Abstract
A tef-Acacia decurrens-charcoal production rotation system, a unique indigenous climate-smart agricultural technology of northwest Ethiopia, is increasingly seen as a promising strategy for improving soil properties. This study investigated the effect of the tef-Acacia decurrens-charcoal production rotation system on soil properties. In total, 112 soil samples (7 treatments × 4 depths × 4 replicates) were collected and analyzed inside and outside randomly selected charcoal production spots in the tef-Acacia decurrens-charcoal production rotation system and from an adjacent tef monocropping system. The soil properties examined generally exhibited significant variation between the tef monocropping system and the tef-Acacia decurrens-charcoal production rotation system, and between soil depths, as well as with respect to charcoal production spots in the system. The system resulted in a significant increase in SOC, TN, available phosphorus, available sodium, available nitrate and ammonium in general, and in total contents of K, P and Mn in the 0–20 cm depth. Charcoal production in the system significantly increased the total content of P, Al, and Fe, as well as the available nitrate and sulfate in the charcoal production spot. The variation in soil proprieties between the land use types and with respect to charcoal production spots in the TACP system were possibly due to the effect of the Acacia decurrens trees, and fire and fine charcoal residues from charcoal production, indicating the capacity of the tef-Acacia decurrens-charcoal production rotation system to improve soil properties.
1. Introduction
Poor land management and plant nutrient depletion are increasingly seen as a fundamental biophysical causes of declining food security among smallholder farm households in sub-Saharan Africa (SSA) [1,2,3,4]. In addition, anthropogenic climate change, the increasing costs of mineral fertilizers and land grabbing are continuously exacerbating this situation [5,6]. In Ethiopia, the problems are even more severe. The majority of Ethiopian farmers are living on land prone to degradation due to low nutrient inputs, soil erosion and droughts, the extent of which is increasing due to an increasing population density [7,8]. Moreover, due to the associated loss of soil fertility and resilience to climate change, soil degradation is one of the most serious constraints limiting crop productivity in Ethiopia [9,10,11].
The most prevalent land management activities for fighting these problems and improving food security in smallholder crop farming are the expansion and intensification of agricultural practices [12,13,14]. However, the increased expansion and intensification of agricultural practices could result in declining soil fertility due to severe soil disturbance, thus increasing the decomposition of soil organic matter (SOM) and soil nutrient depletion [15,16]. A feasible option for addressing this problem may be the adoption of climate-smart agricultural technologies (CSAT) [17,18]. Climate-smart agriculture (CSA) is an approach that helps to guide the actions needed to transform and reorient agricultural systems to effectively support development and ensure food security in a changing climate [17,19]. The three pillars of CSA are: to sustainably increase agricultural productivity and improve the income and livelihood of farmers; to build resilience and adaptation to climate change; and to reduce and/or remove greenhouse gas (GHG) emissions, where possible [19].
Smallholder farmers in SSA have developed a number of alternative CSAT that they can use either alone or in combination to build resilience against climate-induced calamities such as severe drought [20,21,22,23]. Agroforestry is one of the paradigmatic alternatives to climate-smart agriculture [22]. It is a land use practice that introduces trees and/or shrubs to croplands and/or livestock [24,25]. It is one of the beneficial land management practices for increasing soil carbon (C) storage, potentially mitigating GHG emissions [26,27] and improving soil fertility [28,29] in agricultural landscapes. In SSA, agroforestry practices can increase crop yields by 2 to 4 times compared with monocropping [30]. In their reviews, [31] found that the absolute rate of soil organic carbon (SOC) sequestration of agroforestry systems in SSA was up to 14 Mg C ha−1 y−1.
The tef-Acacia decurrens-charcoal production rotation system (referred hereafter as the TACP system) is a unique (and rapidly expanding) agroforestry system in the Fagita Lekoma district of the northwestern highlands of Ethiopia. It involves the intercropping of a tef crop, A. decurrens (a fast-growing acacia tree species that is adapted to acidic soil conditions [32]) and the production of charcoal on the same piece of land in rotation. The TACP system has been adopted by the local farmers in the study area for the last 30 years; it is one of the two agricultural systems in the study area, along with a tef monocropping system (TM system), from which the TACP system was developed. The charcoal is produced using traditional mound kilns. In this system, the charcoal is produced for marketing by burning wood of A. decurrens by a method explained in detail by [33]. In the TACP system, charcoal residues and charred biomass left on the kiln sites could help to ameliorate and improve the fertility of the soils by direct addition and retention of nutrients [34].
The application of charcoal (biochar) to soil is an emerging strategy for sequestering carbon, reducing GHG emissions and improving soil quality in recent years [35,36,37,38]. Evidence has shown that the application of biochar can play a significant role in enhancing SOC content and hence carbon sequestration [39], water-holding capacity [40,41], soil aeration, base saturation, nutrient retention and availability; decreasing fertilizer needs and nutrient leaching [37]; stimulating microbial biomass and activity [42]; enhancing crop yield and reducing anthropogenic GHG fluxes [35,43]. Significant changes in soil properties, such as soil pH, base saturation, electrical conductivity, exchangeable Ca, Mg, K, Na, and available P (Pav) in the soil were also observed in studies carried out to investigate the effect of charcoal production at kiln sites in agricultural and forestry systems in different parts of the world [44,45,46,47]. Furthermore, biochar can reduce the risk of environmental pollutants (organic and inorganic) in soils by forming complexes or through sorption of heavy metals and/or organic compounds [48,49,50]. Nonetheless, biochar may contain contaminates itself, either introduced by its feedstock (e.g., heavy metals) or co-produced during pyrolysis.
In general, trees can exert positive, negative or neutral effects on soil properties and plant communities in agroforestry systems, depending on the local environmental conditions and the position in the landscape. The inclusion of nitrogen-fixing trees, such as A. decurrens, in the TACP system can potentially improve the physicochemical and biological soil conditions through numerous processes, including biological N2 fixation, maintenance of or increases in SOM, uptake of nutrients from below the reach of crop roots, increased water infiltration and storage, reduced loss of nutrients by erosion and leaching, improved the soil’s physical properties, reduced soil acidity and improved soil biological activity [25,51]. Several authors have reported on the effect of trees on soil fertility and the associated crop yields in Ethiopia [52,53,54,55,56]. For example, the effect of Acacia trees on the soil’s physical, chemical and biological properties have been studied extensively in different settings [57,58,59].
While much is known about the independent effects of biochar and nitrogen-fixing trees on soil properties, few studies have examined the synergetic effect of charcoal production and nitrogen-fixing trees such as A. decurrens for modification of the soil properties in agroforestry. This study was designed (1) to assess the impact of the TACP system (in three rotations) on soil properties as compared with the TM system and (2) to evaluate the influence of charcoal production on soil properties in the TACP system under the charcoal production spots.
2. Materials and Methods
The study site is located in the Fagita Lekoma district in the Awi zone of the Amhara region of northwestern Ethiopia (10°57′23″ N to 11°11′21″ N, 36°40′01″ E to 37°05′21″ E, between 1800 and 2900 m above mean sea level) (Figure 1). The mean monthly rainfall between 2007 to 2017 was 1328 mm [60] with an estimated average annual temperature of 17.5 °C [61] (Figure 2). The area is part of the moist subtropical agro-ecological zone of the northwestern highlands of Ethiopia. Farmers in the district practice mixed subsistence cropping–livestock farming systems. The major crops grown are tef (Eragrostis tef Zucc.), barley (Hordeum vulgare L.), wheat (Triticum aestivum L.) and potato (Solanum tuberosum L.). The predominant soil types are Nitisols and Acrisols, according to the World Reference Base for Soil Resources (WRB), which are characterized by moderately and strongly acidic conditions, respectively [10,62]. The soil textural fractions of the study area are presented in Table 1. The area is characterized by flatlands and a moderately steep rolling topography, which covers 65% of the district [63]. The major land use categories of the district are agriculture (60.8%) and forestry (19.5%), while the remaining area is used for grazing land and settlement [64].
