani-Sancho, A.J.; Ros-Mangriñan, E.; Hansen, L.D. Thermodynamics of Soil Organic Matter Decomposition in Semi-Natural Oak (Quercus) Woodland in Southwest Ireland. Oikos 2020, 129, 1632–1644. [Google Scholar] [CrossRef]
- Śpitalniak, M.; Bogacz, A.; Zięba, Z. The Assessment of Water Retention Efficiency of Different Soil Amendments in Comparison to Water Absorbing Geocomposite. Materials 2021, 14, 6658. [Google Scholar] [CrossRef]
- Panagea, I.S.; Berti, A.; Čermak, P.; Diels, J.; Elsen, A.; Kusá, H.; Piccoli, I.; Poesen, J.; Stoate, C.; Tits, M.; et al. Soil Water Retention as Affected by Management Induced Changes of Soil Organic Carbon: Analysis of Long-Term Experiments in Europe. Land 2021, 10, 1362. [Google Scholar] [CrossRef]
- Veevaete, M. Applications of Earth’s Field NMR to Porous Systems and Polymer Gels. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2008. [Google Scholar]
- Bayer, J.V.; Jaeger, F.; Schaumann, G.E. Proton Nuclear Magnetic Resonance (NMR) Relaxometry in Soil Science Applications. Open Magn. Reson. J. 2010, 3, 15–26. [Google Scholar] [CrossRef]
- Climate Change Information Center Rhineland-Palatinate Annual Weather Report for Rhineland Palatinate. Available online: https://www.kwis-rlp.de/service/witterungsrueckblick/ (accessed on 24 October 2022).
- State Agency for Geology and Mining Soil Information for Hesse and Rhineland-Palatinate. Available online: https://www.lgb-rlp.de/fileadmin/service/lgb_downloads/boden/boden_allgemein/grossmassstaebige_bodeninformationen.pdf (accessed on 16 November 2022).
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Buchmann, C.; Bentz, J.; Schaumann, G.E. Intrinsic and Model Polymer Hydrogel-Induced Soil Structural Stability of a Silty Sand Soil as Affected by Soil Moisture Dynamics. Soil Tillage Res. 2015, 154, 22–33. [Google Scholar] [CrossRef]
- Holthusen, D.; Batistão, A.C.; Reichert, J.M. Amplitude Sweep Tests to Comprehensively Characterize Soil Micromechanics: Brittle and Elastic Interparticle Bonds and Their Interference with Major Soil Aggregation Factors Organic Matter and Water Content. Rheol. Acta 2020, 59, 545–563. [Google Scholar] [CrossRef]
- Bachmann, J.; Ellies, A.; Hartge, K.H. Development and Application of a New Sessile Drop Contact Angle Method to Assess Soil Water Repellency. J. Hydrol. 2000, 231–232, 66–75. [Google Scholar] [CrossRef]
- Bachmann, J.; Goebel, M.-O.; Woche, S.K. Small-Scale Contact Angle Mapping on Undisturbed Soil Surfaces. J. Hydrol. Hydromech. 2013, 61, 3–8. [Google Scholar] [CrossRef]
- Kristl, M.; Muršec, M.; Šuštar, V.; Kristl, J. Application of Thermogravimetric Analysis for the Evaluation of Organic and Inorganic Carbon Contents in Agricultural Soils. J. Therm. Anal. Calorim. 2016, 123, 2139–2147. [Google Scholar] [CrossRef]
- Tamimi, N.; Schaumann, G.E.; Diehl, D. The Fate of Organic Matter Brought into Soil by Olive Mill Wastewater Application at Different Seasons. J. Soils Sediments 2017, 17, 901–916. [Google Scholar] [CrossRef]
- Merino, A.; Ferreiro, A.; Salgado, J.; Fontúrbel, M.T.; Barros, N.; Fernández, C.; Vega, J.A. Use of Thermal Analysis and Solid-State 13C CP-MAS NMR Spectroscopy to Diagnose Organic Matter Quality in Relation to Burn Severity in Atlantic Soils. Geoderma 2014, 226–227, 376–386. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial Hotspots and Hot Moments in Soil: Concept & Review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernandez, J.M.; Haddix, M.L.; Steinweg, J.M.; Conant, R.T. Biological, Chemical and Thermal Indices of Soil Organic Matter Stability in Four Grassland Soils. Soil Biol. Biochem. 2011, 43, 1051–1058. [Google Scholar] [CrossRef]
- Barre, P.; Plante, A.F.; Cecillon, L.; Lutfalla, S.; Baudin, F.; Bernard, S.; Christensen, B.T.; Eglin, T.; Fernandez, J.M.; Houot, S.; et al. The Energetic and Chemical Signatures of Persistent Soil Organic Matter. Biogeochemistry 2016, 130, 1–12. [Google Scholar] [CrossRef]
