The Influence of Tree Species on the Recovery of Forest Soils from Acidification in Lower Saxony, Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Estimation of Total Sulfur Deposition
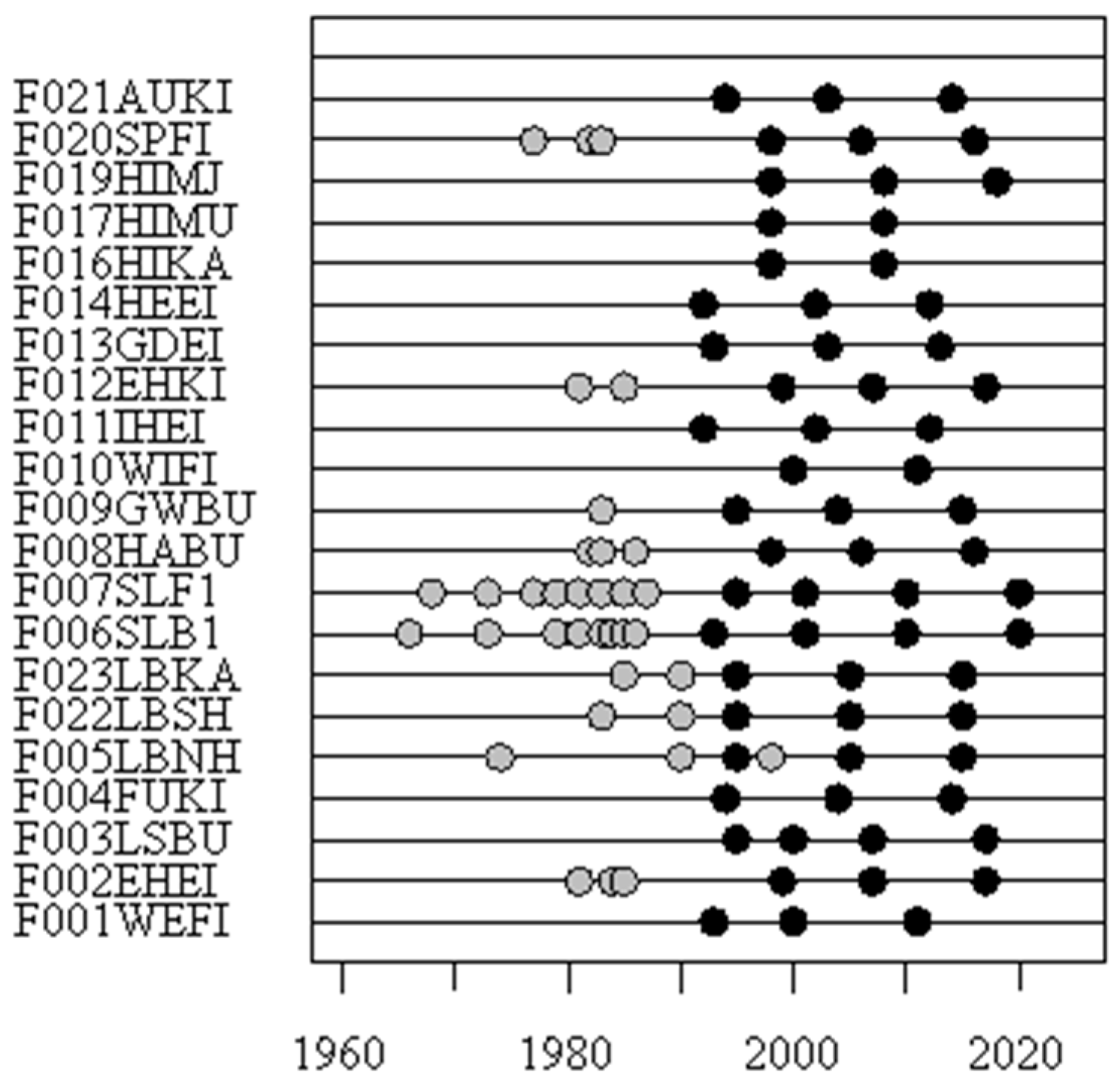
2.3. Sampling Procedures and Chemical Analysis
2.4. Data Handling
2.5. Derivation of Meteorological Data
2.6. Statistical Analysis
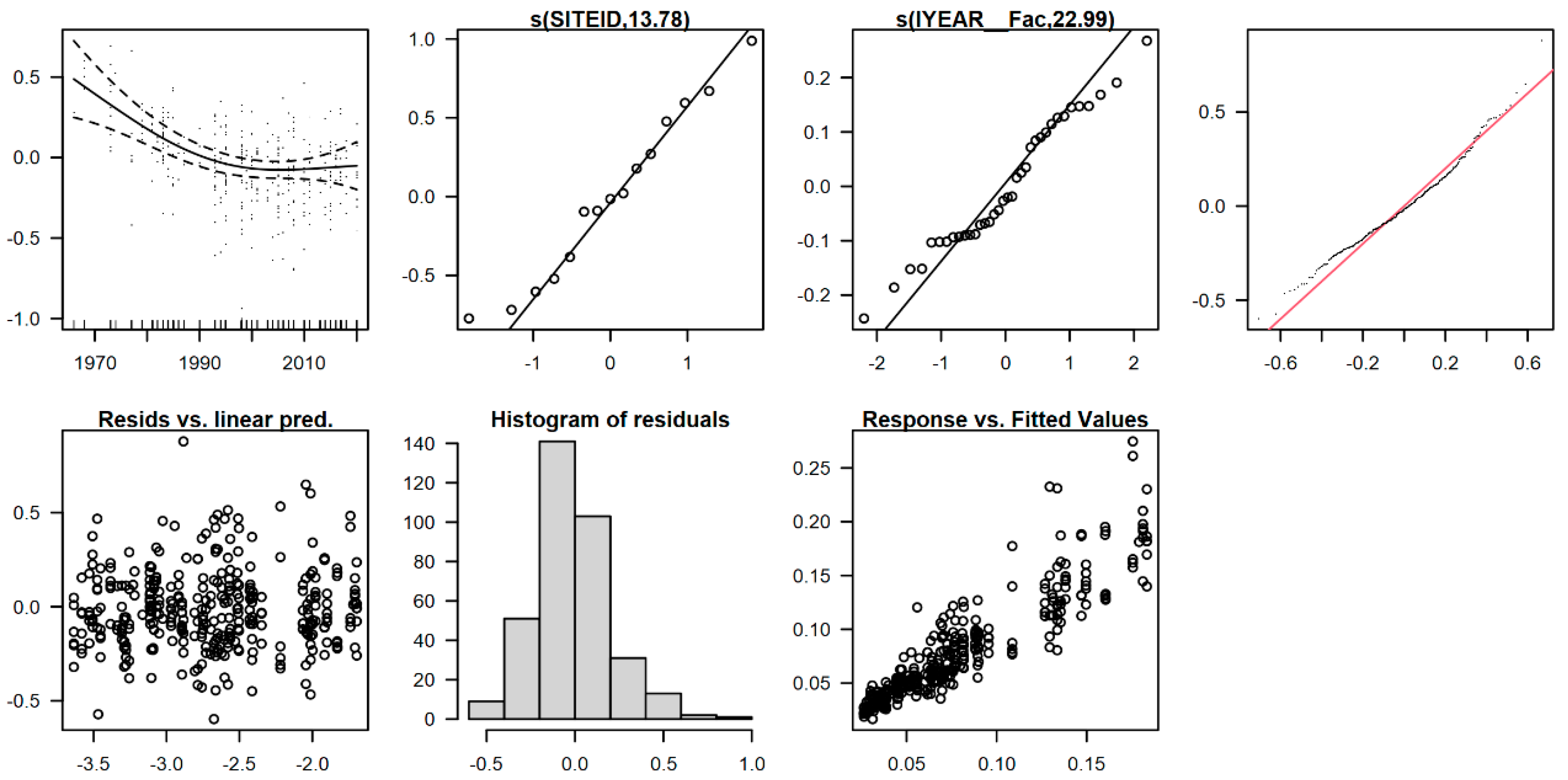
2.6.1. Detection of “Atypical” Plots Using Principal Component Analysis (PCA)
2.6.2. Mixed-Effects Models
3. Results
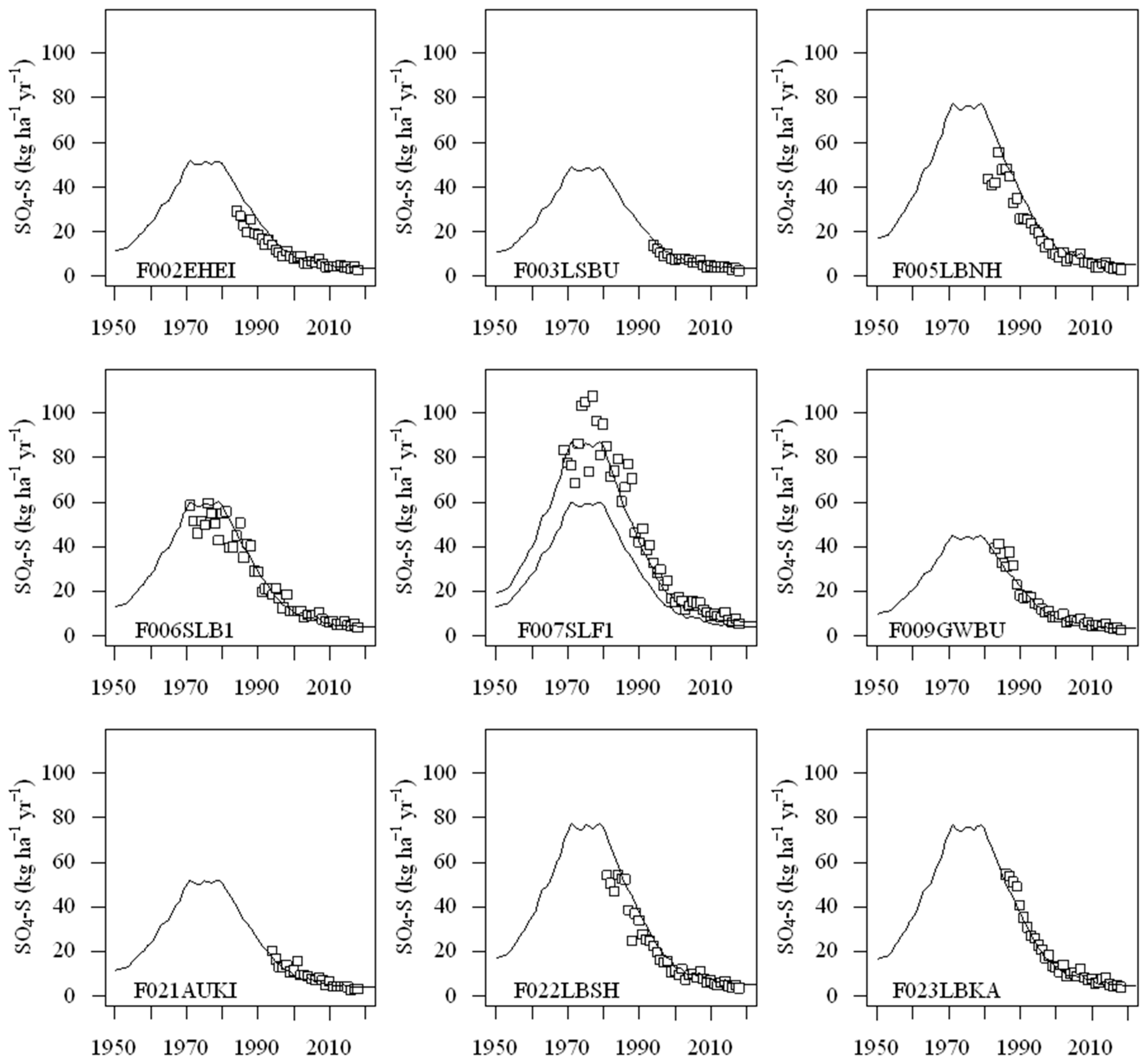
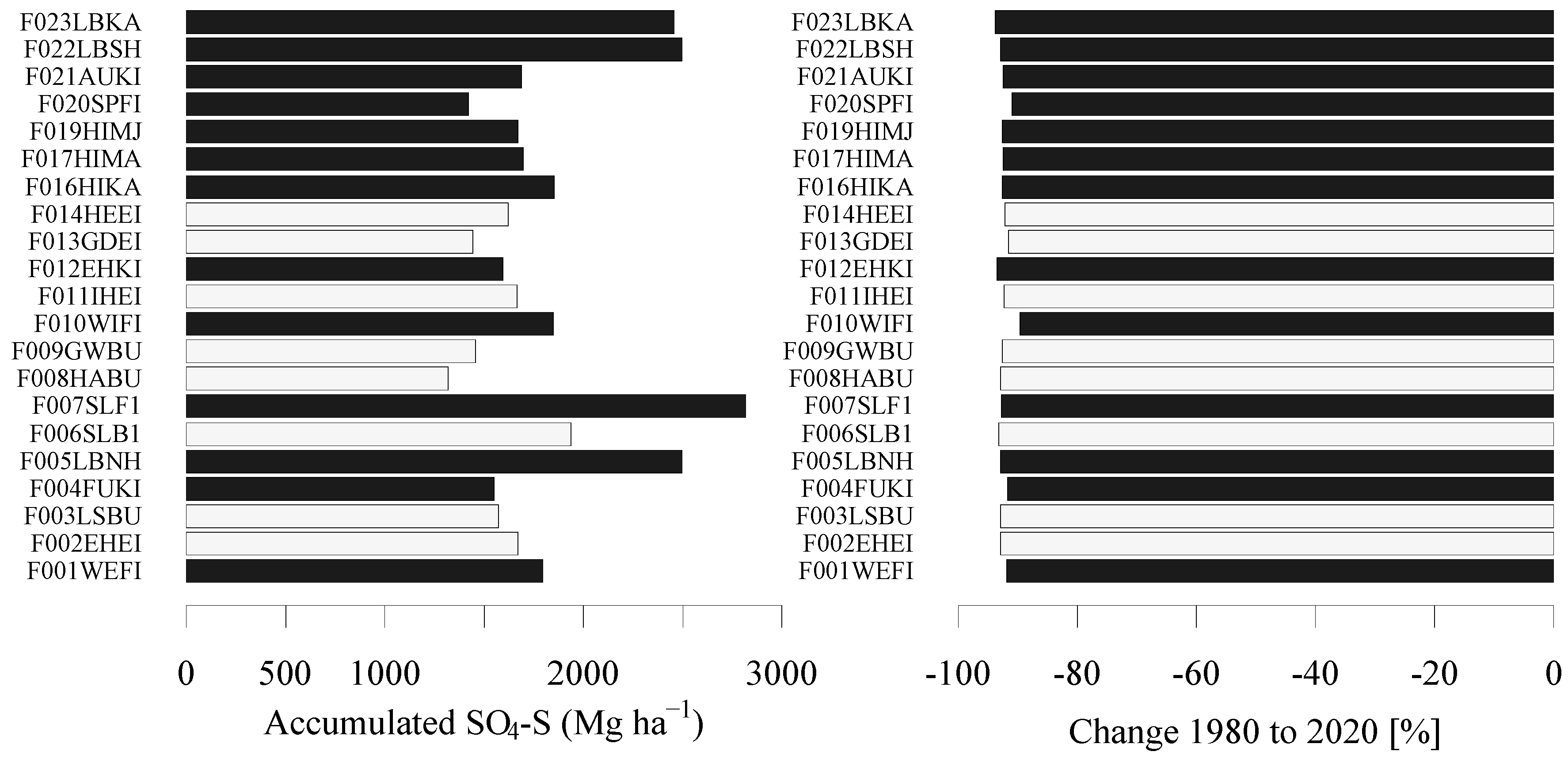
3.1. Site-Specific Load and Reduction of Sulfur Deposition
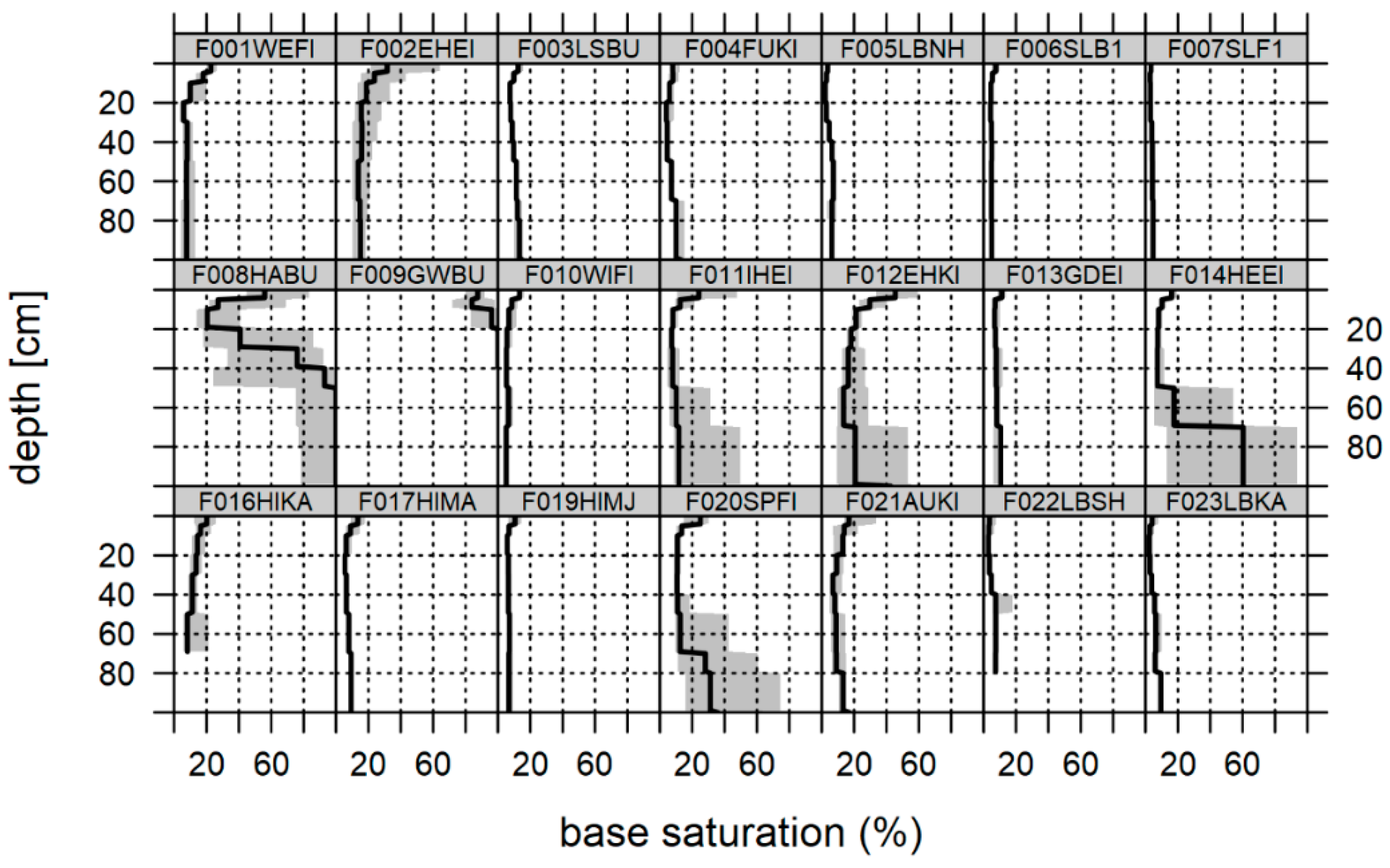
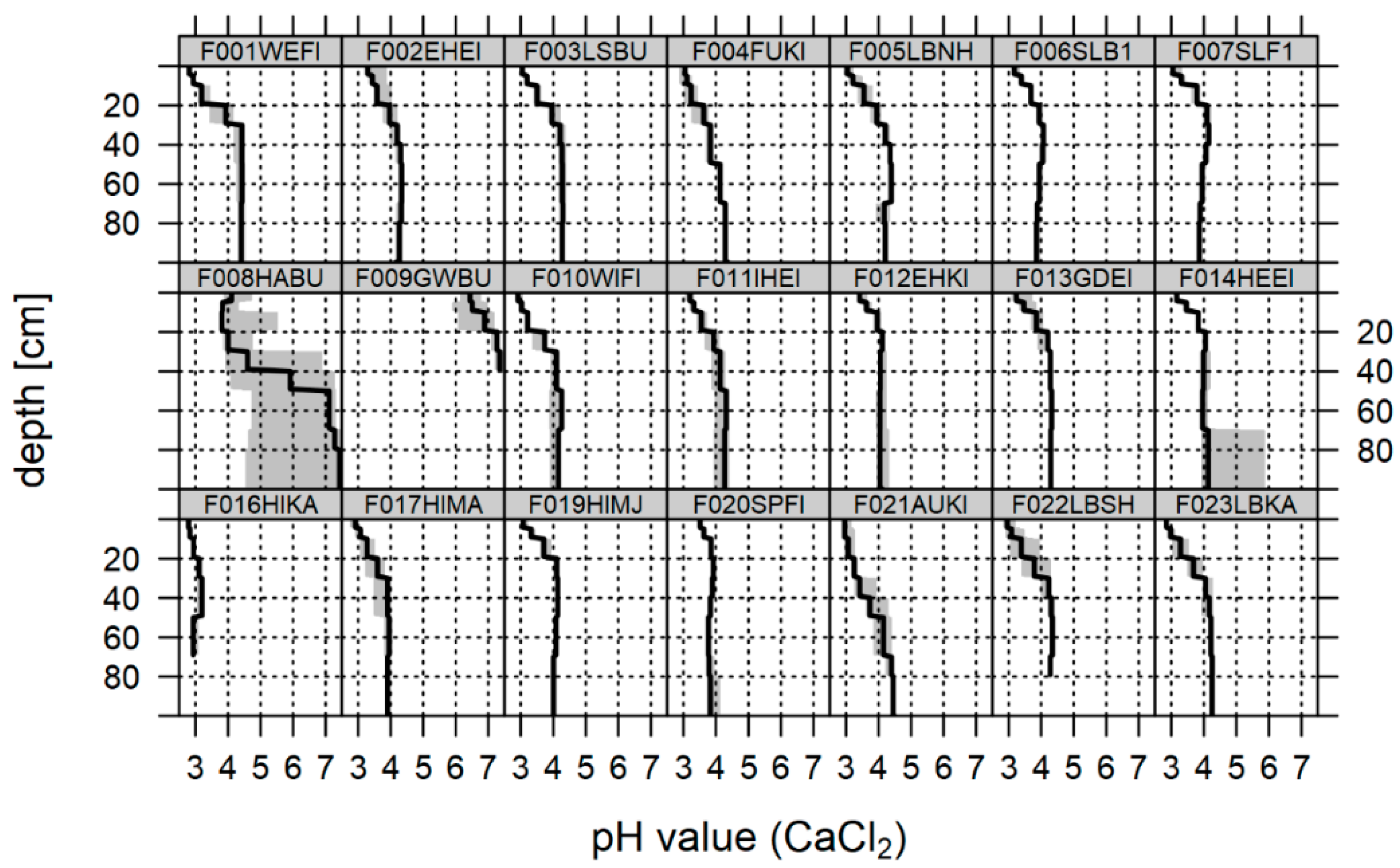
3.2. Soil Chemical Status at the Time of the Last Soil Inventory
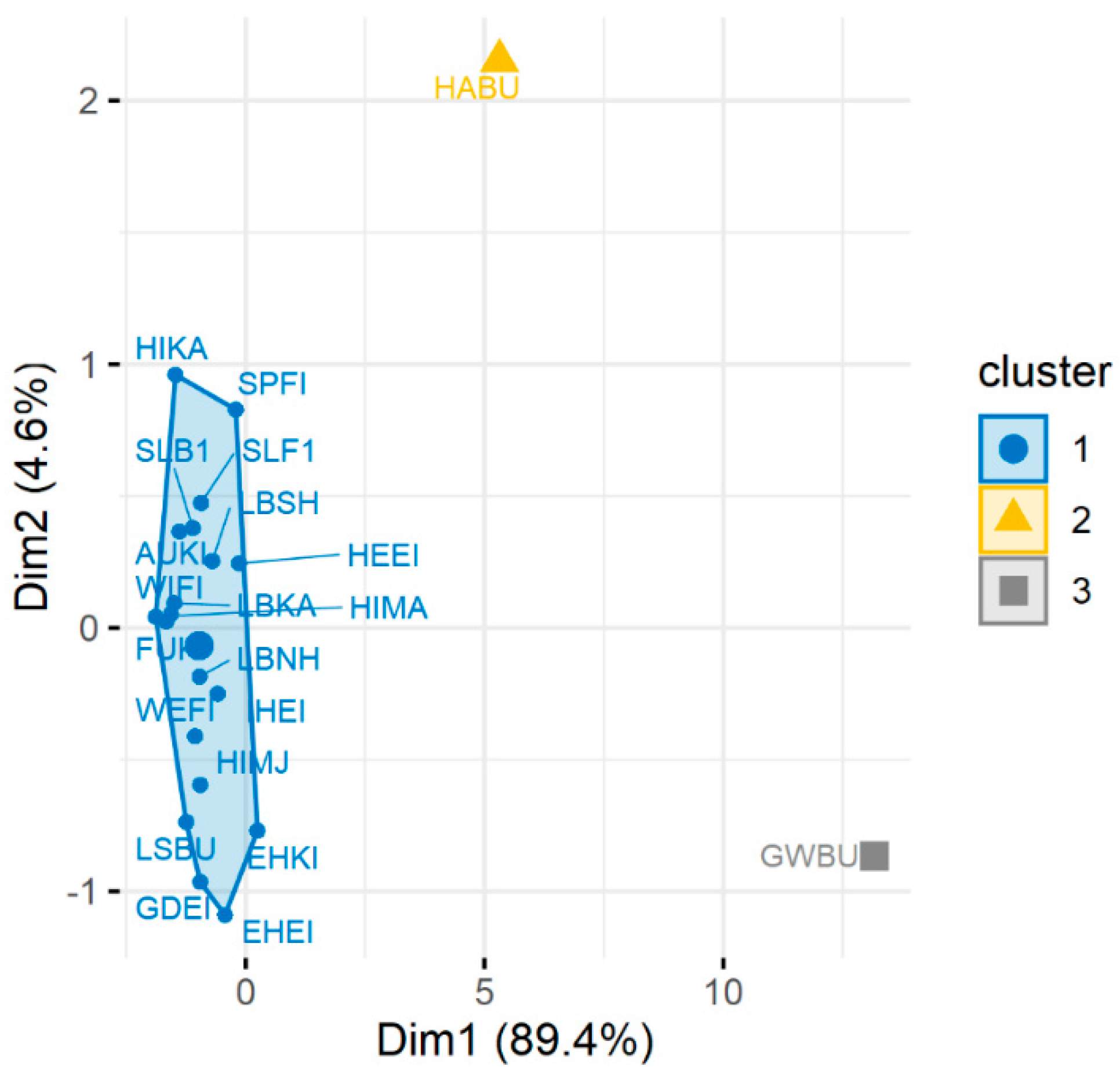
3.3. Statistical Detection of “Atypical” Monitoring Sites (Main Cluster Groups)
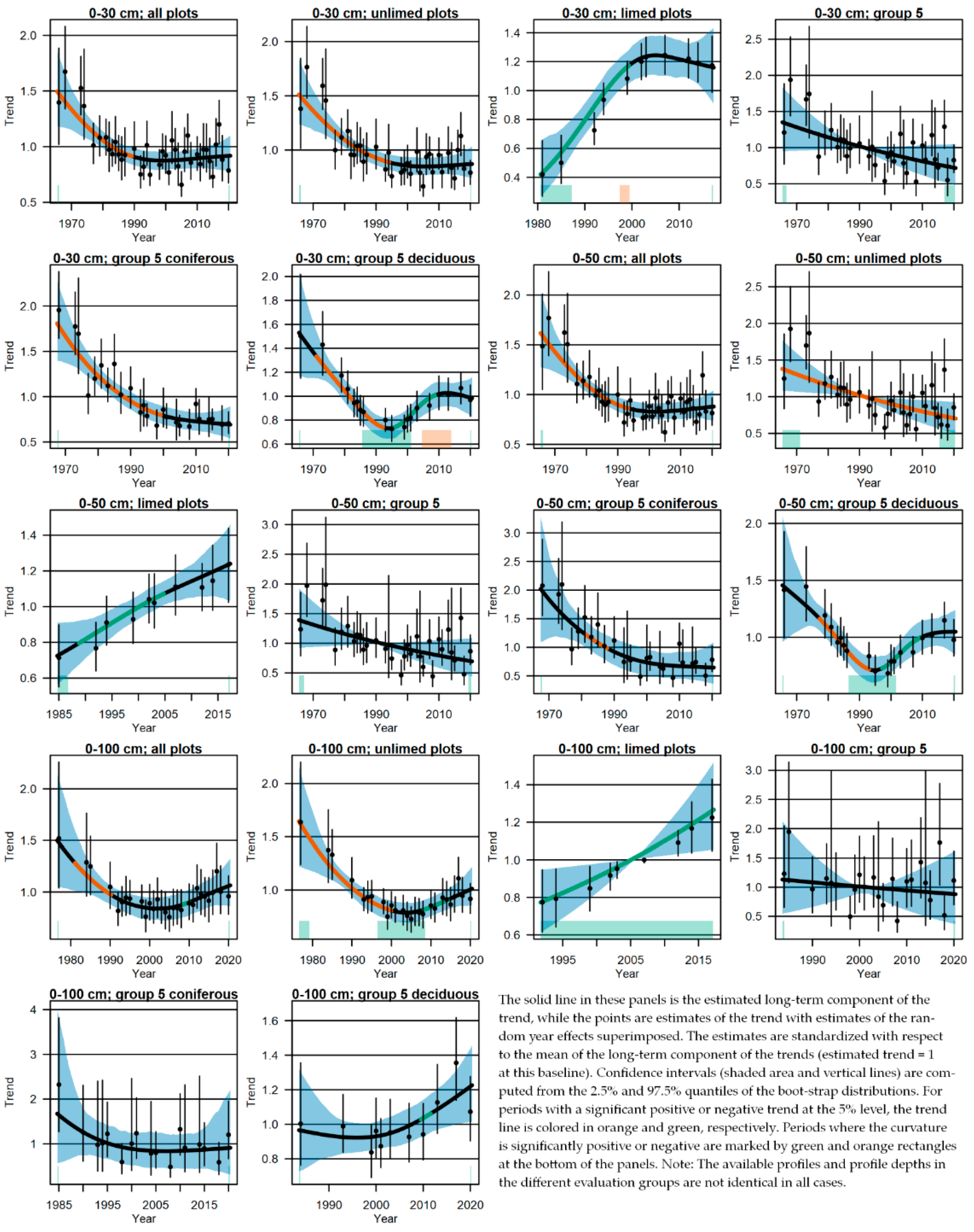
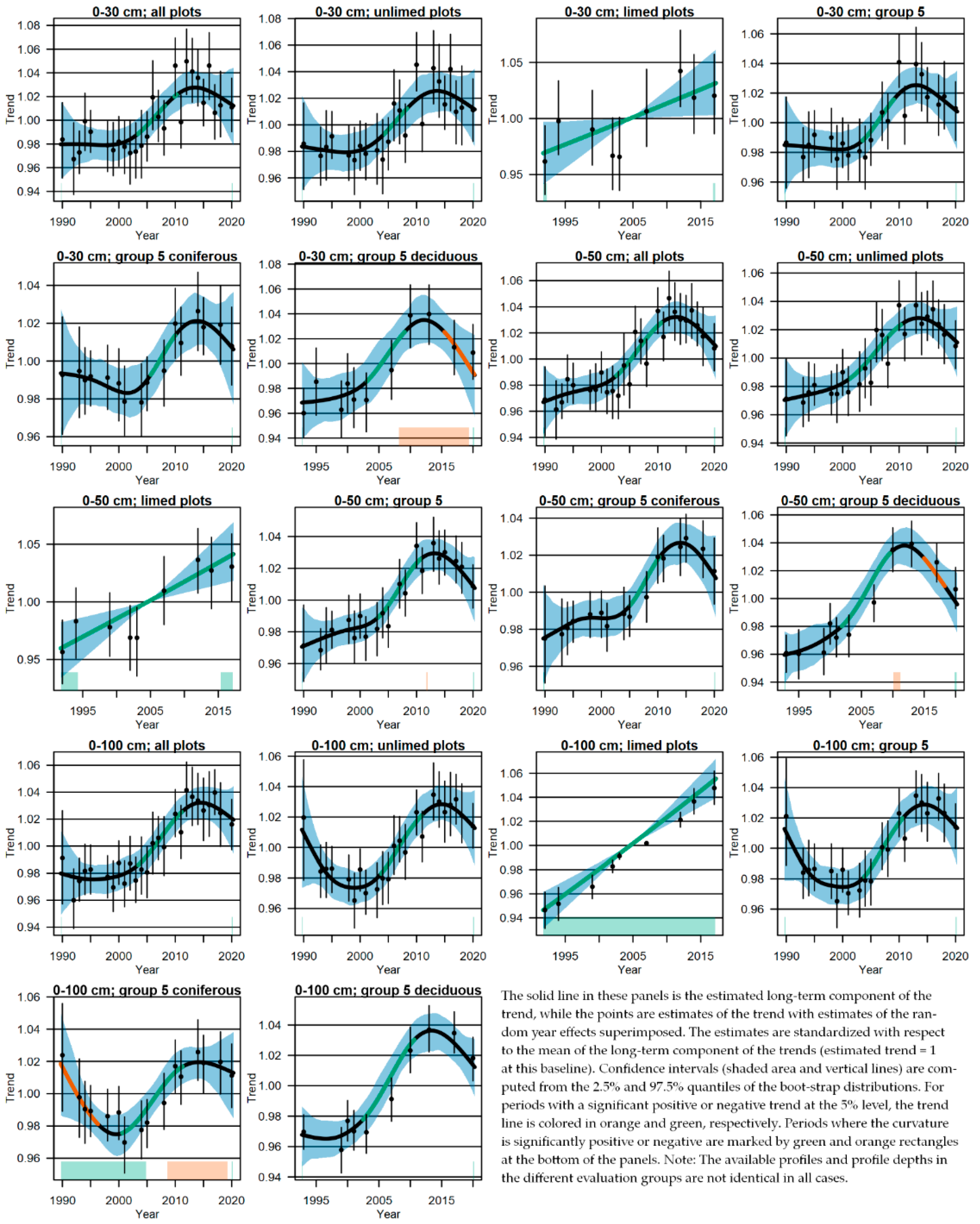
3.4. Changes of the Acid-Base Status and Indications of Recovery
4. Discussion
4.1. Site-Specific Sulfur Deposition Time-Series
4.2. Soil Chemical Status, Sampling Design and Statistical Approach
4.3. Change in Soil Chemical Variables and Indications of Recovery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Brumme, R.; Ahrends, B.; Block, J.; Schulz, C.; Meesenburg, H.; Klinck, U.; Wagner, M.; Khanna, P.K. Cycling and retention of nitrogen in European beech (Fagus sylvatica L.) ecosystems under elevated fructification frequency. Biogeosciences 2021, 18, 3763–3779. [Google Scholar] [CrossRef]
- Wilpert, K.V. Forest Soils—What’s Their Peculiarity? Soil Syst. 2022, 6, 5. [Google Scholar] [CrossRef]
- Oulehle, F.; Evans, C.D.; Hofmeister, J.; Krejci, R.; Tahovska, K.; Persson, T.; Cudlin, P.; Hruska, J. Major changes in forest carbon and nitrogen cycling caused by declining sulphur deposition. Glob. Chang. Biol. 2011, 17, 3115–3129. [Google Scholar] [CrossRef]
- Ulrich, B. Ökologische Gruppierung von Böden nach ihrem chemischen Bodenzustand. Z. Pflanzenernähr. Bodenkd. 1981, 149, 289–305. [Google Scholar] [CrossRef]
- Ulrich, B. Soil Acidity and its Relations to Acid Deposition. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Ulrich, B., Pankrath, J., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 127–146. [Google Scholar]
- Hauhs, M. Lange Bramke: An Ecosystem Study of a Forested Catchment. In Acidic Precipitation. Volume I: Case Studies; Adriano, D.C., Havas, M., Eds.; Springer: New York, NY, USA, 1989; pp. 275–305. [Google Scholar]