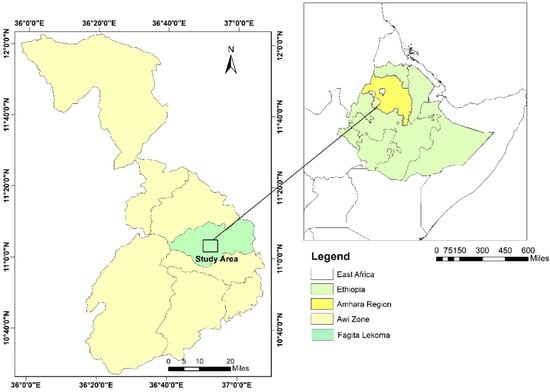
Figure 1.
Map of the study area.
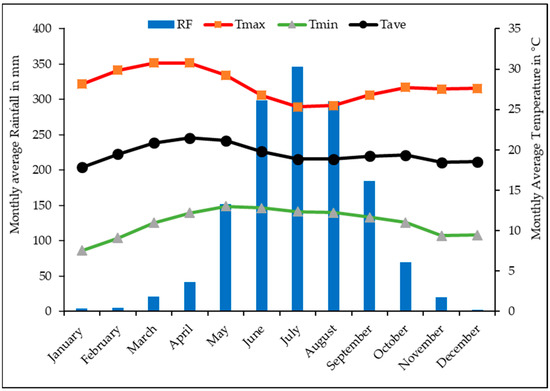
Figure 2.
Maximum, minimum and average temperatures, and mean monthly rainfall of Fagita Lekoma district from 2007 to 2017.The data used in this figure were extracted with permission from [65].

Table 1.
Soil textural fractions (%) of soil samples studied in relation to land use types (TM and TACP system) and soil depths (mean ± standard error of the mean).
The main fertilizers used in the TM system are urea and diammonium phosphate (DAP). Both are applied mostly at a rate of about 50 kg ha−1, but the rate varies (40–60 kg ha−1) depending on the socioeconomic status of the farmers [63,64]. In the TACP system, A. decurrens trees are planted in the fields at about 25–50 cm spacing immediately after tef is cultivated. The tef is harvested after 3 to 5 months, whereas A. decurrens is grown for about 4 to 5 years. Thereafter, the trees are harvested to produce charcoal in the same field (Figure 3).
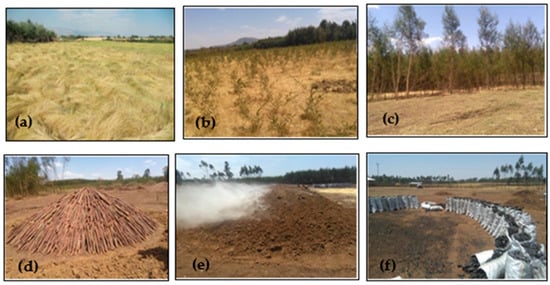
Figure 3.
Photographs of (a) the TM system; (b) the TACP system: [(c) Acacia decurrens seedlings planted with tef, showing Acacia decurrens at the tree stage; (d) piles of Acacia decurrens wood; (e) a charcoal production kiln and (f) harvesting of the charcoal, in the TACP system].
2.1. Soil Sampling and Laboratory Analysis
The soil samples were taken from the TM and the TACP system (in the first, second and third rotations) with four replicates from four depths (0–20, 20–40, 40–60 and 60–100 cm). In the TACP system, the subsoil samples containing charcoal debris (biochar) from charcoal production were taken from inside and outside six randomly selected charcoal production spots at the end of the cropping period. The six subsoil samples were combined into a single composite soil sample for each depth and replication. The sampling design covered seven different areas (treatments) (1) the TM system with no charcoal production, plus (2) the first rotation inside a charcoal production spot, (3) the first rotation outside a charcoal production spot, (4) the second rotation inside a charcoal production spot, (5) the second rotation outside a charcoal production spot, (6) the third rotation inside a charcoal production spot and (7) the third rotation outside a charcoal production spot in the TACP system, resulting in a total of 112 soil samples (7 treatments × 4 depths × 4 replicates).
Soil samples for chemical analysis were passed through a 2 mm soil sieve. Soil texture was determined using the hydrometric method, after removing the SOM using hydrogen peroxide and thereafter dispersing the soil with sodium hexametaphosphate [66]. The USDA particle size classes, viz. sand (2.0–0.05 mm), silt (0.05–0.002 mm) and clay (<0.002 mm), were used for assigning the textural classes. Bulk density was determined by the core method [66]. Soil pH was measured with combined electrodes in a 1:2.5 soil/water suspension. Soil organic carbon (SOC) was determined by the Walkley–Black oxidation method [67]. Total nitrogen (TN) was measured by the Kjeldahl digestion method [68]. Available phosphorus (Pav), available potassium (Kav), available nitrate (NO3−), available magnesium (Mgav), available sulfate (SO42−), available ammonium (NH4+) and available phosphate (PO43−) were extracted by the calcium acetate lactate (CAL) method [69]. Total nutrients (Na, K, P, Mg, Ca, Al, Fe and Mn) were extracted by the aqua regia (concentrated HCl:HNO3 3:1) digestion method, followed by ICP-OES analysis [70]. The soil samples were analyzed at the Central Analytical Laboratory (ZEA-3) of the Forschungszentrum Jülich, Jülich, Germany.
2.2. Data Analysis
The data were then grouped according to the land use types (the TM and the TACP system, with three rotations) and soil depth classes. Two comparisons were conducted: (i) a comparison of the soil properties under the TACP system in the first, second and third rotations with those under the TM system, and (ii) a comparison of soil properties inside the charcoal production spots with the area outside them in the TACP system.
Statistical differences were tested using one-way analysis of variance (ANOVA) following the general linear model (GLM) procedure of SPSS version 20.0 for Windows [71]. Tukey’s honestly significance difference (HSD) test was used for means separation when the analysis of variance showed statistically significant differences (p < 0.05). Linear regression analysis was performed to examine the relationship between TN and SOC content.
3. Results
3.1. Variation in the Soil Properties with the TACP and TM Systems and Soil Depth
3.1.1. Soil Organic Carbon, Total Nitrogen, C:N Ratio and Bulk Density
Soil organic carbon (SOC) content varied significantly with the land use type and soil depth (Table 2). Generally, SOC content was higher under the TACP system than under the TM system, and it was higher in the first rotation than in the two other rotations. SOC content was higher overall (up to 225%) in the topsoil than in the adjacent subsurface soil layer for all land use types (Table 2). In the 0–20 cm soil layer, SOC was significantly higher in the first and the second rotations than in the TM system.

Table 2.
pH, soil organic carbon (SOC) (%), total nitrogen (TN) (%) content, C/N ratio and bulk density (Bd) (g cm−3) of soil under the TM and TACP systems (mean ± standard error of the mean).
The total nitrogen content of the soil showed significant differences between land use type and soil depth (Table 2). Soil TN was 91.7% higher in the first rotation of the TACP system than under the TM system, while it did not show significant differences from the second and third rotations of TACP system. Depthwise, soil TN was higher by up to 255% in the topsoil than in the other depths under both the TM and TACP systems. TN was significantly and positively correlated with SOC content (r2 = 0.96) (Figure 4).