- Rovira, P.; Kurz-Besson, C.; Coûteaux, M.-M.; Vallejo, V.R. Changes in Litter Properties during Decomposition: A Study by Differential Thermogravimetry and Scanning Calorimetry. Soil Biol. Biochem. 2008, 40, 172–185. [Google Scholar] [CrossRef]
- Donnelly, P.K.; Entry, J.A.; Crawford, D.L.; Cromack, K. Cellulose and Lignin Degradation in Forest Soils: Response to Moisture, Temperature, and Acidity. Microb. Ecol. 1990, 20, 289–295. [Google Scholar] [CrossRef]
- Coûteaux, M.-M.; Bottner, P.; Berg, B. Litter Decomposition, Climate and Liter Quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Mousavioun, P.; George, G.A.; Doherty, W.O.S. Environmental Degradation of Lignin/Poly(Hydroxybutyrate) Blends. Polym. Degrad. Stab. 2012, 97, 1114–1122. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The Potential of Stinging Nettle (Urtica dioica L.) as a Crop with Multiple Uses. Ind. Crop. Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Kuglerová, L.; García, L.; Pardo, I.; Mottiar, Y.; Richardson, J.S. Does Leaf Litter from Invasive Plants Contribute the Same Support of a Stream Ecosystem Function as Native Vegetation? Ecosphere 2017, 8, e01779. [Google Scholar] [CrossRef]
- Gaggini, L.; Rusterholz, H.-P.; Baur, B. The Invasive Plant Impatiens glandulifera Affects Soil Fungal Diversity and the Bacterial Community in Forests. Appl. Soil Ecol. 2018, 124, 335–343. [Google Scholar] [CrossRef]
- Jensen, J.L.; Schjønning, P.; Watts, C.W.; Christensen, B.T.; Peltre, C.; Munkholm, L.J. Relating Soil C and Organic Matter Fractions to Soil Structural Stability. Geoderma 2019, 337, 834–843. [Google Scholar] [CrossRef]
- Buchmann, C.; Schaumann, G.E. The Contribution of Various Organic Matter Fractions to Soil–Water Interactions and Structural Stability of an Agriculturally Cultivated Soil. J. Plant Nutr. Soil Sci. 2018, 181, 586–599. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Pértile, P.; Holthusen, D.; Gubiani, P.I.; Reichert, J.M. Microstructural Strength of Four Subtropical Soils Evaluated by Rheometry: Properties, Difficulties and Opportunities. Sci. Agric. 2018, 75, 154–162. [Google Scholar] [CrossRef]
- Le Bissonnais, Y. Aggregate Stability and Assessment of Soil Crustability and Erodibility: I. Theory and Methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Orts, W.J.; Sojka, R.E.; Glenn, G.M.; Gross, R.A. Preventing Soil Erosion with Polymer Additives. Polym. News 1999, 24, 406–413. [Google Scholar]
- Marin, M.; Hallett, P.D.; Feeney, D.S.; Brown, L.K.; Naveed, M.; Koebernick, N.; Ruiz, S.; Bengough, A.G.; Roose, T.; George, T.S. Impact of Root Hairs on Microscale Soil Physical Properties in the Field. Plant Soil 2022, 476, 491–509. [Google Scholar] [CrossRef]
- Koebernick, N.; Daly, K.R.; Keyes, S.D.; George, T.S.; Brown, L.K.; Raffan, A.; Cooper, L.J.; Naveed, M.; Bengough, A.G.; Sinclair, I.; et al. High-resolution Synchrotron Imaging Shows That Root Hairs Influence Rhizosphere Soil Structure Formation. New Phytol. 2017, 216, 124–135. [Google Scholar] [CrossRef]
- Dexter, A.R. Compression of Soil around Roots. Plant Soil 1987, 97, 401–406. [Google Scholar] [CrossRef]
- Woche, S.K.; Goebel, M.-O.; Kirkham, M.B.; Horton, R.; Van der Ploeg, R.R.; Bachmann, J. Contact Angle of Soils as Affected by Depth, Texture, and Land Management. Eur. J. Soil Sci. 2005, 56, 239–251. [Google Scholar] [CrossRef]
- Prietzel, J.; Müller, S.; Kögel-Knabner, I.; Thieme, J.; Jaye, C.; Fischer, D. Comparison of Soil Organic Carbon Speciation Using C NEXAFS and CPMAS 13C NMR Spectroscopy. Sci. Total Environ. 2018, 628–629, 906–918. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Hobley, E.; Hurraß, J.; Rotard, W. H-NMR Relaxometry to Monitor Wetting and Swelling Kinetics in High-Organic Matter Soils. Plant Soil 2005, 275, 1–20. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Werner, A.D.; Li, Y.; Jiang, S.; Tan, Z. Root-Induced Changes of Soil Hydraulic Properties—A Review. J. Hydrol. 2020, 589, 125203. [Google Scholar] [CrossRef]