- Hauck, M.; Zimmermann, J.; Mascha, J.; Dulamsuren, C.; Bade, C.; Ahrends, B.; Leuschner, C. Rapid recovery of stem growth at reduced SO2 levels suggests a major contribution of foliar damage in the pollutant-caused dieback of Norway spruce during the late 20th century. Environ. Pollut. 2012, 164, 132–141. [Google Scholar] [CrossRef]
- Landmann, G.; Hunter, I.R.; Hendershot, W. Temporal and spatial development of magnesium deficiency in forest stands in Europe, North America and New Zealand. In Magnesium Deficiency in Forest Ecosystems; Hüttl, R.F., Schaaf, W., Eds.; Nutrients in Ecosystems; Springer: Dordrecht, The Netherlands, 1997; Volume 1, pp. 23–64. [Google Scholar]
- Engardt, M.; Simpson, D.; Schwikowski, M.; Granat, L. Deposition of sulphur and nitrogen in Europe 1900–2050. Model calculations and comparison to historical observations. Tellus Ser. B 2017, 69, 1328945. [Google Scholar] [CrossRef] [Green Version]
- Meesenburg, H.; Eichhorn, J.; Meiwes, K.J. Atmospheric Deposition and Canopy Interactions. In Functioning and Management of European Beech Ecosystems; Brumme, R., Khanna, P.K., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2009; Volume 208, pp. 265–302. [Google Scholar]
- Müller, F.; Bergmann, M.; Dannowski, R.; Dippner, J.W.; Gnauck, A.; Haase, P.; Jochimsen, M.C.; Kasprzak, P.; Kröncke, I.; Kümmerlin, R.; et al. Assessing resilience in long-term ecological data sets. Ecol. Indic. 2016, 65, 10–43. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, G.B.; Bailey, S.W. Recovery Processes of Acidic Soils Experiencing Decreased Acidic Deposition. Soil Syst. 2021, 5, 36. [Google Scholar] [CrossRef]
- Lange, H.; Hauhs, M.; Schmidt, S. Long-term sulfate dynamics at Lange Bramke (Harz) used for testing two acidification models. Water Air Soil Pollut. 1995, 79, 339–351. [Google Scholar] [CrossRef]
- Meesenburg, H.; Riek, W.; Ahrends, B.; Eickenscheidt, N.; Grüneberg, E.; Evers, J.; Fortmann, H.; Köng, N.; Lauer, A.; Meiwes, K.J.; et al. Soil Acidification in German Forest Soils. In Status and Dynamics of Forests in Germany; Wellbrock, N., Bolte, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 237, pp. 93–120. [Google Scholar]
