Selenium Uptake by Lettuce Plants and Se Distribution in Soil Chemical Phases Affected by the Application Rate and the Presence of a Seaweed Extract-Based Biostimulant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Selection and Characterization of Soil Physicochemical Properties
2.2. Experimental Design
2.3. Plant Tissue Analysis
2.4. Selenium Fractionation
2.5. Statistical Analysis
3. Results
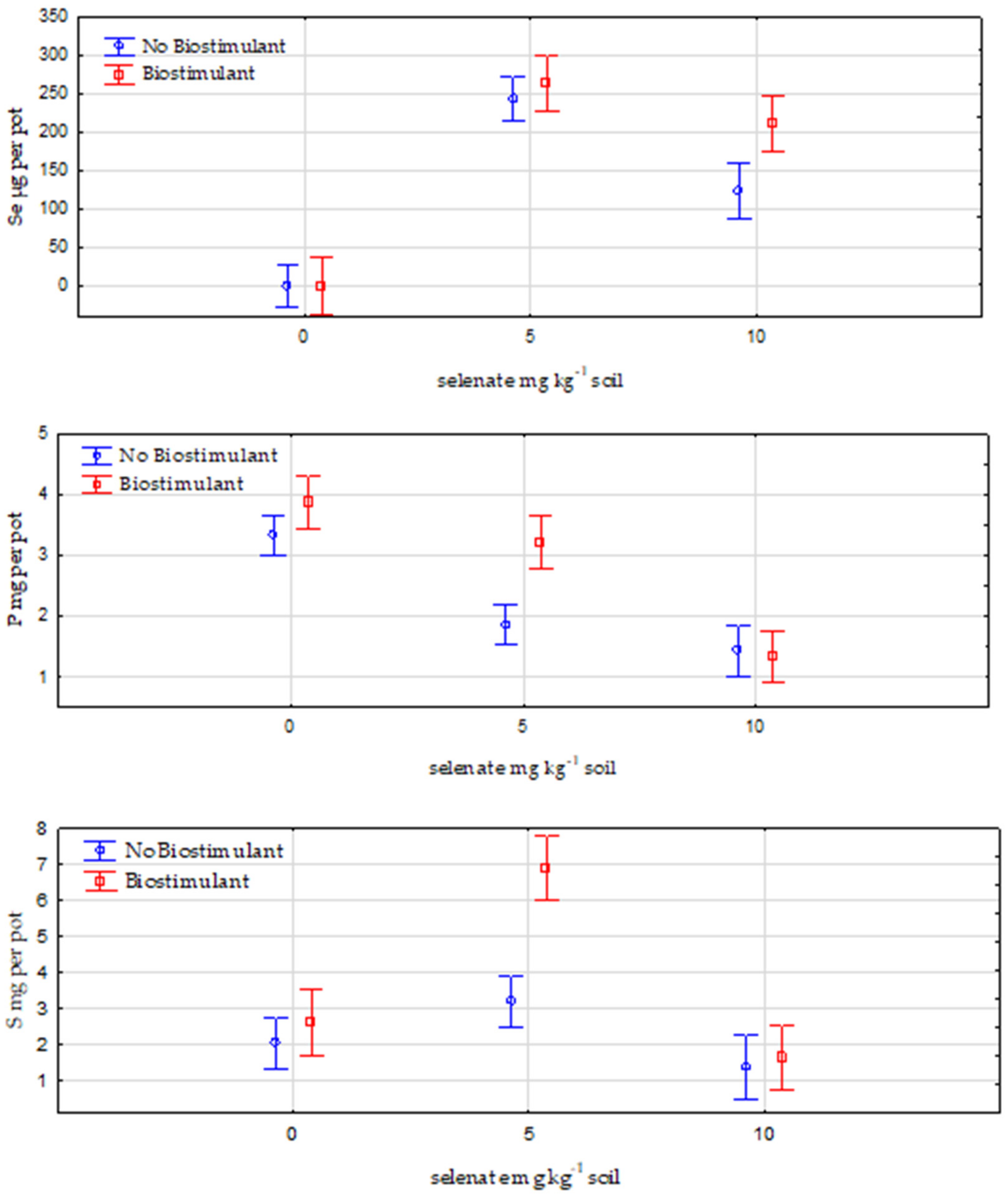
3.1. Plant Tissues
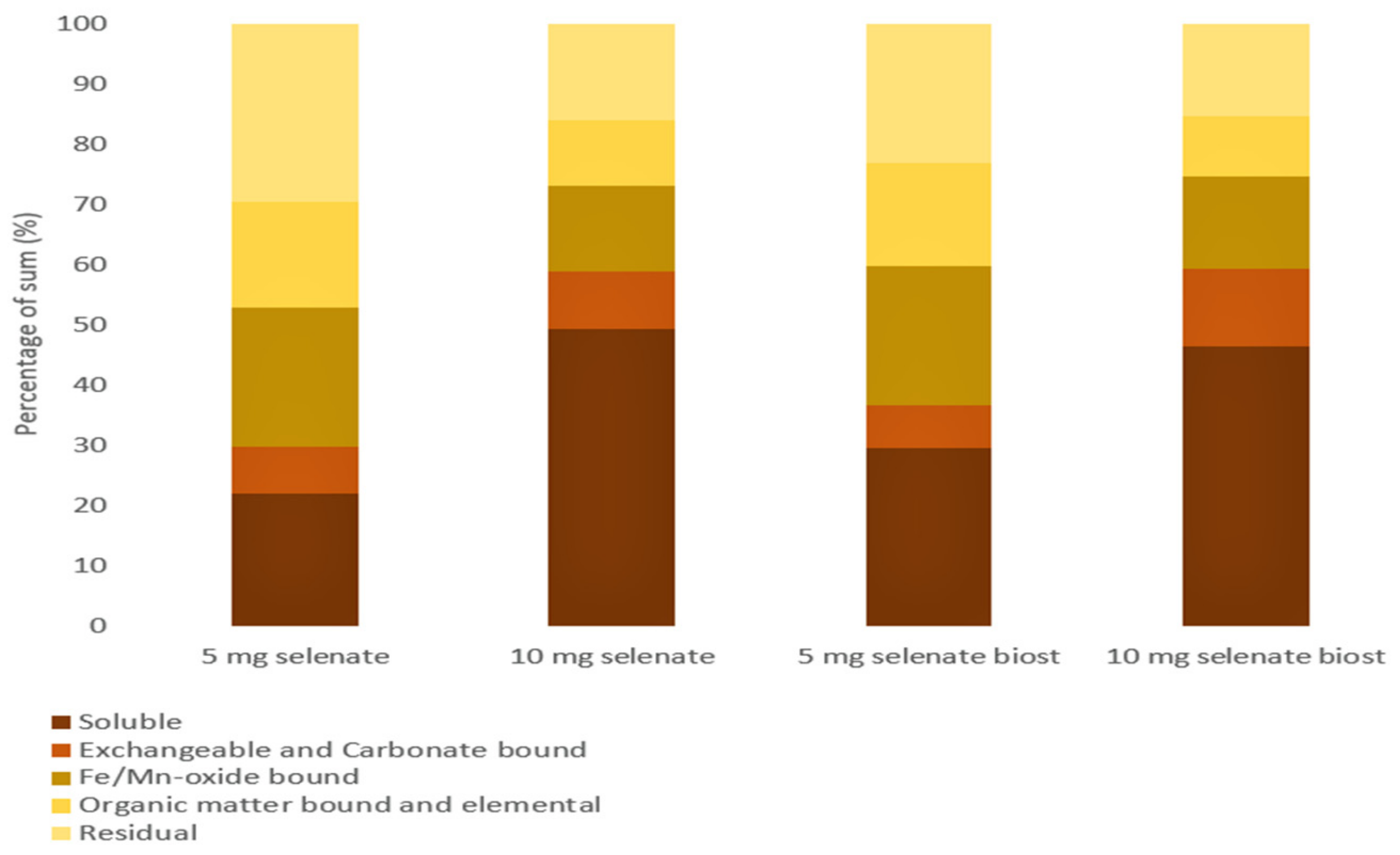
3.2. Se Fractions
4. Discussion
Total Selenium, Phosphorus and Sulfur Uptake by Lettuce Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.D.; Garg, U. Chapter 17—Disorders of trace metals. In Clinical Aspects and Laboratory Determination, Biomarkers in Inborn Errors of Metabolism; Garg, U., Smith, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 399–426. ISBN 9780128028964. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium Biofortification of Lettuce Plants (Lactuca sativa L.) as Affected by Se Species, Se Rate, and a Biochar Co-Application in a Calcareous Soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Natasha Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Massas, I. Adsorption/Desorption Patterns of Selenium for Acid and Alkaline Soils of Xerothermic Environments. Environments 2020, 7, 72. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Wang, D.; Wei, W.; Fu, D.; Lin, Z. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427–428, 159–164. [Google Scholar] [CrossRef]
- Dhillon, K.; Dhillon, S.; Pareek, N. Distribution and bioavailability of selenium fractions in some seleniferous soils of Punjab, India. Arch. Agron. Soil Sci. 2005, 51, 633–643. [Google Scholar] [CrossRef]
- De Temmerman, L.; Waegeneers, N.; Thiry, C.; Du Laing, G.; Tack, F.; Ruttens, A. Selenium content of Belgian cultivated soils and its uptake by field crops and vegetables. Sci. Total Environ. 2014, 468–469, 77–82. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef]
- Page, A.L. (Ed.) Methods of Soil Analysis, Part. 2, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Bouyoucos, G.J. A recalibration of the hydrometer method for making mechanical analysis of soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part. 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA-SSSA: Madison, WI, USA, 1982. [Google Scholar]