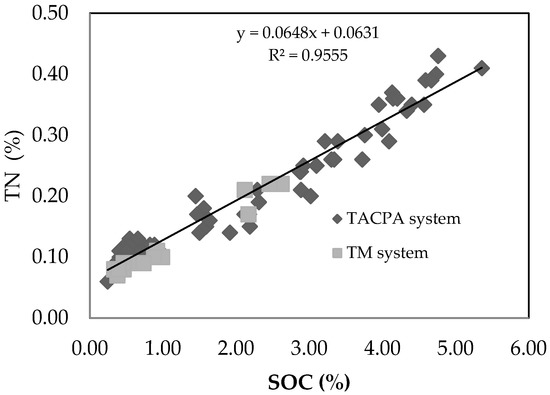
Figure 4.
Relationship between total nitrogen and the soil organic carbon content of the two land-use systems.
The carbon to nitrogen (C/N) ratio varied with land use type and soil depth (Table 2). The C/N ratio was higher in the first rotation of the TACP system than under the TM system. Under both the TACP and TM systems, the C/N ratio was higher in the topsoil compared with the other soil depths. In the 0–20 cm and 40–60 cm soil layers, the C/N ratio was significantly higher under the TACP system than under the TM system.
There was a significant variation in Bd with land use type and soil depth (Table 2). The Bd was 15% higher in the first rotation of the TACP system than under the TM system. It declined with depth in all rotations of the TACP system.
3.1.2. Soil pH, Pav, Kav, Naav and Mgav
Significant differences in pH were observed between land use types (Table 2). There was no significant difference in soil pH with soil depth under the TACP system, in contrast to the TM system (Table 2). Under the TM system, the soil pH was higher compared with that under the TACP system and declined with soil depth (Table 2). The soil pH under the TACP system and the TM system was within the medium to strongly acidic range (Table 2).
Available phosphorus varied significantly with land use type (Table 3), and was 159.1% and 77.8% higher in the first and second rotations of the TACP system, respectively, compared with the TM system. Variation in Pav was also observed across soil depths under the TACP system but not under the TM system (Table 3). Available phosphorus was higher in the topsoil compared with the other depths under the TACP system. In the 0–20 cm soil layer, Pav was significantly higher in the first and second rotations of the TACP system than that under the TM system.

Table 3.
Available phosphorus (Pav), available potassium (Kav), available sodium (Naav), available (Mgav), available ammonium (NH4+), nitrate (NO3−) and sulfate (SO42−) content in soils in the TM and TACP systems (mean ± standard error of the mean., all contents in mg kg−1).
There was a significant variation in available potassium (Kav) with land use type and soil depth (Table 3). Kav decreased by about 56.7% in the third rotation of the TACP system compared with the TM system. The mean Kav in the topsoil (0–20 cm) was higher than in the soil depth immediately below it (20–40 cm) under both the TM and TACP systems.
The ANOVA for available sodium (Naav) and available magnesium (Mgav) revealed that they were significantly affected by land use type but not by soil depth (Table 3). Available Na increased by 152.1% and 184.7% in the first and third rotations, respectively, whereas Mgav decreased by 66.0% and 44.1% in the first and second rotations of the TACP system, respectively, compared with the TM system.
3.1.3. Available Ammonium, Nitrate, Phosphate and Sulfate
Available ammonium (NH4+), available nitrate (NO3−) and available sulfate (SO42−) varied significantly with land use type but not with soil depth, except in the 20–40 cm and 40–60 cm soil layers of the TM system (Table 3). Available NH4+ and NO3− increased by up to 300% and 10% in the TACP system in different rotations, while available sulfate (SO42−) decreased by up to 77%. Available phosphate (PO43−) was below the detection limit under both the TM and TACP systems across all depths (Table 3).
3.1.4. Total Contents of Soil Na, K, P, Mg, Ca, Al, Fe and Mn
Total soil contents of K, Mg, Ca, Fe, P and Al overall varied significantly between the TM and the TACP system, and with soil depth (Table 4). In the TACP system, the mean total contents of Ca were higher after three rotations, whereas K, Mg and P decreased with increasing number of rotations; while Mn remained unaffected. In contrast, total Na, Fe and Al contents showed an inconsistent pattern. The total contents of K, Mg and Mn were higher overall in the 0–20 cm depth of the third rotation of the TACPA system, whereas for other elements and rotations of the TACPA system no clear pattern could be found. In the 0–20 cm soil layer, the total Na content was significantly higher in the second and the third rotations of the TACP system than in the TM system. The total content of P (20–40 cm layer) was significantly higher in the first rotation of the TACP system than in the TM system. The total content of Mn was significantly higher in the 0–20 soil layer of the third rotation of the TACP system than in the TM system.

Table 4.
Total element content of Na, K, P, Mg, Ca, Al, Fe and Mn (in %) of soil under the TM and TACP systems at different depths (mean ± standard error of the mean), (* Total nutrient contents below the detection limit have not been presented in the table).
3.2. Variation of Soil Properties between Areas inside and outside Charcoal Production Spots
3.2.1. Soil Organic Carbon and Bulk Density, Total Nitrogen and C:N Ratio
There was no difference in soil SOC, TN or C:N ratio between the soils inside and outside charcoal production spots in the three rotations of the TACP system (Table 5). Moreover, no significant differences were observed in Bd between the soils from inside and outside charcoal production spots under the TACP system (Table 5).

Table 5.
Bulk density (Bd) (g cm−3), soil organic carbon (SOC) (%), total nitrogen (TN) (%), carbon to nitrogen (C/N) ratio, available nitrate (NO3−), available phosphate (PO43−), sulfate (SO42−), available ammonium (NH4+) and nutrient content (%) (all available nutrient contents in mg kg−1 ) and total element contents (%) in soils from inside and outside charcoal production spots (CPS) in the three rotations of the TACP system. (* Total element contents below the detection limit have not been presented in the table).
3.2.2. Soil pH, and Available Phosphorus, Potassium, Magnesium and Sodium
No significant differences were observed in soil pH (Table 5), Pav and Kav (Figure 5a,b) between soils inside and outside the CPS. Available Na varied significantly between soils inside and outside the charcoal production spots of the TACP system (Figure 5) and was up to 139% higher inside the charcoal production spots compared with outside them.
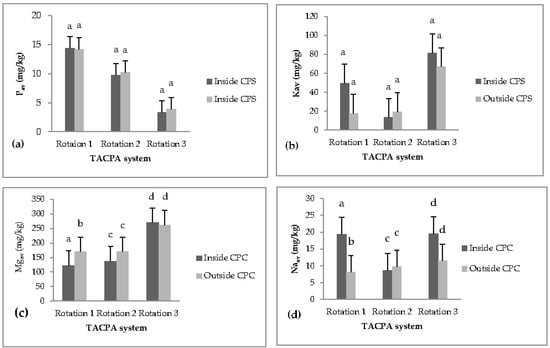
Figure 5.
(a) Available phosphorus (Pav, mg kg−1), (b) available potassium (Kav, mg kg−1), (c) available magnesium (Mgav, mg kg−1), (d) available sodium (Naav, mg kg−1), from soil inside and outside charcoal production spots (CPS) of the three rotations of the TACP system. Different letters indicate significant differences between soils inside and outside charcoal production spots (CPS) within each rotation.
