Biodegradability of Disposable Surgical Face Masks Littered into Soil Systems during the COVID 19 Pandemic—A First Approach Using Microcosms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microcosm Experiment
2.2. Solid-State CPMAS 13C NMR Spectroscopy
2.3. Statistical Design
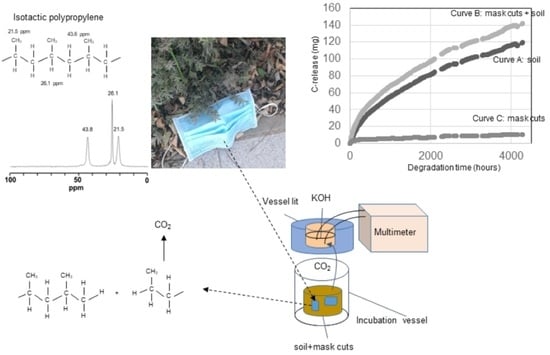
3. Results and Discussion
3.1. Composition of the Starting Mask Material
3.2. Microbial Decomposition of Mask Material
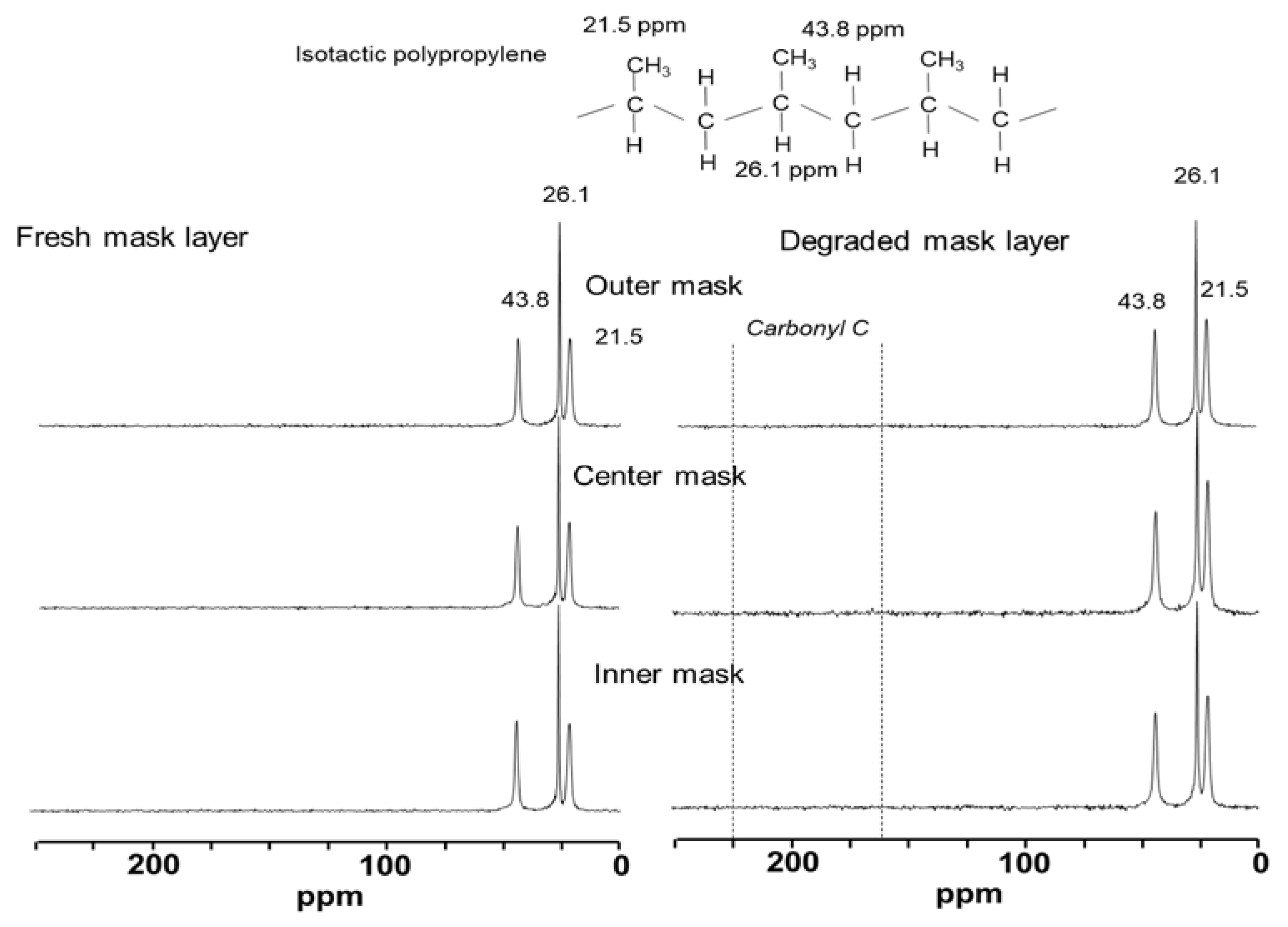
3.3. Chemical Changes during Microbial Degradation of Single-Use Face Masks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worby, C.J.; Chang, H.-H. Face mask use in the general population and optimal resource allocation during the COVID-19 pandemic. Nat. Commun. 2020, 11, 4049. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef] [PubMed]
- Chellamani, K.P.; Veerasubramanian, D.; Vignesh Balaji, R.S. Surgical face masks: Manufacturing methods and classification. J. Acad. Ind. Res. 2013, 6, 320–324. [Google Scholar]
- Saliu, F.; Veronelli, M.; Raguso, C.; Barana, D.; Galli, P.; Lasagni, M. The release process of microfibers: From surgical face masks into the marine environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Arkatkar, A.; Arutchelvi, J.; Bhaduri, S.; Uppara, P.V.; Doble, M. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegrad. 2009, 63, 106–111. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Giovannozzi Sermanni, G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef] [Green Version]
- Longo, C.; Savaris, M.; Zeni, M.; Brandalise, R.N.; Grisa, A.M.C. Degradation study of polypropylene (PP) and bioriented polypropylene (BOPP) in the environment. Mater. Res. 2011, 14, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Isolation of mesophilic bacterium for biodegradation of polypropylene. Int. Biodeterior. Biodegrad. 2016, 115, 244–249. [Google Scholar] [CrossRef]
- Pang, M.-M.; Pun, M.-Y.; Ishak, Z.A.M. Degradation studies during water absorption, aerobic biodegradation, and soil burial of biobased thermoplastic starch from agricultural waste/polypropylene blends. J. Appl. Polym. Sci. 2013, 129, 3656–3664. [Google Scholar] [CrossRef]
- Jain, K.; Bhunia, H.; Reddy, M.S. Degradation of polypropylene–poly-L-lactide blend by bacteria isolated from compost. Bioremediat. J. 2018, 22, 73–90. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef]