- NF ISO 10693; Détermination de la Teneuren Carbonate—MéthodeVolumétrique. Qualité des Sols AFNOR: Paris, France, 1995; pp. 177–186.
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis, Part. 3, Chemical Methods; Bigham, J.M., Bartels, J.M., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 437–474. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939, pp. 1–19.
- Schwertmann, U.; Taylor, R.M. Iron oxides. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 379–438. [Google Scholar]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clay by a dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Min. 2013, 7, 317–327. [Google Scholar] [CrossRef]
- Gasparatos, D.; Haidouti, C.; Tarenidis, D. Characterization of iron oxides in Fe-rich concretions from an imperfectly-drained Greek soil: A study by selective-dissolution techniques and X-ray diffraction. Arch. Agron. Soil Sci. 2004, 50, 485–493. [Google Scholar] [CrossRef]
- Bonfil, D.J.; Gitelson, A.A. RapidScan and CropCircle radiometers: Opportunities and limitation in assessing wheat biomass and nitrogen. In Proceedings of the 12th International Conference on Precision Agriculture, Sacramento, CA, USA, 20–23 July 2014. [Google Scholar]
- Jones, J.B.; Case, V.W. Sampling, Handling and Analysing Plant Tissue Samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Book Series No. 3; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Cartes, P.; Jara, A.; Pinilla, L.; Rosas, A.; Mora, M. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Esringu, A.; Ekinci, M.; Usta, S.; Turan, M.; Dursun, A.; Ercisli, S.; Yildirim, E. Selenium supplementation affects the growth, yield and selenium accumulation in lettuce (Lactuca sativa L.). C. R. Acad. Bulg. Sci. 2015, 68, 801. [Google Scholar]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Massas, I. Selenium uptake by rocket plants (Eruca sativa) grown in a calcareous soil as affected by Se species, Se rate and a seaweed extract-based biostimulant application. Crop Pasture Sci. 2022. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Karapouloutidou, S.; Gasparatos, D. Effects of Biostimulant and Organic Amendment on Soil Properties and Nutrient Status of Lactuca Sativa in a Calcareous Saline-Sodic Soil. Agriculture 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Sinka, L.; Zsembeli, J.; Ragán, P.; Duzs, L.; Hájos, M.T. Effect of different seedling growing methods on the SPAD, NDVI values and some morphological parameters of four sweet corn (Zea mays L.) hybrids. Agriculture 2021, 67, 177–190. [Google Scholar] [CrossRef]