- De Vries, W.; Dobbertin, M.H.; Solberg, S.; van Dobben, H.F.; Schaub, M. Impacts of acid deposition, ozone exposure and weather conditions on forest ecosystems in Europe: An overview. Plant Soil 2014, 380, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, A.; Sanders, T.G.M.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J.; et al. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 2019, 244, 980–994. [Google Scholar] [CrossRef]
- Schrijver, D.A.; Geudens, G.; Augusto, L.; Staelens, J.; Mertens, J.; Wuyts, K.; Gielis, L.; Verheyen, K. The effect of forest type on throughfall deposition and seepage flux: A review. Oecologia 2007, 153, 663–674. [Google Scholar] [CrossRef]
- Zhang, L.; Brook, J.R.; Vet, R. A revised parameterization for gaseous dry deposition in air quality models. Atmos. Chem. Phys. 2003, 3, 2067–2082. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, P.L. Deposition Velocities of SO2 and O3 over Agricultural and Forest Ecosystems. Water Air Soil Pollut. Focus 2001, 1, 49–57. [Google Scholar] [CrossRef]
- Hazlett, P.W.; Emilson, C.E.; Lawrence, G.B.; Fernandez, I.J.; Ouimet, R.; Bailey, S.W. Reversal of forest soil acidification in the northeastern United States and eastern Canada: Site and soil factors contributing to recovery. Soil Syst. 2020, 4, 54. [Google Scholar] [CrossRef]
- Meesenburg, H.; Ahrends, B.; Fleck, S.; Wagner, M.; Fortmann, H.; Scheler, B.; Klinck, U.; Dammann, I.; Eichhorn, J.; Mindrup, M.; et al. Long-term changes of ecosystem services at Solling, Germany: Recovery from acidification, but increasing nitrogen saturation? Ecol. Indic. 2016, 65, 103–112. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Hazlett, P.W.; Fernandez, I.J.; Ouimet, R.; Bailey, S.W.; Shortle, W.C.; Smith, K.T.; Antidormi, M.R. Declining Acidic Deposition Begins Reversal of Forest-Soil Acidification in the Northeastern U.S. and Eastern Canada. Environ. Sci. Technol. 2015, 49, 13103–13111. [Google Scholar] [CrossRef]
- Pihl Karlsson, G.; Akselsson, C.; Hellsten, S.; Karlsson, P.E. Reduced European emissions of S and N—Effects on air concentrations, deposition and soil water chemistry in Swedish forests. Environ. Pollut. 2011, 159, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.; Graf Pannatier, E.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Sucker, C.; Wilpert, K.; Puhlmann, H. Acidification reversal in low mountain range streams of Germany. Environ. Monit. Assess. 2011, 174, 65–89. [Google Scholar] [CrossRef]
- Wright, R.F.; Larssen, T.; Camarero, L.; Cosby, B.J.; Ferrier, R.C.; Helliwell, R.; Forsius, M.; Jenkins, A.; Kopáěek, J.; Majer, V.; et al. Recovery of Acidified European Surface Waters. Environ. Sci. Technol. 2005, 39, 64A–72A. [Google Scholar] [CrossRef] [Green Version]