3.2.3. Available Ammonium, Sulfate, Nitrate and Phosphate
Available ammonium (NO3−) and available sulfate (SO42−) increased significantly inside the CPS of the TACP system compared with outside them in different rotations by up to 88% and 75%, respectively; no statistical difference was observed for available ammonium (NH4+) (Table 5). Available phosphate (PO43−) was less than 0.0008 mg kg−1 inside the CPS of all the three rotations of the TACP system.
3.2.4. Total Soil Element Contents
Our results showed that total P varied significantly between soils inside and outside charcoal production spots in the TACP system (Table 5). It was 19% higher inside the charcoal production spots compared with outside them. No significant difference was observed in the total contents of Na, K, B, S, Mg, Ca, Mn and Cu.
Our results also showed that total Al and Fe varied significantly between soils inside and outside the charcoal production spots in the TACP system (Table 5). Total Al was up to 7% higher in the second rotation and 25% higher in the third rotation, while the total Fe was up to 11% higher in the second rotation and up to 14% higher in the third rotation.
4. Discussion
4.1. Variation in the Soil Properties between the TACP and TM Systems and with Soil Depth
We found that SOC, TN, the C/N ratio, Bd, and the contents of Al, K, Mg, Ca, Fe, Pav, Pav, Naav, available NO3− and NH4+ were significantly higher under the TACP than under the TM system; SOC, TN, the C/N ratio, Pav and Kav were higher in topsoil than in the rest of the soil depths (Table 2 and Table 3, Figure 5).
The significant improvement in SOC and TN under the TACP system could be explained by the progressive accumulation of litter [72] and root biomass [73,74] under the canopy of A. decurrens and probably the effects of tef crop residues left after harvest. Many authors have reported higher SOC levels and TN concentrations under the canopy of trees and in topsoil for other species, which is in concert with the present investigation (e.g., [66,67,68]). For instance, a study on the impacts of Millettia ferruginea on soil fertility [75] observed significantly higher SOC and TN beneath the canopy compared with the open field outside the canopy.
Soil enrichment in terms of SOC and TN in soils under trees in an agricultural system is concentrated in a few centimeters of the soil, especially under leguminous trees [76,77,78,79], which is in agreement with the increases in SOC and TN of up to 225% and 255% in the topsoil of the TACP system in the present study, respectively. For instance, [75] found significantly higher soil SOC and TN in both the topsoil and subsoil under the canopy of Millettia ferruginea compared with the open area outside the canopy zone of M. ferruginea. The increase in SOC and TN under the TACP system could also be explained by the addition of charcoal debris to the soil [80,81]. In several studies, adding biochar when cultivating the soil of farmland has been shown to significantly increase SOC [81,82,83] and TN [84,85] in the surface soil.
The C/N ratio in the studied soils was higher under the TACP system and in the topsoil under both the TM and TACP systems, probably due to the input of litter from the A. decurrens trees, which could have a high C/N ratio, and/or due to the formation of recalcitrant nitrogen compounds in the soil during charcoal production [86]. Tree litter and biochars with high C/N ratios are low in quality [82]. The C/N ratios of the current study are contradictory to the findings of different authors [75,82]. For instance, [75,87] observed a lower C/N ratio under M. ferruginea than in open areas and in soil treated with biochar, which they attributed to the high quality of the litter of M. ferruginea trees and biochar.
Contrary to our expectations, the TACP system brought about a significant increase in terms of Bd in the first and second rotations, as well as with depth. The result is, however, not entirely surprising. Several previous studies have found that biochar does not always rapidly improve Bd and other physical soil properties, depending on the management (e.g., [88,89]) or because of the slower decomposition rate of wood-derived biochar, such as that of A. decurrens, as a result of its high C content [90,91].
The accumulation of aboveground biomass and the associated cation uptake by the tree component of agroforestry systems are among the possible indirect causes of the decreased pH in soils [92]. Studies have also indicated the rise of soil pH due to application of biochar (e.g., [84,93,94]). On the contrary, in our study, soil pH was found to be higher under the TM system compared with the TACP system, which may be an indication of overcultivation leading to prolonged uptake of basic cations [95]. The relatively higher pH under the TACP system could also be due to other mechanisms that release H+ ions, such as soil base cation uptake (or depletion) by the decomposition of organic matter to organic acids and CO2, root respiration and nitrification. The findings of this research are in agreement with [77], who found a higher pH under the canopy of Balanites aegyptiaca.
The observed higher Pav in the soil of rotation 1 of the TACP system compared with the soil under TM, and in the topsoil could be due to higher SOM accumulation from litterfall from A. decurrens and due to the charcoal debris in the soil. The increase in Pav could also be associated with the relatively higher pH under the TACP system, which could make phosphorus more available in the soil [96]. Higher Pav content was also reported by [97] and [98] as result of the presence of trees in the agricultural land use systems, and by [96] in soil treated with biochar, which confirms the relatively higher P found here.
Trees in an agroforestry system can potentially improve nutrient availability via nutrient-enriched litterfall [25,51]. In the present study, however, Kav was observed to be higher under the TM system compared with the TACP system in the 0–20 cm depth. This could be due to the application of K fertilizer in the TM system by the local farmers. In contrast, Naav was higher under the TACP system, which could be attributed to the ability of the fine charcoal residues in the soil of the TACP system to retain nutrients [35,99]. Contrary to our study, [55,100] reported a significant decrease in Naav in soil treated with charcoal debris.
The increased availability of NO3− and NH4+ in the TACP system could be due to change in microbial abundance [63] and the increased nitrification and oxidation of ammonia [96] as result of the charcoal residues. The high capacity of the charcoal residues to absorb nutrients enables their effective absorption of ammonia (NH3), reducing its loss through volatilization [34,101]. The higher SO42− under the TACP system compared with the TM system in the present study could also be attributed to the effect of the microbially mediated transformation of nutrients in the soil by the addition of the charcoal residue [96]. Our findings of higher NO3− and NH4+ in the soil of TACP corroborate the findings of previous studies that compared soil nutrients in agroforestry systems versus agricultural fields [102,103,104].
In our study, the TACP system increased the total contents of K, Mg and Ca overall, while it decreased the total content of P and Al in the different rotations. However, notice that due to charcoal production, the total content of P and Al was significantly higher inside the charcoal production spots under the TACP system compared with outside them (see Table 4). Previous studies revealed that application of biochar in agroforestry systems could significantly increase the total nutrient contents in the soil (e.g., [93,94,95]), though beneficial effects depend on the soil type and properties [105]. For example, [82] found a 60 to 670% increase in the total contents of K, Mg and Ca after application of biochar. The higher total content of K, P and Mg in the 0–20 cm depth of the third rotation of the TACP system in the present study could be attributed to the release of nutrients to the soil by the decomposition of leaf litter of A. decurrens. The findings on K, P, Mg and Ca were also similar to those reported by [106] but for trees other than A. decurrens.
4.2. Comparison of Soil Properties between Areas inside and outside the Charcoal Production Spots
Charcoal production under the TACP system resulted in a significant increase in the total contents of P, Al, Fe, available NO3− and SO42− inside the CPS compared with outside them, but no increases in SOC, TN, Bd, Pav and Kav (Table 4 and Table 5).
The effect of fire on soil SOC is highly dependent on the type and intensity of the fire, among other factors, such as soil moisture, the soil type and the nature of the burned materials. Therefore, the effects on soil processes and their intensity by fire are highly variable [107]. At the site studied here, charcoal production did not bring about the expected change in SOC. This result contradicts the findings of [46,108], who reported a significant change in soil SOC in their studies of on the effect of charcoal production on soil properties. This result is also not in line with [109], who found higher SOC in charcoal production areas compared with non-charcoal sites in southwest Ethiopia.