- Wang, J.; Bailey, E.H.; Sanders, H.K.; Izquierdo, M.; Crout, N.M.J.; Shaw, G.; Yang, L.; Li, H.; Wei, B.; Young, S.D. Using chemical fractionation and speciation to describe uptake of technetium, iodine and selenium by Agrostis capillaris and Lolium perenne. J. Environ. Radioact. 2020, 212, 106131. [Google Scholar] [CrossRef]
- Shao, Y.; Xie, B.; Li, M.; Zhang, X.; Xiao, H. Selenium Fractionation and Speciation in Paddy Soils and Accumulation in Rice under Field Conditions in Jinhua Zhejinang Province, China. In Proceedings of the E3S Web of Conferences, Guilin, China, 20–22 December 2019. [Google Scholar]
- Tokunaga, T.K.; Lipton, D.S.; Benson, S.M.; Yee, A.W.; Oldfather, J.M.; Duckart, E.C.; Johannis, P.W.; Halvorsen, K.E. Soil selenium fractionation, depth profiles and time trends in a vegetated site at Kesterson Reservoir. Water Air Soil Pollut. 1991, 57–58, 31–41. [Google Scholar] [CrossRef]
- Bassil, J.; Naveau, A.; Bueno, M.; Tullo, P.D.; Grasset, L.; Kazpard, V.; Razack, M. Determination of the distribution and speciation of selenium in an argillaceous sample using chemical extractions and post-extractions analyses: Application to the hydrogeological experimental site of Poitiers. Environ. Sci. Pollut. Res. 2016, 23, 9598–9613. [Google Scholar] [CrossRef]
- Lyu, C.H.; Qin, Y.J.; Zhao, Z.Q.; Liu, X.W. Characteristics of selenium enrichment and assessment of selenium bioavailability using the diffusive gradients in thin-films technique in seleniferous soils in Enshi, Central China. Environ. Pollut. 2021, 273, 116507. [Google Scholar] [CrossRef]
- Ali, F.; Peng, Q.; Wang, D.; Cui, Z.W.; Liang, D.L. Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2017, 24, 8315.e8325. [Google Scholar] [CrossRef]
- Li, Z.; Man, N.; Wang, S.S.; Liang, D.L.; Liu, J.J. Selenite adsorption and desorption in main chinese soils with their characteristics and physicochemical properties. J. Soils Sediments 2015, 15, 1150–1158. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Li, Z.; Tran, T.A.T.; Wang, D.; Liang, D.L. Role of organic acids on the bioavailability of selenium in soil: A review. Chemosphere 2017, 184, 618–663. [Google Scholar] [CrossRef]