- Stoddard, J.L.; Jeffries, D.S.; Lükewille, A.; Clair, T.A.; Dillon, P.J.; Driscoll, C.T.; Forsius, M.; Johannessen, M.; Kahl, J.S.; Kellogg, J.H.; et al. Regional trends in aquatic recovery from acidification in North America and Europe. Nature 1999, 401, 575–578. [Google Scholar] [CrossRef]
- Watmough, S.A.; Eimers, M.C. Rapid Recent Recovery from Acidic Deposition in Central Ontario Lakes. Soil Syst. 2020, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Berger, T.W.; Türtscher, S.; Berger, P.; Lindebner, L. A slight recovery of soils from Acid Rain over the last three decades is not reflected in the macro nutrition of beech (Fagus sylvatica) at 97 forest stands of the Vienna Woods. Environ. Pollut. 2016, 216, 624–635. [Google Scholar] [CrossRef] [Green Version]
- Reininger, D.; Fiala, P.; Samek, T. Acidification of Forest Soils in the Hrubý Jeseník Region. Soil Water Res. 2011, 6, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Cools, N.; De Vos, B. Availability and evaluation of European forest soil monitoring data in the study on the effects of air pollution on forests. Iforest—Biogeosciences For. 2011, 4, 205–211. [Google Scholar] [CrossRef]
- Grigal, D.F.; McRoberts, R.E.; Ohmann, L.F. Spatial Variation in Chemical Properties of Forest Floor and Surface Mineral Soil in the North Central United States. Soil Sci. 1991, 151, 282–290. [Google Scholar] [CrossRef]
- Bruelheide, H.; Udelhoven, P. Correspondence of the fine-scale spatial variation in soil chemistry and the herb layer vegetation in beech forests. For. Ecol. Manag. 2005, 210, 205–223. [Google Scholar] [CrossRef]
- Penne, C.; Ahrends, B.; Deurer, M.; Böttcher, J. The impact of the canopy structure on the spatial variability in forest floor carbon stocks. Geoderma 2010, 158, 282–297. [Google Scholar] [CrossRef]
- Arrouays, D.; Bellamy, P.H.; Paustian, K. Soil inventory and monitoring. Current issues and gaps. Eur. J. For. Res. 2009, 60, 721–722. [Google Scholar] [CrossRef]
- Mobley, M.L.; Yang, Y.; Yanai, R.D.; Nelson, K.A.; Bacon, A.R.; Heine, P.R.; Richter, D.D. How to Estimate Statistically Detectable Trends in a Time Series: A Study of Soil Carbon and Nutrient Concentrations at the Calhoun LTSE. Soil Sci. Soc. Am. J. 2019, 83, S133–S140. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Fernandez, I.J.; Richter, D.D.; Ross, D.S.; Hazlett, P.W.; Bailey, S.W.; Oiumet, R.; Warby, R.A.F.; Johnson, A.H.; Lin, H.; et al. Measuring environmental change in forest ecosystems by repeated soil sampling: A North American perspective. J. Environ. Qual. 2013, 42, 623–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, R.P.; Swanson, A.K. Forest soil characteristics in a chronosequence of harvested Douglas-fir forests. Can. J. For. Res. 2001, 31, 1871–1879. [Google Scholar] [CrossRef]
- Bens, O.; Buczko, U.; Sieber, S.; Hüttl, R.F. Spatial variability of O layer thickness and humus forms under different pine beech-forest transformation stages in NE Germany. J. Plant Nutr. Soil Sci. 2006, 169, 5–15. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U. Long-term changes in pH of forest soils in southern Sweden. Environ. Pollut. 1987, 43, 79–90. [Google Scholar] [CrossRef]
- Blake, L.; Goulding, K.W.T.; Mott, C.J.B.; Johnston, A.E. Changes in soil chemistry accompanying acidification over more than 100 years under woodland and grass at Rothamsted Experimental Station, UK. Eur. J. Soil Sci. 1999, 50, 401–412. [Google Scholar] [CrossRef]
- Johnson, A.H.; Andersen, S.B.; Siccama, T.G. Acid rain and soils of the Adirondacks. I. Changes in pH and available calcium, 1930–1984. Can. J. For. Res. 1994, 24, 39–45. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar] [CrossRef] [Green Version]
- Ingersoll, T.E.; Sewall, B.J.; Amelon, S.K. Improved Analysis of Long-Term Monitoring Data Demonstrates Marked Regional Declines of Bat Populations in the Eastern United States. PLoS ONE 2013, 8, e65907. [Google Scholar] [CrossRef] [Green Version]
- Knape, J. Decomposing trends in Swedish bird populations using generalized additive mixed models. J. Appl. Ecol. 2016, 53, 1852–1861. [Google Scholar] [CrossRef]
- Höper, H.; Meesenburg, H. Das Bodendauerbeobachtungsprogramm. GeoBerichte 2012, 23, 6–18. [Google Scholar]
- De Vries, W.; Vel, E.; Reinds, G.J.; Dellstra, H.; Klap, J.M.; Leeters, E.E.J.M.; Hendricks, C.M.A.; Kerkvoorden, M.; Landmann, G.; Herkendell, J.; et al. Intensive monitoring of forest ecosystems in Europe: 1. Objectives, set-up and evaluation strategy. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Clarke, N.; Zlindra, D.; Ulrich, E.; Mosello, R.; Derome, J.; Derome, K.; König, N.; Lövblad, G.; Draaijers, G.; Hansen, K.; et al. Part XIV: Sampling and Analysis of Deposition. Available online: https://storage.ning.com/topology/rest/1.0/file/get/9995560266?profile=original (accessed on 17 January 2022).