The nonsignificant difference in soil Bd between areas inside and outside charcoal production spots in the TACP system indicates that the charcoal production spots in the TACP system were not sufficiently exposed to fire. The bulk density did not change inside the CPS, which may be because the soil’s physical properties do not change after fire unless a very severe fire occurs [110]. These results are not in agreement with the findings of [109], who found significantly higher SOC and a 13% reduction in Bd at kiln sites compared with the adjacent field.
Charcoal production under the TACP system also brought about a change in soil TN, demonstrating inadequate volatilization of nitrogen during charcoal production, which is the dominant mechanism of N loss from soil systems, usually in the form of ammonia and other related N gases [111]. This result agreed with [46] but was inconsistent with [109], who found a significant change in soil TN in their studies on the effect of charcoal production on soil properties in southwestern Ethiopia.
Though the soil pH decreased significantly from the initial value of 5.18 under TM to 4.49, 4.63 and 5.00 in the first, second and third rotations of the TACP system, respectively, no significant difference was observed in soil pH between soils inside and outside the CPS, which may be due to insufficient accumulation of base-forming cations or the production of alkaline primary oxides, carbonates and the loss of organic acids in the CPS [112]. In contrast to this result, [113] found increases of up to 1.2 pH units due to an accumulation of ashes from charcoal production.
In this study, no significant difference was observed in Pav and Kav between areas inside and outside CPS. The nonsignificant difference in Pav suggests that it might have not volatilized enough at the temperature generated during the charcoal production in the TACP system to make a significant variation. This result is inconsistent with [34,100,114], who found an increase in Pav in a slash-and-burn experiment. The nonsignificant difference in Kav could be attributed the direct release of fine charcoal residues from charcoal production under the TACP system. In disagreement with our result, [100] reported a significant increase in Kav in soil exposed to fire.
The results of this study indicated that Nav inside the charcoal production spots was higher compared with that outside them under the TACP system. This could be due to the accumulation of charred biomass. Orguntunde et al. [34,39,109] also reported a significant increase in the availability of Na at kiln sites compared with the adjacent agricultural fields.
Two different mechanisms responsible for the increase in the availability of NO3− inside the charcoal production spots are the direct addition of NO3− from charcoal residues or enhanced nitrification following charcoal production [96]. The rise in the available SO42− in this study inside the charcoal production spots was presumably due to the degradation of SOM by the heat of charcoal production, which increased the concentration of SO42− in the soil solution [115]. This result is consistent with other studies (e.g., [28,37,39,107]) that compared the soil nutrient status of kiln sites with adjacent land use systems.
In the present study, charcoal production increased the Al and Fe content in the charcoal production spots. This is presumably due to the release of large quantities of ashes, which are rich in nutrients, during biomass burning [116,117]. Similar results were obtained in studies related to charcoal production in Ghana [46], slash-and-burn management and soil amended with charcoal [34]. Oguntunde et al. [46] and [118] attributed the increase in these elements in charcoal production spots to decrease of nutrient leaching, especially Al and Fe which are mediated by the high adsorption capacity of charcoal [119]. According to [118], addition of charcoal in a soil system inhibits leaching of nutrients by up to 68% after depending on soil types.
5. Conclusions
Taken together, the studied soil of the TACP system in northern Ethiopia showed marked variations in SOC, TN, the C/N ratio, Bd, and the contents of Al, K, Mg, Ca, Fe, Pav, Pav, Naav, available NO3− and NH4+ with land use type, and variations in SOC, TN, C/N ratio, Pav and Kav with respect to soil depth. The TACP system resulted in improvements in SOC, TN and soil nutrients compared with the TM system. The marked improvement could be associated with the effect of litter accumulation, belowground root degradation of the acacia trees and the addition of charcoal residues to the soil. Under the TACP system, charcoal production increased the total contents of P, Al and Fe, as well as the available NO3− and available SO42− in the CPS. The increases could be related to impact of fine charcoal residues and ashes from charcoal production. Therefore, it is concluded that in general, the TACP system improves some soil properties and nutrients generally and in the charcoal production spots specifically. In the future, the joint effect of soil-improving trees and charcoal production on soil proprieties still need to be studied further to ascertain our research.
Author Contributions
Conceptualization, M.B., F.Y. and N.B.; methodology, M.B., F.Y. and N.B.; software, M.B.; formal analysis, M.B.; investigation, M.B.; resources, N.B.; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, M.B., F.Y., N.B. and M.T.; supervision, F.Y., N.B. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Forschungszentrum Jülich.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author on reasonable request.
Acknowledgments
M.B. acknowledges funding by the German Academic Exchange Service (DAAD) through the funding program “Research Grants—Bi-nationally Supervised Doctoral Degrees, 2019/20” (57440919). M.B is also grateful to all field workers and lab technicians for their field and lab assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanchez, P.A.; Shepherd, K.D.; Soule, M.J.; Place, F.M.; Buresh, R.J.; Izac, A.-M.N.; Uzo Mokwunye, A.; Kwesiga, F.R.; Ndiritu, C.G.; Woomer, P.L. Soil Fertility Replenishment in Africa: An Investment in Natural Resource Capital. In SSSA Special Publications; Buresh, R.J., Sanchez, P.A., Calhoun, F., Eds.; Soil Science Society of America American Society of Agronomy: Madison, WI, USA, 2015; pp. 1–46. ISBN 978-0-89118-946-6. [Google Scholar]
- Vlek, P.L.G.; Khamzina, A.; Tamene, L. Land Degradation and the Sustainable Development Goals: Threats and Potential Remedies.; International Center for Tropical Agriculture (CIAT): Nairobi, Kenya, 2017. [Google Scholar]
- Dagnachew, M.; Kebede, A.; Moges, A.; Abebe, A. Land Use Land Cover Changes and Its Drivers in Gojeb River Catchment, Omo Gibe Basin, Ethiopia. J. Agric. Environ. Int. Dev. (JAEID) 2020, 114, 33–56. [Google Scholar]
- Ejigu, W.; Selassie, Y.G.; Elias, E.; Damte, M. Integrated Fertilizer Application Improves Soil Properties and Maize (Zea mays L.) Yield on Nitisols in Northwestern Ethiopia. Heliyon 2021, 7, e06074. [Google Scholar] [CrossRef] [PubMed]
- Matondi, P.B.; Havnevik, K.; Beyene, A. Biofuels, Land Grabbing and Food Security in Africa; Zed Books: London, UK, 2011. [Google Scholar]