- Wang, M.K.; Cui, Z.W.; Xue, M.Y.; Peng, Q.; Zhou, F.; Wang, D.; Dinh, Q.T.; Liu, Y.X.; Liang, D.L. Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ. 2019, 689, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.Y.; Gu, M.H.; Li, H.; Shohag, M.J.I.; Shen, F.K.; Wang, X.L.; Wei, Y.Y. Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Sci. Total Environ. 2020, 748, 141166. [Google Scholar] [CrossRef]
- Di Tullo, P.; Pannier, F.; Thiry, Y.; Le Hecho, I.; Bueno, M. Filed study of time-dependent selenium partitioning in soil using isotopically enriched selenite tracer. Sci. Total Environ. 2016, 562, 280.e288. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Clay (g kg−1) | 180 |
| Silt (g kg−1) | 420 |
| Sand (g kg−1) | 400 |
| pH (1:1) | 7.40 |
| EC (mS cm−1) | 0.575 |
| CaCO3 (g kg−1) | 184 |
| Act. CaCO3 (g kg−1) | 47.5 |
| Org. matter (g kg−1) | 13.0 |
| P-Olsen (mg kg−1) | 15.40 |
| CEC (cmolc kg−1) | 22.40 |
| Fed (g kg−1) | 11.6 |
| Feo (g kg−1) | 1.4 |
| Mnd (g kg−1) | 0.7 |
| Mno (g kg−1) | 0.4 |
| Ald (g kg−1) | 0.5 |
| Alo (g kg−1) | 0.6 |
| Total Se (μg kg−1) | 156 |
| Step | Fraction | Reagents | Procedure |
|---|---|---|---|
| 1 | Soluble | 10 mL 0.25 mol L−1 KCl | 1 h shaking 200 rpm T = 25 °C |
| 2 | Exchangeable and carbonate bound | 10 mL 0.7 mol L−1 KH2PO4 (pH = 5) | 4 h shaking 200 rpm T = 25 °C |
| 3 | Fe/Mn-oxide bound | 10 mL 2.5 mol L−1 HCl | 50 min heating in a water bath shaking intermittently T = 90 °C |
| 4 | Organic matter bound and elemental | 8 mL 5% K2S2O8 2 mL conc. HNO3 | 3 h heating in a water bath Capped vials Shaking intermittently T = 90 °C |
| 5 | Residual | 8 mL conc. HNO3 2 mL conc. HClO4 | Transferring into Teflon crucibles with the reagents Heating in a sand bath until the soil turns white or gray in color. Covered crucibles T = 170 °C. Transfer remaining solution in 25 mL volumetric flask with DI water |
| Treatment | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|
| mg Se kg−1 Soil | 0 | 5 | 10 | 0 | 5 | 10 |
| No Biostimulant | Biostimulant | |||||
| Se (μg g−1) | 0.14 a | 196.74 c | 110.9 b | 0.15 a | 147.6 bc | 325.42 d |
| P (mg g−1) | 2.41 c | 1.5 ab | 1.27 a | 2.48 c | 1.8 b | 2.04 b |
| S (mg g−1) | 1.47 a | 2.58 b | 1.24 a | 1.66 a | 3.77 c | 2.5 b |
| F.W. (g) 1 | 12.28 b | 13.32 b | 7.55 ab | 14.6 b | 21.16 c | 2.43 a |