- Ru, J.; Huo, Y.; Yang, Y. Microbial Degradation and Valorization of Plastic Wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Lopéz-Martín, M.; Velasco-Molina, M.; Knicker, H.; Lopez-Martin, M.; Velasco-Molina, M.; Knicker, H.; López-Martín, M.; Velasco-Molina, M.; Knicker, H.; Lopéz-Martín, M.; et al. Variability of the quality and quantity of organic matter in soil affected by multiple wildfires. J. Soils Sediments 2016, 16, 360–370. [Google Scholar] [CrossRef]
- Veihmeyer, F.J.; Hendrickson, A.H. Methods of measuring field capacity and permament wilting percentage of soils. Soil Sci. 1949, 68, 75–94. [Google Scholar] [CrossRef]
- Nordgren, A. Apparatus for the continuous, long-term monitoring of soil respiration rate in large numbers of samples. Soil Biol. Biochem. 1988, 20, 955–957. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press, Inc.: San Diego, CA, USA, 1996. [Google Scholar]
- Knicker, H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011, 42, 867–890. [Google Scholar] [CrossRef]
- Wilhelm, M.; Neidhöfer, M.; Spiegel, S.; Spiess, H.W. A collection of solid-state 13C CP/MAS NMR spectra of common polymers. Macromol. Chem. Phys. 1999, 200, 2205–2207. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Spiess, H.W. Multidimensional Solid-State NMR and Polymers, 1st ed.; Academic Press: London, UK, 2012; ISBN 9780080925622. [Google Scholar]
- Aragaw, T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H.; González-Vila, F.J.; González-Vázquez, R. Biodegradability of organic matter in fire-affected mineral soils of Southern Spain. Soil Biol. Biochem. 2013, 56, 31–39. [Google Scholar] [CrossRef]
- Jeon, J.-M.; Park, S.-J.; Choi, T.-R.; Park, J.-H.; Yang, Y.-H.; Yoon, J.-J. Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym. Degrad. Stab. 2021, 191, 109662. [Google Scholar] [CrossRef]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Yang, J.; Kong, M.; Yang, H.; Zhao, J.; Li, G. Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study. Polym. Degrad. Stab. 2015, 112, 145–159. [Google Scholar] [CrossRef]




| 50–40 ppm | 30–24 ppm | 24–15 ppm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outer | Center | Inner | Outer | Center | Inner | Outer | Center | Inner | |
| St0 | 32.6 ± 0.0 a | 32.6 ± 0.2 a | 32.6 ± 0.1 a | 32.8 ± 0.1 a | 32.8 ± 0.2 ab | 32.8 ± 0.1 a | 34.6 ± 0.0 a | 34.7 ± 0.4 a | 34.6 ± 0.1 a |
| St1 | 31.7 ± 0.1 ab | 31.0 ± 0.4 bc | 31.4 ± 0.4 bc | 32.0 ± 0.3 abc | 31.5 ± 0.3 c | 31.7 ± 0.3 bc | 36.6 ± 0.4 ab | 37.5 ± 0.7 bc | 36.9 ± 0.7 b |
| Mt1 | 31.4 ± 0.1 bc | 31.8 ± 0.2 ab | 31.7 ± 0.1 ab | 31.8 ± 0.3 abc | 32.1 ± 0.2 abc | 31.8 ± 0.8 abc | 36.8 ± 0.3 b | 36.1 ± 0.3 ab | 36.5 ± 0.8 ab |