- Palombi, L.; Sessa, R. Climate-Smart Agriculture: Sourcebook; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Barber, R. United Nations. Economic Commission for Africa (1984-03). An Assessment of the Dominant Soil Degration Processes in the Ethiopian Highlands—Their Impacts and Hazards. Addis Ababa; EHRS Working; FAO: Addis Ababa, Ethiopia, 1984. [Google Scholar]
- Josephson, A.L.; Ricker-Gilbert, J.; Florax, R.J.G.M. How Does Population Density Influence Agricultural Intensification and Productivity? Evidence from Ethiopia. Food Policy 2014, 48, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Amede, T.; Takele, B.; Endris, G. Reversing the Degradation of Arable Land in the Ethiopian Highlands; Managing Africa’s Soils No. 23; Areka Research Centre: Areka, Ethiopia, 2001. [Google Scholar]
- FAO. Assistance to Land Use Planning Ethiopia Geomorphology and Soils; FAO: Addis Ababa, Ethiopia, 1984. [Google Scholar]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy Metal Contamination and Accumulation in Soil and Wild Plant Species from Industrial Area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Headey, D.D.; Jayne, T.S. Adaptation to Land Constraints: Is Africa Different? Food Policy 2014, 48, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Droppelmann, K.J.; Snapp, S.S.; Waddington, S.R. Sustainable Intensification Options for Smallholder Maize-Based Farming Systems in Sub-Saharan Africa. Food Sec. 2017, 9, 133–150. [Google Scholar] [CrossRef]
- Gebrehiwot, T.; Teklewold, H. Determinants of Farmland Expansion in the Forest Margins of Ethiopia; IIED; Sentinel: London, UK, 2022. [Google Scholar]
- Kim, D.-G.; Kirschbaum, M.U.F.; Beedy, T.L. Carbon Sequestration and Net Emissions of CH4 and N2O under Agroforestry: Synthesizing Available Data and Suggestions for Future Studies. Agric. Ecosyst. Environ. 2016, 226, 65–78. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-Smart Agriculture for Food Security. Nat. Clim Change 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Schaller, M.; Barth, E.; Blies, D.; Schümmelfeder, M.; Felicitas, R. Climate Smart Agriculture (CSA): Farmyard Compost; International Center for Tropical Agriculture (CIAT): Cali, Colombia; The Centre for Rural Development (SLE): Berlin, Germany, 2017. [Google Scholar]
- FAO. Land and Environmental Degradation and Desertification in Africa: Issues and Options for Sustainable Economic Development with Transformation; FAO: Addis Ababa, Ethiopia, 1995. [Google Scholar]
- Kahsay, G.A.; Hansen, L.G. The Effect of Climate Change and Adaptation Policy on Agricultural Production in Eastern Africa. Ecol. Econ. 2016, 121, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Teklewold, H.; Mekonnen, A.; Kohlin, G.; Di Falco, S. Does adoption of multiple climate-smart practices improve farmers’ climate resilience? Empirical evidence from the nile basin of ethiopia. Clim. Change Econ. 2017, 8, 1750001. [Google Scholar] [CrossRef]
- Blaser, W.J.; Oppong, J.; Hart, S.P.; Landolt, J.; Yeboah, E.; Six, J. Climate-Smart Sustainable Agriculture in Low-to-Intermediate Shade Agroforests. Nat. Sustain. 2018, 1, 234–239. [Google Scholar] [CrossRef]
- Nyagumbo, I.; Mutenje, M.; Setimela, P.; Chipindu, L.; Chisaka, A.; Simwaka, P.; Mwale, B.; Ngwira, A.; Mupangwa, W. Evaluating the Merits of Climate Smart Technologies under Smallholder Agriculture in Malawi. Soil Use Manag. 2022, 38, 890–906. [Google Scholar] [CrossRef]
- Schroeder, P. Carbon Storage Benefits of Agroforestry Systems. Agrofor. Syst 1994, 27, 89–97. [Google Scholar] [CrossRef]
- Young, A. Agroforestry for Soil Management; CAB International: Wallingford, UK, 1997. [Google Scholar]
- Baah-acheamfour, M.; Chang, S.X.; Bork, E.; Carlyle, C. The Potential of Agroforestry to Reduce Atmospheric Greenhouse Gases in Canada: Insight from Pairwise Comparisons with Traditional Agriculture, Data Gaps and Future Research. For. Chron. 2017, 93, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Eddy, W.C.; Yang, W.H. Improvements in Soil Health and Soil Carbon Sequestration by an Agroforestry for Food Production System. Agric. Ecosyst. Environ. 2022, 333, 107945. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating Root and Soil Microbial Contributions to Soil Respiration: A Review of Methods and Observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Wang, W.J.; Dalal, R.C.; Moody, P.W.; Smith, C.J. Relationships of Soil Respiration to Microbial Biomass, Substrate Availability and Clay Content. Soil Biol. Biochem. 2003, 35, 273–284. [Google Scholar] [CrossRef]
- Akinnifesi, F.K.; Ajayi, O.C.; Sileshi, G.; Chirwa, P.W.; Chianu, J. Fertiliser Trees for Sustainable Food Security in the Maize-Based Production Systems of East and Southern Africa. A Review. Agron. Sustain. Dev. 2010, 30, 615–629. [Google Scholar] [CrossRef]
- Corbeels, M.; Cardinael, R.; Naudin, K.; Guibert, H.; Torquebiau, E. The 4 per 1000 Goal and Soil Carbon Storage under Agroforestry and Conservation Agriculture Systems in Sub-Saharan Africa. Soil Tillage Res. 2019, 188, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Muche, M.; Eyasu, M. Assessing the Physicochemical Properties of Soil under Different Land Use Types. J. Env. Anal. Toxicol. 2015, 5, 309. [Google Scholar]
- Gómez-Luna, B.E.; Rivera-Mosqueda, M.C.; Dendooven, L.; Vázquez-Marrufo, G.; Olalde-Portugal, V. Charcoal Production at Kiln Sites Affects C and N Dynamics and Associated Soil Microorganisms in Quercus Spp. Temperate Forests of Central Mexico. Appl. Soil Ecol. 2009, 41, 50–58. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a Strategy to Sequester Carbon and Increase Yield in Durum Wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Xu, X.; Harris, W. Phosphorus Release from Dairy Manure, the Manure-Derived Biochar, and Their Amended Soil: Effects of Phosphorus Nature and Soil Property. J. Environ. Qual. 2014, 43, 1504–1509. [Google Scholar] [CrossRef]
- Schmidt, H.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in Agriculture—A Systematic Review of 26 Global Meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Tan, K.; Qin, Y.; Wang, J. Evaluation of the Properties and Carbon Sequestration Potential of Biochar-Modified Pervious Concrete. Constr. Build. Mater. 2022, 314, 125648. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of Biochar and Hydrochar Addition on Water Retention and Water Repellency of Sandy Soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black Carbon Affects the Cycling of Non-Black Carbon in Soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Lal, R. Encyclopedia of Soil Science, 3rd ed.; Taylor and Francis: Columbus, OH, USA, 2016. [Google Scholar]
- Chidumayo, E.N. Effects of Wood Carbonization on Soil and Initial Development of Seedlings in Miombo Woodland, Zambia. For. Ecol. Manag. 1994, 70, 353–357. [Google Scholar] [CrossRef]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems, 2nd ed.; CABI Pub: Wallingford, UK; New York, NY, USA, 2001; ISBN 978-0-85199-417-8. [Google Scholar]
- Oguntunde, P.G.; Fosu, M.; Ajayi, A.E.; van de Giesen, N. Effects of Charcoal Production on Maize Yield, Chemical Properties and Texture of Soil. Biol. Fertil. Soils 2004, 39, 295–299. [Google Scholar] [CrossRef]