| D.W. (g) 2 | 1.38 c | 1.31 c | 1.12 b | 1.57 c | 1.81 c | 0.65 a |
| SPAD | 24.36 b | 20.04 ab | 15.26 a | 26.3 b | 36.16 c | 16 a |
| NDRE | 0.342 b | 0.253 ab | 0.329 b | 0.351 b | 0.324 b | 0.188 a |
| NDVI | 0.9118 b | 0.8218 b | 0.791 b | 0.899 b | 0.912 b | 0.561 a |
| Treatment | Soluble (Sol-Se) (μg kg−1) | Exchangeable and Carbonate Bound (EXC-Se) (μg kg−1) | Fe/Mn-Oxide Bound (Fe/Mn-Se) (μg kg−1) | Organic Matter Bound and Elemental (OM-Se) (μg kg−1) | Residual (Res-Se) (μg kg−1) | Recovery Factor (%) |
|---|---|---|---|---|---|---|
| T2 | 992 | 361 | 1050 | 794 | 1350 | 114 |
| T3 | 3850 | 760 | 1100 | 860 | 1260 | 112 |
| T5 | 1300 | 310 | 1020 | 750 | 1020 | 111 |
| T6 | 3130 | 870 | 1040 | 670 | 1050 | 109 |
| Se μg kg−1 | P mg kg−1 | S mg kg−1 | D.W. | SPAD | NDVI | NDRE | |
|---|---|---|---|---|---|---|---|
| Se μg kg−1 | −0.48 | +0.55 | −0.57 | −0.82 | −0.73 | ||
| P mg kg−1 | −0.48 | ||||||
| S mg kg−1 | +0.55 | +0.46 | |||||
| D.W. | −0.57 | +0.82 | +0.76 | +0.53 | |||
| SPAD | +0.46 | +0.82 | +0.62 | ||||
| NDVI | −0.82 | +0.76 | +0.62 | +0.57 | |||
| NDRE | −0.73 | +0.53 | +0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Massas, I. Selenium Uptake by Lettuce Plants and Se Distribution in Soil Chemical Phases Affected by the Application Rate and the Presence of a Seaweed Extract-Based Biostimulant. Soil Syst. 2022, 6, 56. https://doi.org/10.3390/soilsystems6020056
Zafeiriou I, Gasparatos D, Ioannou D, Massas I. Selenium Uptake by Lettuce Plants and Se Distribution in Soil Chemical Phases Affected by the Application Rate and the Presence of a Seaweed Extract-Based Biostimulant. Soil Systems. 2022; 6(2):56. https://doi.org/10.3390/soilsystems6020056
Chicago/Turabian StyleZafeiriou, Ioannis, Dionisios Gasparatos, Dafni Ioannou, and Ioannis Massas. 2022. "Selenium Uptake by Lettuce Plants and Se Distribution in Soil Chemical Phases Affected by the Application Rate and the Presence of a Seaweed Extract-Based Biostimulant" Soil Systems 6, no. 2: 56. https://doi.org/10.3390/soilsystems6020056
APA StyleZafeiriou, I., Gasparatos, D., Ioannou, D., & Massas, I. (2022). Selenium Uptake by Lettuce Plants and Se Distribution in Soil Chemical Phases Affected by the Application Rate and the Presence of a Seaweed Extract-Based Biostimulant. Soil Systems, 6(2), 56. https://doi.org/10.3390/soilsystems6020056