- Gebauer, G.; Rehder, H.; Wollenweber, B. Nitrate, Nitrate Reduction and Organic Nitrogen in Plants from Different Ecological and Taxonomic Groups of Central Europe. Oecologia 1988, 75, 371–385. [Google Scholar] [CrossRef]
- Olsen, C. The Ecology of Urtica dioica. J. Ecol. 1921, 9, 1–18. [Google Scholar] [CrossRef]
- Rosnitschek-Schimmel, I. Effect of Ammonium and Nitrate Supply on Dry Matter Production and Nitrogen Distribution in Urtica dioica. Z. Pflanzenphysiol. 1982, 108, 329–341. [Google Scholar] [CrossRef]
- Lükewille, A.; Alewell, C. Acidification. In Encyclopedia of Ecology; Fath, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–241. ISBN 9780444641304. [Google Scholar]
- Tanner, R.A.; Gange, A.C. The Impact of Two Non-Native Plant Species on Native Flora Performance: Potential Implications for Habitat Restoration. Plant Ecol. 2013, 214, 423–432. [Google Scholar] [CrossRef]
- Rinklebe, J.; Langer, U. Microbial Diversity in Three Floodplain Soils at the Elbe River (Germany). Soil Biol. Biochem. 2006, 38, 2144–2151. [Google Scholar] [CrossRef]
- Tate, R.L.I. Effect of Flooding on Microbial Activities in Organic Soils: Carbon Metabolism. Soil Sci. 1979, 128, 267–273. [Google Scholar] [CrossRef]
- Dassonville, F.; Renault, P. Interactions between Microbial Processes and Geochemical Transformations under Anaerobic Conditions: A Review. Agronomie 2002, 22, 51–68. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Pietri, J.C.A.; Brookes, P.C. Relationships between Soil PH and Microbial Properties in a UK Arable Soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Friedman, S.P. Soil Properties Influencing Apparent Electrical Conductivity: A Review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Rhoades, J.D. Electrical Conductivity Methods for Measuring and Mapping Soil Salinity. In Advances in Agronomy; Sparks, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 49, pp. 201–251. ISBN 9780120007493. [Google Scholar]
- Lambs, L. Interactions between Groundwater and Surface Water at River Banks and the Confluence of Rivers. J. Hydrol. 2004, 288, 312–326. [Google Scholar] [CrossRef]
- Ontl, T.A.; Cambardella, C.A.; Schulte, L.A.; Kolka, R.K. Factors Influencing Soil Aggregation and Particulate Organic Matter Responses to Bioenergy Crops across a Topographic Gradient. Geoderma 2015, 255–256, 1–11. [Google Scholar] [CrossRef]
- Brax, M.; Buchmann, C.; Schaumann, G.E. Biohydrogel Induced Soil–Water Interactions: How to Untangle the Gel Effect? A Review. J. Plant Nutr. Soil Sci. 2017, 180, 121–141. [Google Scholar] [CrossRef]
- Diehl, D. The Role of the Organic Matter for Hydrophobicity in Urban Soils. Ph.D. Thesis, Universität Koblenz-Landau, Campus Landau, Landau, Germany, 2009. [Google Scholar]
- Leifeld, J.; Von Lützow, M. Chemical and Microbial Activation Energies of Soil Organic Matter Decomposition. Biol. Fertil. Soils 2014, 50, 147–153. [Google Scholar] [CrossRef]




| Parameter | |
|---|---|
| Texture | Loam silt |
| Sand (%) | 21.83 |
| Silt (%) | 68.80 |
| Clay (%) | 9.37 |
| Soil unit (WRB) [32] | gleysol |
| WHCmax (g kg−1) | 681 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamin, J.; Diehl, D.; Meyer, M.; David, J.; Schaumann, G.E.; Buchmann, C. Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study. Soil Syst. 2022, 6, 93. https://doi.org/10.3390/soilsystems6040093
Jamin J, Diehl D, Meyer M, David J, Schaumann GE, Buchmann C. Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study. Soil Systems. 2022; 6(4):93. https://doi.org/10.3390/soilsystems6040093
Chicago/Turabian StyleJamin, Jellian, Dörte Diehl, Michele Meyer, Jan David, Gabriele Ellen Schaumann, and Christian Buchmann. 2022. "Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study" Soil Systems 6, no. 4: 93. https://doi.org/10.3390/soilsystems6040093
APA StyleJamin, J., Diehl, D., Meyer, M., David, J., Schaumann, G. E., & Buchmann, C. (2022). Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study. Soil Systems, 6(4), 93. https://doi.org/10.3390/soilsystems6040093