- Oguntunde, P.G.; Abiodun, B.J.; Ajayi, A.E.; van de Giesen, N. Effects of Charcoal Production on Soil Physical Properties in Ghana. Z. Pflanzenernähr. Bodenk. 2008, 171, 591–596. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane Bagasse-Derived Biochar Reduces the Cadmium and Chromium Bioavailability to Mash Bean and Enhances the Microbial Activity in Contaminated Soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Peng, Q.; Wu, L.; Van Zwieten, L.; Khalid, M.S.; Younas, A.; Lin, S.; Zhao, J.; Bashir, S.; et al. The Interactive Effects of Dolomite Application and Straw Incorporation on Soil N2O Emissions: Nitrous Oxide Emissions from an Acidic Soil. Eur. J. Soil Sci. 2018, 69, 502–511. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pal, A.; Chen, C.-W.; Pandey, A.; Dong, C.-D. Advances on Tailored Biochar for Bioremediation of Antibiotics, Pesticides and Polycyclic Aromatic Hydrocarbon Pollutants from Aqueous and Solid Phases. Sci. Total Environ. 2022, 817, 153054. [Google Scholar] [CrossRef]
- Daniel, H.; Agena, A.; Abdu, A. Evaluation of the Effect of Ficus Thonningii (Blume) on Soil Physicochemical Properties in Ahferom District of Tigray, Ethiopia. J. Soil Sci. Environ. Manage. 2013, 4, 35–45. [Google Scholar]
- Poschen, P. An Evaluation of the Acacia Albida-Based Agroforestry Practices in the Hararghe Highlands of Eastern Ethiopia. Agrofor. Syst. 1986, 4, 129–143. [Google Scholar] [CrossRef]
- Haque, I. Use of Legume Biological Nitrogen Fixation in Crop/Livestock Production Systems; Chichester: Nigeria, Africa, 1992. [Google Scholar]
- Smit, G.N. An Approach to Tree Thinning to Structure Southern African Savannas for Long-Term Restoration from Bush Encroachment. J. Environ. Manag. 2004, 71, 179–191. [Google Scholar] [CrossRef]
- Hadgu, K.M.; Kooistra, L.; Rossing, W.A.H.; van Bruggen, A.H.C. Assessing the Effect of Faidherbia Albida Based Land Use Systems on Barley Yield at Field and Regional Scale in the Highlands of Tigray, Northern Ethiopia. Food Sec. 2009, 1, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Manjur, B.; Abebe, T.; Abdulkadir, A. Effects of Scattered, F. Albida (Del) and C. Macrostachyus (Lam) Tree Species on Key Soil Physicochemical Properties and Grain Yield of Maize (Zea mays): A Case Study at Umbulo Wacho Watershed, Southern Ethiopia. Wudpecker J. Agric. Res. 2014, 3, 63–73. [Google Scholar]
- Deans, J.D.; Diagne, O.; Nizinski, J.; Lindley, D.K.; Seck, M.; Ingleby, K.; Munro, R.C. Comparative Growth, Biomass Production, Nutrient Use and Soil Amelioration by Nitrogen-Fixing Tree Species in Semi-Arid Senegal. For. Ecol. Manag. 2003, 176, 253–264. [Google Scholar] [CrossRef]
- Abule, E.; Smit, G.N.; Snyman, H.A. The Influence of Woody Plants and Livestock Grazing on Grass Species Composition, Yield and Soil Nutrients in the Middle Awash Valley of Ethiopia. J. Arid Environ. 2005, 60, 343–358. [Google Scholar] [CrossRef]
- Burke, D.J.; Kretzer, A.M.; Rygiewicz, P.T.; Topa, M.A. Soil Bacterial Diversity in a Loblolly Pine Plantation: Influence of Ectomycorrhizas and Fertilization: Soil Bacterial Diversity in a Loblolly Pine Plantation. FEMS Microbiol. Ecol. 2006, 57, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Worku, T.; Mekonnen, M.; Yitaferu, B.; Cerdà, A. Conversion of Crop Land Use to Plantation Land Use, Northwest Ethiopia. Trees For. People 2021, 3, 100044. [Google Scholar] [CrossRef]
- NWEMA. Northwest Ethiopia Meteorological Agency; NWEMA: Bahir Dar, Ethiopia, 2017. [Google Scholar]
- Yihenew, G. Selected Physical and Chemical Characteristics of Soils of Adet Research Center and Its Testing Sites in Northwestern Ethiopia. Ethiop. J. Nat. Resour. 2002, 4, 199–215. [Google Scholar]
- Nigussie, Z.; Tsunekawa, A.; Haregeweyn, N.; Adgo, E.; Nohmi, M.; Tsubo, M.; Aklog, D.; Meshesha, D.T.; Abele, S. Factors Affecting Small-Scale Farmers’ Land Allocation and Tree Density Decisions in an Acacia Decurrens-Based Taungya System in Fagita Lekoma. Small-Scale For. 2016, 16, 219–233. [Google Scholar] [CrossRef]
- EYASU, E. Farmers’ Perceptions of Soil Fertility Changes and Managment; Institute for Sustainable Development: Addis Ababa, Ethiopia, 2002. [Google Scholar]
- Tegegn, Z.; Abebe, A.; Agide, Z. Understanding Catchments’ Hydrologic Response Similarity of Upper Blue Nile (Abay) Basin through Catchment Classification. Model. Earth Syst. Environ. 2021, 1–19. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Esingoger, L.E.; White, J.L.; Clark, F.E. Physical Properties. In Methods of Soil Analysis, Part I and II; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Schnitzer, M.; Schulten, H.R. The Analysis of Soil Organic Matter by Pyrolysis-Field Ionization Mass Spectrometry. Soil Sci. Soc. Am. J. 1992, 56, 1811–1817. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Schüller, H. Die CAL-Methode, Eine Neue Methode Zur Bestimming Des Pflanzenverfugbaren Phosphates Im Boden. Z. Planzenernaehr. Bodenkd. 1969, 123, 48–63. [Google Scholar] [CrossRef]
- Chen, M.; Lena, Q. Compasrsion of Three Aqua Regia Digestin Methods for Twenty Florida Soils. Soil Sci. Soc. Am. 2001, 65, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Coakes, S.J. SPSS 20.0 for Windows: Analysis without Anguish; Wiley: Milton, Australia, 2013; ISBN 978-1-118-33776-9. [Google Scholar]
- Agena, A. Component Interactions and Their Influence on the Production of Apple Based Agroforestry System in Wet Temperate Zone of Himachal Himalayas; University of Horticulture and Forestry: Solan, India, 2009. [Google Scholar]
- Berg, B.; McClaughert, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2003; 286p, ISBN 3-540-44329-0. [Google Scholar]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar Additions Alter Phosphorus and Nitrogen Availability in Agricultural Ecosystems: A Meta-Analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Negash, L.; Olsson, M. Millettia Ferruginea from Southern Ethiopia: Impacts on Soil Fertility and Growth of Maize. Agrofor. Syst. 2000, 48, 9–24. [Google Scholar]
- Loumeto, F.; Bernhard-Reversat, J. The Litter System in African Forest-Tree Plantations, Chapter 2. In Management of Tropical Plantation-Forests and Their Soil-Litter Systems; Science Publishers: Enfield, NH, USA, 2002. [Google Scholar]
- Hailemariam, K.; Kindeya, G.; Yamoah, C. Balanites Aegyptiaca, a Potential Tree for Parkland Agroforestry Systems with Sorghum in Northern Ethiopia. Soil Sci. Environ. Manag. 2010, 1, 107–114. [Google Scholar]
- Jiregn, G.; Rozanov, A.; Negash, L. Trees on Farms and Their Contribution to Soil Fertility Parameters in Badessa, Eastern Ethiopia. Biol. Fertil. Soils 2005, 42, 66–71. [Google Scholar]
- Enideg, D.T. Importance OfFicus Thonningii Blume in Soil Fertility Improvement and Animal Nutrition in Gondar Zuria, Ethiopia. Master’s Thesis, University of Natural Resources and Applied Life Science, Vienna, Austria, 2008. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 105, pp. 47–82. ISBN 978-0-12-381023-6. [Google Scholar]
- Wang, C.; Liu, J.; Shen, J.; Chen, D.; Li, Y.; Jiang, B.; Wu, J. Effects of Biochar Amendment on Net Greenhouse Gas Emissions and Soil Fertility in a Double Rice Cropping System: A 4-Year Field Experiment. Agric. Ecosyst. Environ. 2018, 262, 83–96. [Google Scholar] [CrossRef]