- Schaap, M.; Hendriks, C.; Kranenburg, R.; Kuenen, J.; Segers, A.; Schlutow, A.; Nagel, H.-D.; Ritter, A.; Banzhaf, S. PINETI-3: Modellierung atmosphärischer Stoffeinträge von 2000 bis 2015 zur Bewertung der ökosystem-spezifischen Gefährdung von Biodiversität durch Luftschadstoffe in Deutschland. Texte Umweltbundesamt 2018, 79, 149. [Google Scholar]
- Alveteg, M.; Walse, C.; Warfvinge, P. Reconstructing historic atmospheric deposition and nutrient uptake from present day values using MAKEDEP. Water Air Soil Pollut. 1998, 104, 269–283. [Google Scholar] [CrossRef]
- Barth, N.; Brandtner, W.; Cordsen, E.; Dann, T.; Emmerisch, K.-H.; Feldhaus, D.; Kleefisch, B.; Schilling, B.; Utermann, J. Boden-Dauerbeobachtung—Einrichtung und Betrieb von Boden-Dauerbeobachtungsflächen. In Bodenschutz. Ergänzbares Handbuch der Maßnahmen und Empfehlungen für Schutz, Pflege und Sanierung von Böden, Landschaft und Grundwasser; Rosenkranz, D., Bachmann, G., König, W., Einsele, G., Eds.; Erich Schmidt Verlag: Berlin, Germany, 2000; Volume Bd3: 9152, pp. 1–127. [Google Scholar]
- Cools, N.; De Vos, B. Part X: Sampling and Analysis of Soil. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Coordinating Centre; Thünen Institute of Forest Ecosystems: Eberswalde, Germany; Available online: https://storage.ning.com/topology/rest/1.0/file/get/9995584862?profile=original (accessed on 17 February 2022).
- König, N.; Forstmann, H. Probenvorbereitungs-Untersuchungs-und Elementbestimmungs-Methoden des Umweltanalytik-Labors der Niedersächsischen Forstlichen Versuchsanstalt und des Zentrallabor II des Forschungszentrums Waldökosysteme; Forest Ecosystem Research Center: Göttingen, Germany, 1996; pp. 46–49. [Google Scholar]
- König, N.; Fortmann, H. Probenvorbereitungs-, Untersuchungs- und Elementbestimmungsmethoden des Umweltanalytiklabors der Niedersächsischen Forstlichen Versuchsanstalt und des Zentrallabor II des Forschungszentrums Waldökosysteme; Forest Ecosystem Research Center: Göttingen, Germany, 1999; pp. 58–60. [Google Scholar]
- König, N.; Forstmann, H.; Lüter, K.L. Probenvorbereitungs-, Untersuchungs- und Elementbestimmungs-Methoden des Umweltanalytik-Labors der Niedersächsischen Forstlichen Versuchsanstalt; Forest Ecosystem Research Center: Göttingen, Germany, 2009; pp. 75–78. [Google Scholar]
- Meiwes, K.J.; Khanna, P.K.; Ulrich, B. Parameter for describing soil acidification and their relevance to the stability of forest ecosystems. For. Ecol. Manage. 1986, 15, 161–179. [Google Scholar] [CrossRef]
- Wellbrock, N.; Ahrends, B.; Bögelein, R.; Bolte, A.; Eickenscheidt, N.; Grüneberg, E.; König, N.; Schmitz, A.; Fleck, S.; Ziche, D. Concept and Methodology of the National Forest Soil Inventory. In Status and Dynamics of Forests in Germany; Wellbrock, N., Bolte, A., Eds.; Ecological Studies; Springer: Cham, Schwitzerland, 2019; Volume 237. [Google Scholar]
- Meiwes, K.J.; Meesenburg, H.; Eichhorn, J.; Jacobsen, C.; Khanna, P.K. Changes in C and N content of soils under beech forests over a period of 35 years. In Functioning and Management of European Beech Ecosystems; Brumme, R., Khanna, P., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2009; Volume 208, pp. 49–63. [Google Scholar]
- Hartmann, P.; Von Wilpert, k. Statistisch definierte Vertikalgradienten der Basensättigung sind geeignete Indikatoren für den Status und die Veränderungen der Bodenversauerung in Waldböden. Allg. Forst-U. J.-Ztg. 2016, 187, 61–69. [Google Scholar]
- Dietrich, H.; Wolf, T.; Kawohl, T.; Wehberg, J.; Kändler, G.; Mette, T.; Röder, A.; Böhner, J. Temporal and spatial high-resolution climate data from 1961 to 2100 for the German National Forest Inventory (NFI). Ann. For. Sci. 2019, 76, 6. [Google Scholar] [CrossRef] [Green Version]
- Beaudette, D.E.; Roudier, P.; O’Geen, A.T. Algorithms for quantitative pedology: A toolkit for soil scientists. Comput. Geosci. 2013, 52, 258–268. [Google Scholar] [CrossRef]
- Jackson, D.A.; Chen, Y. Robust principal component analysis and outlier detection with ecological data. Environmetrics 2004, 15, 129–139. [Google Scholar] [CrossRef]
- Pascoal, C.; Oliveira, M.R.; Pacheco, A.; Valadas, R. Detection of Outliers Using Robust Principal Component Analysis: A Simulation Study. In Combining Soft Computing and Statistical Methods in Data Analysis; Borgelt, C.E.A., Ed.; Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2010; Volume 77, pp. 499–507. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 18. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman & Hall: Boca Raton, FL, USA, 2006; p. 410. [Google Scholar]
- Dixon, W.J. Analysis of Extreme Values. Comput. Geosci. 1950, 21, 488–506. [Google Scholar] [CrossRef]
- Harrison, P.J.; Buckland, S.T.; Yuan, Y.; Elston, D.A.; Brewer, M.J.; Johnston, A.; Pearce-Higgins, J.W. Assessing trends in biodiversity over space and time using the example of British breeding birds. J. Appl. Ecol. 2014, 51, 1650–1660. [Google Scholar] [CrossRef]
- Kölling, C.; Hoffmann, M.; Gulder, H.-J. Bodenchemische Vertikalgradienten als charakteristische Zustandsgrößen von Waldökosystemen. Z. Pflanzenernähr. Bodenk. 1996, 159, 69–77. [Google Scholar] [CrossRef]
- Schaap, M.; Timmermans, R.M.A.; Roemer, M.; Boersen, G.A.C.; Builtjes, P.J.H. The LOTOS–EUROS model: Description, validation and latest developments. Int. J. Environ. Pollut. 2008, 32, 270–290. [Google Scholar] [CrossRef]
