Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical and Biological Soil Characterization
2.2. Treatments and Experimental Protocol
2.3. Quantification of Mn, Mg, Ca, and K in the Soil Solution and Wheat Shoot Tissues
2.4. Data Analysis
3. Results
3.1. Wheat Growth
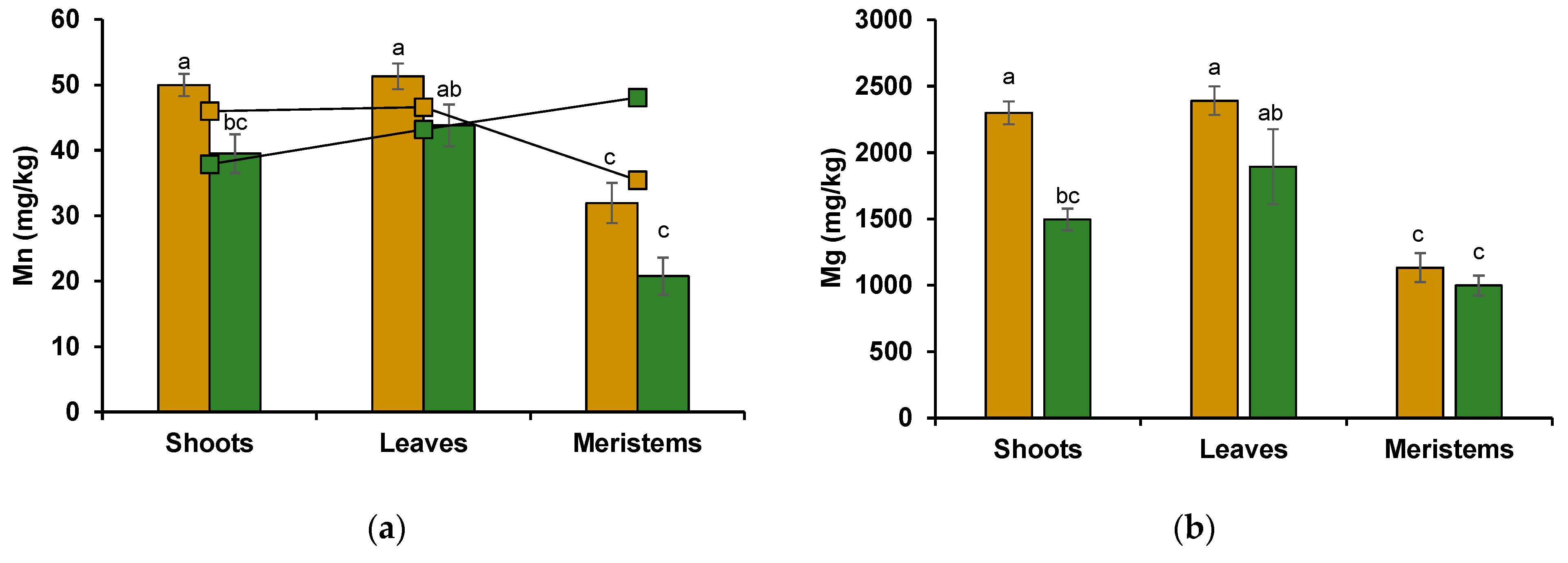
3.2. Mn, Mg, Ca, and K Concentration
3.2.1. Soil Solution
3.2.2. Wheat Shoot Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trinchera, A.; Ciaccia, C.; Testani, E.; Baratella, V.; Campanelli, G.; Leteo, F.; Canali, S. Mycorrhiza-mediated interference between cover crop and weed in organic winter cereal agroecosystems: The mycorrhizal colonization intensity indicator. Ecol. Evol. 2019, 9, 5593–5604. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2011, 84, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The Future Prospect for Sustainable Agriculture; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 102, pp. 36–45. [Google Scholar]
- Web of Science. Available online: www.webofscience.com (accessed on 29 January 2022).