- Zhang, A.; Cheng, G.; Hussain, Q.; Zhang, M.; Feng, H.; Dyck, M.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; et al. Contrasting Effects of Straw and Straw–Derived Biochar Application on Net Global Warming Potential in the Loess Plateau of China. Field Crops Res. 2017, 205, 45–54. [Google Scholar] [CrossRef]
- Joseph, S.; Pow, D.; Dawson, K.; Rust, J.; Munroe, P.; Taherymoosavi, S.; Mitchell, D.R.G.; Robb, S.; Solaiman, Z.M. Biochar Increases Soil Organic Carbon, Avocado Yields and Economic Return over 4 Years of Cultivation. Sci. Total Environ. 2020, 724, 138153. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using Poultry Litter Biochars as Soil Amendments. Soil Res. 2008, 46, 437. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Xifré-Salvadó, M.À.; Prat-Guitart, N.; Francos, M.; Úbeda, X.; Castellnou, M. Effects of Fire on the Organic and Chemical Properties of Soil in a Pinus Halepensis Mill. Forest in Rocallaura, NE Spain. Sustainability 2021, 13, 5178. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Taylor and Francis: London, UK, 2012; ISBN 978-1-84977-055-2. [Google Scholar]
- Blanco-Canqui, H. Biochar and Phisycial Soil Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Prober, S.M.; Stol, J.; Melissa, P. Enhanicng Soil Biophysical Condition for Climate-Resileicne Restoraiton in Mesic Woodlands. Ecol. Eng. 2014, 71, 246–255. [Google Scholar] [CrossRef]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralisation and Structural Changes during the Initial Phase of Microbial Degradation of Pyrogenic Plant Residues in Soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Singh, N.; Abiven, S.; Maestrini, B.; Bird, J.A.; Torn, M.S.; Schmidt, M.W.I. Transformation and Stabilization of Pyrogenic Organic Matter in a Temperate Forest Field Experiment. Glob. Change Biol. 2014, 20, 1629–1642. [Google Scholar] [CrossRef]
- Rhoades, C.C. Single-Tree Influences on Soil Properties in Agroforestry: Lessons from Natural Forest and Savanna Ecosystems. Agrofor. Syst 1996, 35, 71–94. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of Soil-Applied Black Carbon: Downward Migration, Leaching and Soil Respiration. Glob. Change Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of Biochar from Slow Pyrolysis of Papermill Waste on Agronomic Performance and Soil Fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Tegenu, A.; Habitabmu, T.; Collick, A.S. Soil Fertility Status Dynamics of Northwestern Ethiopia as Influcned by Land Use Changes; Cornnel University: Ithaca, NY, USA, 2008. [Google Scholar]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J. Biochar Effects on Soil Nutrient Transformations. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 251–270. ISBN 978-0-415-70415-1. [Google Scholar]
- Bambrick, A.D.; Whalen, J.K.; Bradley, R.L.; Cogliastro, A.; Gordon, A.M.; Olivier, A.; Thevathasan, N.V. Spatial Heterogeneity of Soil Organic Carbon in Tree-Based Intercropping Systems in Quebec and Ontario, Canada. Agrofor. Syst. 2010, 79, 343–353. [Google Scholar] [CrossRef]
- Follain, S.; Walter, C.; Legout, A.; Lemercier, B.; Dutin, G. Induced Effects of Hedgerow Networks on Soil Organic Carbon Storage within an Agricultural Landscape. Geoderma 2007, 142, 80–95. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.H.; Zech, W. Long Term Effects of Manure, Charcoal and Mineral Fertilization on Crop Production and Fertility on a Highly Weathered Central Amazonian Upland Soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Ketterings, Q.M.; Bigham, J.M. Soil Color as an Indicator of Slash-and-Burn Fire Severity and Soil Fertility in Sumatra, Indonesia. Soil Sci. Soc. Am. J. 2000, 64, 1826–1833. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar Impact on Nutrient Leaching from a Midwestern Agricultural Soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Gautam, D.K.; Bajracharya, R.M.; Sitaula, B.K. Effects of Biochar and Farm Yard Manure on Soil Properties and Crop Growth in an Agroforestry System in the Himalaya. SAR 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Tammeorg, P.; Luukkanen, O.; Helenius, J.; Starr, M. Effects of Acacia Seyal and Biochar on Soil Properties and Sorghum Yield in Agroforestry Systems in South Sudan. Agrofor. Syst. 2017, 91, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Chang, S.; Bork, E.; Carlyle, C. Enrichment Planting and Soil Amendments Enhance Carbon Sequestration and Reduce Greenhouse Gas Emissions in Agroforestry Systems: A Review. Forests 2018, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Smider, B.; Singh, B. Agronomic Performance of a High Ash Biochar in Two Contrasting Soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Singh, J.S. Impact of Post-Mining Subsidence on Nitrogen Transformation in Southern Tropical Dry Deciduous Forest, India. Environ. Res. 2009, 109, 258–266. [Google Scholar] [CrossRef]
- Gonzalez-Perez, J.A.; Gonzalez, V. The Effect of Fire on Organic Matter-a Review. Enviro. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar Application to Soil for Climate Change Mitigation by Soil Organic Carbon Sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef] [Green Version]
- Abebe, N.; Endalkachew, K. Effect of Charcoal Production on Soil Properties in Southwestern Ethiopia. Middle-East J. Sci. Res. 2011, 9, 807–813. [Google Scholar]
- Terefe, T.; Mariscal-Sancho, I.; Peregrina, F.; Espejo, R. Influence of Heating on Various Properties of Six Mediterranean Soils. A Laboratory Study. Geoderma 2008, 143, 273–280. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme Activiteis as a Component of Soil Biodiversity—A Reivew. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C. Forest Fire Effects on Soil Color and Texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Cox, D.; Bezdicek, D.; Fauci, M. Effects of Compost, Coal Ash, and Straw Amendments on Restoring the Quality of Eroded Palouse Soil. Biol. Fertil. Soils 2001, 33, 365–372. [Google Scholar] [CrossRef]
- Singh, R. Changes in Soil Nutrients Following Burning Burning of Dry Tropical Savanna. Int. J. Wildland Fire 1994, 4, 187. [Google Scholar] [CrossRef]
- Moritsuka, N.; Yanai, J.; Kosaki, T. Effect of Soil Heating on the Dynamics of Soil Available Nutrients in the Rhizosphere. Soil Sci. Plant Nutr. 2001, 47, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Ayodele, A.; Oguntunde, P.; Joseph, A.; de Souza Dias, M., Jr. Numerical Analysis of the Impact of Charcoal Production on Soil Hydrological Behavior, Runoff Response and Erosion Susceptibility. Rev. Bras. Ciênc. Solo 2009, 33, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Fontodji, J.; Mawussi, G.; Nuto, Y.; Kokou, K. Effects of Charcoal Production on Soil Biodiversity and Soil Physical and Chemical Properties in Togo, West Africa. Int. J. Biol. Chem. Sci. 2010, 3, 51051. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Lee, C.-H.; Jien, S.-H. Reduction of Nutrient Leaching Potienal in Coarse-Textured Soil by Using Biochar. Water 2020, 12, 2012. [Google Scholar] [CrossRef]
- Baigorri, R.; San Francisco, S.; Urrutia, Ó.; García-Mina, J.M. Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity Are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils. Agronomy 2020, 10, 968. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).