- Manders, A.M.M.; Builtjes, P.J.H.; Curier, L.; Denier van der Gon, H.A.C.; Hendriks, C.; Jonkers, S.; Kranenburg, R.; Kuenen, J.J.P.; Segers, A.J.; Timmermans, R.M.A.; et al. Curriculum vitae of the LOTOS–EUROS (v2.0) chemistry transport model. Geosci. Model Dev. 2017, 10, 4145–4173. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.I.; Fowler, D. Uncertainty in Estimation of Wet Deposition of Sulphur. Water Air Soil Pollut. Focus 2001, 1, 341–353. [Google Scholar] [CrossRef]
- Ahrends, B.; Schmitz, A.; Prescher, A.-K.; Wehberg, J.; Geupel, M.; Andreae, H.; Meesenburg, H. Comparison of Methods for the Estimation of Total Inorganic Nitrogen Deposition to Forests in Germany. Front. For. Glob. Change 2020, 3, 103. [Google Scholar] [CrossRef]
- Prechtel, A.; Alewell, C.; Armbruster, M.; Bittersohl, J.; Cullen, J.; Evans, C.D.; Helliwell, R.C.; Kopacek, J.; Marchetto, A.; Matzner, E.; et al. Response of sulphur dynamics in European freshwaters to decreasing sulphate deposition. Hydrol. Earth Syst. Sci. 2001, 5, 311–325. [Google Scholar] [CrossRef]
- Evers, J.; Dammann, I.; König, N.; Paar, U.; Stüber, V.; Schulze, A.; Schmidt, M.; Schönfelder, E.; Eichhorn, J. Waldbodenzustandsbericht für Niedersachsen und Bremen. Ergebnisse der zweiten Bodenzustandserhebung im Wald (BZE II). Beitr. aus der NW-FVA 2019, 19, 498. [Google Scholar]
- Reuss, J.O. Implications of the calcium-aluminum exchange system for the effect of acid precipitation on soils. J. Environ. Qual. 1983, 12, 591–595. [Google Scholar] [CrossRef]
- Kirwan, N.; Oliver, M.A.; Moffat, A.J.; Morgan, G.W. Sampling The Soil In Long-Term Forest Plots: The Implications of Spatial Variation. Environ. Monit. Assess. 2005, 111, 149–172. [Google Scholar] [CrossRef]
- Braun, S.; Tresch, S.; Augustin, S. Soil solution in Swiss forest stands: A 20 year’s time series. PLoS ONE 2020, 15, e0227530. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Li, Y.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Penttinen, P. Spatial variability of soil nutrients in forest areas: A case study from subtropical China. J. Plant Nutr. Soil Sci. 2018, 181, 827–835. [Google Scholar] [CrossRef]
- Li, J.; Richter, D.D.; Mendoza, A.; Heine, P. Effects of land-use history on soil spatial heterogeneity of macro- and trace elements in the Southern Piedmont USA. Geoderma 2010, 156, 60–73. [Google Scholar] [CrossRef]
- Heinze, S.; Ludwig, B.; Piepho, H.-P.; Mikutta, R.; Don, A.; Wordell-Dietrich, P.; Helfrich, M.; Hertel, D.; Leuschner, C.; Kirfel, K.; et al. Factors controlling the variability of organic matter in the top- and subsoil of a sandy Dystric Cambisol under beech forest. Geoderma 2018, 311, 37–44. [Google Scholar] [CrossRef]
- König, N.; Cools, N.; Derome, K.; Kowalska, A.; De Vos, B.; Fürst, A.; Marchetto, A.; O’Dea, P.; Tartari, G.A. Chapter 22—Data Quality in Laboratories: Methods and Results for Soil, Foliar, and Water Chemical Analyses. In Developments in Environmental Science; Ferretti, M., Fischer, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 12, pp. 415–453. [Google Scholar]
- Kirk, G.J.D.; Bellamy, P.H.; Lark, R.M. Changes in soil pH across England and Wales in response to decreased acid deposition. Glob. Chang. Biol. 2010, 16, 3111–3119. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Benham, S.; Pitman, R.; Moffat, A.J.; Broadmeadow, M.; Nisbet, T.; Durrant, D.; Barsoum, N.; Wilkinson, M.; Bochereau, F.; et al. Chemical fluxes in time through forest ecosystems in the UK—Soil response to pollution recovery. Environ. Pollut. 2010, 158, 1857–1869. [Google Scholar] [CrossRef]
- Jansone, L.; von Wilpert, K.; Hartmann, P. Natural Recovery and Liming Effects in Acidified Forest Soils in SW-Germany. Soil Syst. 2020, 4, 38. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Scanga, S.E.; Sabo, R.D. Recovery of Soils from Acidic Deposition May Exacerbate Nitrogen Export from Forested Watersheds. J. Geophys. Res. 2020, 125, e2019JG005036. [Google Scholar] [CrossRef] [Green Version]
- Hedin, L.O.; Granat, L.; Likens, G.E.; Buishand, T.A.; Galloway, J.N.; Butler, T.J.; Rodhe, H. Steep declines in atmospheric base cations in regions of Europe and North America. Nature 1994, 367, 351–354. [Google Scholar] [CrossRef]
- Meesenburg, H.; Meiwes, K.-J.; Rademacher, P. Long term trends in atmospheric deposition and seepage output in northwest german forest ecosystems. Water Air Soil Pollut. 1995, 85, 611–616. [Google Scholar] [CrossRef]
- Guckland, A.; Ahrends, B.; Paar, U.; Dammann, I.; Evers, J.; Meiwes, K.J.; Schönfelder, E.; Ullrich, T.; Mindrup, M.; König, N.; et al. Predicting depth translocation of base cations after forest liming—Results from long-term experiments. Eur. J. For. Res. 2012, 131, 1869–1887. [Google Scholar] [CrossRef]
- Lawrence, G.; Siemion, J.; Antidormi, M.; Bonville, D.; McHale, M. Have Sustained Acidic Deposition Decreases Led to Increased Calcium Availability in Recovering Watersheds of the Adirondack Region of New York, USA? Soil Syst. 2021, 5, 6. [Google Scholar] [CrossRef]
- Greve, M.; Block, J.; Schüler, G.; Werner, W. Long Term Effects of Forest Liming on the Acid-Base Budget. Appl. Sci. 2021, 11, 955. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Shortle, W.C.; David, M.B.; Smith, K.T.; Warby, R.A.F.; Lapenis, A.G. Early indications of soil recovery from acidic deposition in U.S. red spruce forests. Soil Sci. Soc. Am. J. 2012, 76, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Czajkowski, T.; Ahrends, B.; Bolte, A. Critical limits of soil water availability (CL-SWA) in forest trees—An approach based on plant water status. Vti Agric. For. Res. 2009, 59, 87–93. [Google Scholar]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci 2002, 59, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Rumpf, S.; Schönfelder, E.; Ahrends, B. Biometrische Schätzmodelle für Nährelementgehalte in Baumkompartimenten. Freibg. Forstl. Forsch. 2018, 101, 33–73. [Google Scholar]