- Humphreys, C.P.; Franks, P.J.; Rees, M.; Bidartondo, M.I.; Leake, J.R.; Beerling, D.J. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat. Commun. 2010, 1, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glomeromycota PHYLOGENY. Available online: http://amf-phylogeny.com/ (accessed on 29 January 2022).
- Thirkell, T.J.; Pastok, D.; Field, K.J. Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Glob. Chang. Biol. 2020, 26, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Aluminium, Iron and Silicon Subcellular Redistribution in Wheat Induced by Manganese Toxicity. Appl. Sci. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Rengel, Z.; Wilson, R.; Setter, T.L. Variation of tolerance to manganese toxicity in Australian hexaploid wheat. J. Plant Nutr. Soil Sci. 2010, 173, 103–112. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef]
- Faria, J.; Pinto, A.P.; Teixeira, D.; Brito, I.; Dias, L.; Barrulas, P.; Alho, L.; Carvalho, M. Elemental composition and antioxidant enzyme activity of roots and shoots of wheat grown in manganese spiked Montado alentejano soil. Free Radic. Biol. Med. 2018, 120, S151. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Alho, L.; Goss, M.J. Managing arbuscular mycorrhizal fungi for bioprotection: Mn toxicity. Soil Biol. Biochem. 2014, 68, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Alho, L.; Carvalho, M.; Brito, I.; Goss, M.J. The effect of arbuscular mycorrhiza fungal propagules on the growth of subterranean clover (Trifolium subterraneum L.) under Mn toxicity in ex situ experiments. Soil Use Manag. 2015, 31, 337–344. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; Alho, L.; Brígido, C.; van Tuinen, D.; Félix, M.R.; Carvalho, M. Agronomic management of AMF functional diversity to overcome biotic and abiotic stresses—The role of plant sequence and intact extraradical mycelium. Fungal Ecol. 2019, 40, 72–81. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Arbuscular Mycorrhiza Inoculum Type Influences Phosphorus Subcellular Distribution in Shoots of Wheat Grown in Acidic Soil under Sustainable Agricultural Practices. Biol. Life Sci. Forum 2020, 4, 62. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.; Brito, I.; Carvalho, M. Diversity of Native Arbuscular Mycorrhiza Extraradical Mycelium Influences Antioxidant Enzyme Activity in Wheat Grown Under Mn Toxicity. Bull. Environ. Contam. Toxicol. 2021, 108, 451–456. [Google Scholar] [CrossRef]
- Campos, C.; Carvalho, M.; Brígido, C.; Goss, M.J.; Nobre, T. Symbiosis Specificity of the Preceding Host Plant Can Dominate but Not Obliterate the Association Between Wheat and Its Arbuscular Mycorrhizal Fungal Partners. Front. Microbiol. 2018, 9, 2920. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.J.; Carvalho, M.J.G.P.R.; Van Beusichem, M.L.; Kirkby, E.A. The significance of the magnesium to manganese ratio in plant tissues for growth and alleviation of manganese toxicity in tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Ciênc. Agrár. 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.J.G.P.R.; Cosimini, V.; Fearnhead, M.L. An approach to the identification of potentially toxic concentrations of manganese in soils. Soil Use Manag. 1992, 8, 40–43. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Brito, I.; de Carvalho, M.; Goss, M.J. Summer survival of arbuscular mycorrhiza extraradical mycelium and the potential for its management through tillage options in Mediterranean cropping systems. Soil Use Manag. 2011, 27, 350–356. [Google Scholar] [CrossRef]