- Watmough, S.A.; Aherne, J.; Alewell, C.; Arp, P.; Bailey, S.; Clair, T.; Dillon, P.; Duchesne, L.; Eimers, C.; Fernandez, I.; et al. Sulphate, nitrogen and base cation budgets at 21 forested catchments in Canada, the united states and Europe. Environ. Monit. Assess. 2005, 109, 1–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Mitchell, M.J.; Christ, M.; Likens, G.E.; Krouse, H.R. Stable sulfur isotopic biogeochemistry of the Hubbard Brook Experimental Forest, New Hampshire. Biogeochemistry 1998, 41, 259–275. [Google Scholar] [CrossRef]
- Alewell, C.; Mitchell, M.J.; Likens, G.E.; Krouse, H.R. Sources of Stream Sulfate at the Hubbard Brook Experimental Forest: Long-Term Analyses Using Stable Isotopes. Biogeochemistry 1999, 44, 281–299. [Google Scholar] [CrossRef]
- Jandl, R.; Schmidt, S.; Mutsch, F.; Fürst, A.; Zechmeister, H.; Bauer, H.; Dirnböck, T. Acidifiction and nitrogen eutrophication of austrian forest soils. Appl. Envirionmental Soil Sci. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Sokolova, T.A.; Alekseeva, S.A. Adsorption of Sulfate Ions by Soils (A Review). Eurasian Soil Sci. 2008, 41, 140–148. [Google Scholar] [CrossRef]
- Meiwes, K.J.; Khanna, P.K.; Ulrich, B. Retention of sulphate by an acid brown earth and its relationship with the atmospheric impact of sulphur to forest vegetation. J. Plant Nutr. Soil Sci. 1980, 143, 402–411. [Google Scholar] [CrossRef]
- Ulrich, B. Natural and anthropogenic components of soil acidification. J. Plant Nutr. Soil Sci. 1986, 149, 702–717. [Google Scholar] [CrossRef]
- Klaminder, J.; Lucas, R.W.; Futter, M.N.; Bishop, K.H.; Köhler, S.J.; Egnell, G.; Laudon, H. Silicate mineral weathering rate estimates: Are they precise enough to be useful when predicting the recovery of nutrient pools after harvesting? For. Ecol. Manag. 2011, 261, 1–9. [Google Scholar] [CrossRef]
- Futter, M.N.; Klaminder, J.; Lucas, R.W.; Laudon, H.; Köhler, S.J. Uncertainty in silicate mineral weathering rate estimates: Source partitioning and policy implications. Environ. Res. Lett. 2012, 7, 8. [Google Scholar] [CrossRef]
- Fenn, M.E.; Huntington, T.G.; McLaughlin, S.B.; Eager, S.B.; Gomez, A.; Cook, R.B. Status of soil acidification in North America. J. For. Sci. 2006, 52, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Sverdrup, H.; Martinson, L.; Alveteg, M.; Moldan, F.; Kronnäs, V.; Munthe, J. Modeling recovery of Swedish ecosystems from acidification. Ambio 2005, 34, 25–31. [Google Scholar] [CrossRef]
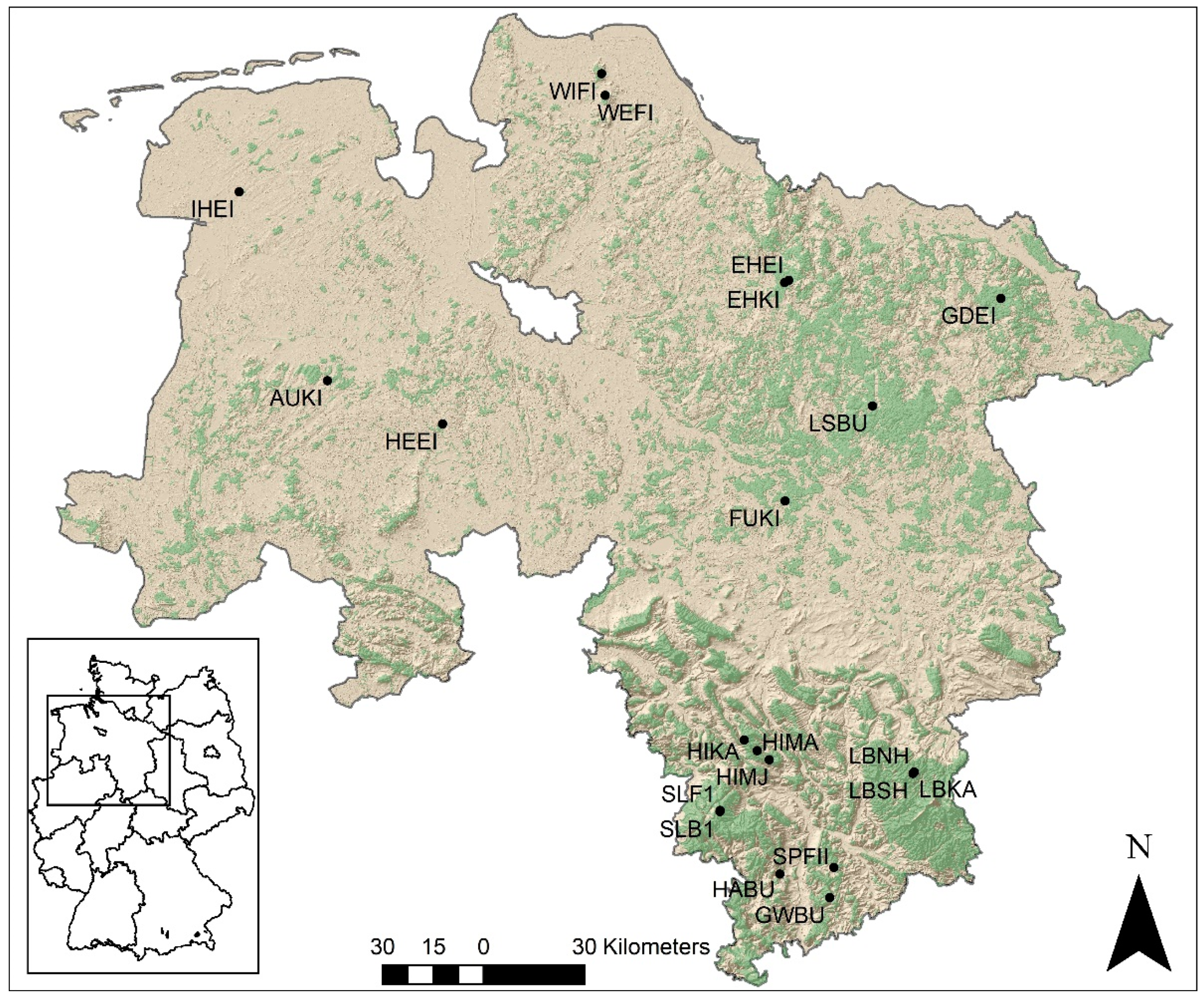


| Location | Plot Code | Lat [°] | Long [°] | MTS [-] | Alt [m] | Slope [°] | Aspect [°] | Stype [WRB] | MAT [°C] | MAP [mm] |
|---|---|---|---|---|---|---|---|---|---|---|
| Westerberg | F001WEFI | 53.67 | 9.09 | spruce | 37 | 1.1 | 311 | Podzol | 8.7 | 903 |
| Ehrhorn | F002EHEI | 53.18 | 9.90 | oak | 110 | 2.9 | 337 | Cambisol | 8.5 | 843 |
| F012EHKI | 53.17 | 9.88 | pine | 82 | 1.1 | 241 | Podzol | 8.8 | 826 | |
| Lüss | F003LSBU | 52.84 | 10.17 | beech | 115 | 2.3 | 310 | Podzol | 8.5 | 859 |
| Fuhrberg | F004FUKI | 52.59 | 9.87 | pine | 37 | 0.1 | 107 | Podzol | 9.7 | 684 |
| Lange Bramke | F005LBNH | 51.86 | 10.42 | spruce | 613 | 15.8 | 19 | Podzol | 6.5 | 1453 |
| F022LBSH | 51.86 | 10.41 | spruce | 603 | 23.0 | 151 | Podzol | 6.4 | 1446 | |
| F023LBKA | 51.86 | 10.42 | spruce | 656 | 8.1 | 109 | Podzol | 6.5 | 1453 | |
| Solling | F006SLB1 | 51.76 | 9.58 | beech | 506 | 1.9 | 207 | Cambisol | 7.0 | 1246 |
| F007SLF1 | 51.76 | 9.58 | spruce | 507 | 1.4 | 92 | Podzol | 7.0 | 1245 | |
| Harste | F008HABU | 51.59 | 9.83 | beech | 250 | 5.5 | 82 | Luvisol | 8.5 | 774 |
| Göttingen | F009GWBU | 51.53 | 10.05 | beech | 421 | 0.3 | 246 | Luvisol | 7.5 | 897 |
| Wingst | F010WIFI | 53.73 | 9.07 | spruce | 34 | 3.7 | 102 | Podzol | 8.7 | 933 |
| Ihlow | F011IHEI | 53.41 | 7.45 | oak | 53 | 0.3 | 274 | Podzol | 9.4 | 846 |
| Göhrde | F013GDEI | 53.12 | 10.84 | oak | 97 | 1.9 | 127 | Podzol | 8.5 | 742 |
| Heerenholz | F014HEEI | 52.80 | 8.37 | oak | 48 | 0.3 | 204 | Fluvisol | 9.4 | 795 |
| Hils | F016HIKA | 51.95 | 9.69 | spruce | 424 | 24.3 | 253 | Podzol | 7.6 | 1196 |
| F017HIMA | 51.92 | 9.74 | spruce | 253 | 6.3 | 244 | Cambisol | 8.2 | 1020 | |
| F019HIMJ | 51.90 | 9.79 | spruce | 317 | 14.0 | 26 | Cambisol | 7.8 | 1075 | |
| Spanbeck | F020SPFI | 51.61 | 10.07 | spruce | 251 | 1.7 | 72 | Planosol | 8.6 | 830 |
| Augustendorf | F021AUKI | 52.91 | 7.86 | pine | 31 | 1.0 | 102 | Podzol | 9.4 | 843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrends, B.; Fortmann, H.; Meesenburg, H. The Influence of Tree Species on the Recovery of Forest Soils from Acidification in Lower Saxony, Germany. Soil Syst. 2022, 6, 40. https://doi.org/10.3390/soilsystems6020040
Ahrends B, Fortmann H, Meesenburg H. The Influence of Tree Species on the Recovery of Forest Soils from Acidification in Lower Saxony, Germany. Soil Systems. 2022; 6(2):40. https://doi.org/10.3390/soilsystems6020040
Chicago/Turabian StyleAhrends, Bernd, Heike Fortmann, and Henning Meesenburg. 2022. "The Influence of Tree Species on the Recovery of Forest Soils from Acidification in Lower Saxony, Germany" Soil Systems 6, no. 2: 40. https://doi.org/10.3390/soilsystems6020040
APA StyleAhrends, B., Fortmann, H., & Meesenburg, H. (2022). The Influence of Tree Species on the Recovery of Forest Soils from Acidification in Lower Saxony, Germany. Soil Systems, 6(2), 40. https://doi.org/10.3390/soilsystems6020040