- Campos, C.; Nobre, T.; Goss, M.J.; Faria, J.; Barrulas, P.; Carvalho, M. Transcriptome Analysis of Wheat Roots Reveals a Differential Regulation of Stress Responses Related to Arbuscular Mycorrhizal Fungi and Soil Disturbance. Biology 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, E.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Influence of soil chemistry and plant physiology in the phytoremediation of Cu, Mn, and Zn. Crit. Rev. Plant Sci. 2014, 33, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Goss, M.J.; Carvalho, M.J.G.P.R. Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant Soil 1992, 139, 91–98. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Zhang, F.; Du, P.; Song, C.; Wu, Q. Alleviation of Mycorrhiza to Magnesium Deficiency in Trifoliate Orange: Changes in Physiological Activity. Emirates J. Food Agric. 2015, 27, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Kothari, S.K.; Marschner, H.; Römeheld, V. Effect of a vesicular—Arbuscular mycorrhizal fungus and rhizosphere micro-organisms on manganese reduction in the rhizosphere and manganese concentrations in maize (Zea mays L.). New Phytol. 1991, 117, 649–655. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Cardoso, E.J.B.N. Mycorrhizal effectiveness and manganese toxicity in soybean as affected by soil type and endophyte. Sci. Agric. 2003, 60, 329–335. [Google Scholar] [CrossRef]


| Elements (mg/L) | Mn | Mg | Ca | K |
|---|---|---|---|---|
| Acidic soil | 110.7 ± 3.1 a | 7566.3 ± 721.4 a | 23,247.6 ± 1351.7 a | 3060.5 ± 8.4 a |
| LOL/Wheat | 74.0 ± 7.5 b | 6700.2 ± 478.4 a | 22,518.3 ± 1783.1 a | 5171.7 ± 515.4 b |
| ORN/Wheat | 138.8 ± 8.7 a | 12,028.4 ± 1290.0 b | 37,445.7 ± 849.0 b | 7900.4 ± 115.1 c |
| Plant Part/Bioconcentration Factor | Mn × 10−1 | Mg × 10−1 | Ca × 10−2 | K | |
|---|---|---|---|---|---|
| Shoot | LOL | 6.9 ± 0.3 a | 3.5 ± 0.1 a | 1.8 ± 0.1 a | 7.6 ± 0.4 a |
| ORN | 2.9 ± 0.1 b,c | 1.3 ± 0.1 a,b | 1.0 ± 0.0 b | 4.0 ± 0.1 b | |
| Leaves | LOL | 7.1 ± 0.3 a | 3.6 ± 0.1 a | 1.8 ± 0.1 a | 7.7 ± 0.4 a |
| ORN | 3.2 ± 0.2 c | 1.6 ± 0.1 b | 1.1 ± 0.0 b | 4.6 ± 0.3 b | |
| Meristems | LOL | 4.4 ± 0.3 c | 1.7 ± 0.1 c | 1.9 ± 0.1 a | 5.9 ± 0.5 c |
| ORN | 1.5 ± 0.1 b | 0.8 ± 0.0 d | 0.9 ± 0.0 b | 3.5 ± 0.1 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Teixeira, D.M.; Ferreira, D.; Barrulas, P.; Brito, I.; Pinto, A.P.; Carvalho, M. Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil. Soil Syst. 2022, 6, 50. https://doi.org/10.3390/soilsystems6020050
Faria JMS, Teixeira DM, Ferreira D, Barrulas P, Brito I, Pinto AP, Carvalho M. Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil. Soil Systems. 2022; 6(2):50. https://doi.org/10.3390/soilsystems6020050
Chicago/Turabian StyleFaria, Jorge M. S., Dora Martins Teixeira, Diana Ferreira, Pedro Barrulas, Isabel Brito, Ana Paula Pinto, and Mário Carvalho. 2022. "Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil" Soil Systems 6, no. 2: 50. https://doi.org/10.3390/soilsystems6020050
APA StyleFaria, J. M. S., Teixeira, D. M., Ferreira, D., Barrulas, P., Brito, I., Pinto, A. P., & Carvalho, M. (2022). Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil. Soil Systems, 6(2), 50. https://doi.org/10.3390/soilsystems6020050