| St6 | 30.9 ± 0.6 bc | 28.9 ± 0.6 d | 30.5 ± 0.3 c | 32.9 ± 0.5 a | 32.0 ± 0.3 abc | 32.5 ± 0.5 abc | 36.2 ± 1.1 ab | 39.1 ± 0.4 c | 37.0 ± 0.9 b |
| Mt6 | 30.5 ± 0.6 c | 31.4 ± 0.7 bc | 30.8 ± 0.6 c | 32.8 ± 0.5 a | 32.9 ± 0.1 a | 32.7 ± 0.2 ab | 36.7 ± 1.1 b | 35.6 ± 0.8 ab | 36.5 ± 0.7 ab |
| 50–40 ppm | 30–24 ppm | 24–15 ppm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outer | Center | Inner | Outer | Center | Inner | Outer | Center | Inner | |
| St0 | 1057 | 1086 | 994 | 1038 | 1065 | 1031 | 1021 | 1066 | 1060 |
| St1 | 1029 | 1048 | 1021 * ± 35 | 995 | 1019 | 1027 * ± 23 | 985 | 1007 | 1010 * ± 19 |
| Mt1 | 1057 | 996 | 1010 | 1017 | 987 | 996 | 945 | 984 | 1008 |
| St6 | 910 * ± 12 | 961 * ± 31 | 955 * ± 26 | 969 * ± 1 | 970 * ± 16 | 933 * ± 37 | 948 * ± 4 | 984 * ± 29 | 931 * ± 24 |
| Mt6 | 1027 * ± 8 | 960 | 959 | 994 * ± 36 | 943 | 950 | 1017 ± 32 | 920 | 949 |
| Csample Loss (mg) | Csample Loss (% of Ctsample) | Cmask Loss (mg) | Cmask Loss (% of Ctmask) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time (Days) | 7 1 | 31 1 | 178 2 | 7 | 31 | 178 | 7 | 31 | 178 | 7 | 31 | 178 |
| Soil (S) | 17.0 ± 1.3 a | 46.6 ± 1.8 a | 119.4 ± 1.6 a | 1.8 | 4.9 | 12.6 | ||||||
| S/outer mask | 25.4 ± 1.9 b | 58.1 ± 3.0 b | 132.9 ± 9.1 b | 2.2 | 5.0 | 11.4 | 8.4 | 11.4 | 13.6 | 3.9 | 5.3 | 6.3 |
| S/center mask | 25.4 ± 1.5 bc | 57.9 ± 2.6 b | 141.8 ± 5.1 b | 2.2 | 5.0 | 12.2 | 8.4 | 11.3 | 22.4 | 3.9 | 5.2 | 10.4 |
| S/inner mask | 26.4 ± 1.4 b | 57.5 ± 2.5 b | 133.4 ± 6.2 b | 2.3 | 5.0 | 11.5 | 9.5 | 10.9 | 14.0 | 4.4 | 5.1 | 6.5 |
| S/complete mask | 22.5 ± 3.6 c | 52.7 ± 6.3 b | 132.5 ± 3.0 b | 1.9 | 4.5 | 11.4 | 5.5 | 6.1 | 13.1 | 2.6 | 2.8 | 6.1 |
| Afast | kfast | MRTfast | Aslow | kslow | MRTslow | R2 | ||
|---|---|---|---|---|---|---|---|---|
| % | Days−1 | Years | Days | % | Days−1 | Years | ||
| soil | 5.2 | 0.02 | 0.114 | 41.7 | 94.4 | 0.00043 | 6 | 0.999 |
| outer mask | 4.4 | 0.40 | 0.007 | 2.5 | 95.1 | 0.00014 | 19 | 0.693 |
| center mask | 3.8 | 0.34 | 0.008 | 3.0 | 95.7 | 0.00040 | 7 | 0.981 |
| inner mask | 4.7 | 0.38 | 0.008 | 2.8 | 94.8 | 0.00010 | 28 | 0.952 |
| complete mask | 4.0 | 0.14 | 0.019 | 6.9 | 95.1 | 0.00013 | 21 | 0.946 |
| 50–40 ppm | 30–24 ppm | 24–15 ppm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outer | Center | Inner | Outer | Center | Inner | Outer | Center | Inner | |
| St0 | 32.6 | 32.6 | 32.6 | 32.8 | 32.8 | 32.8 | 34.6 | 34.7 | 34.6 |
| St1 | 30.1 | 29.5 | 29.6 | 30.3 | 29.9 | 30.0 | 34.7 | 35.6 | 35.4 |
| Mt1 | 30.5 | 30.8 | 30.7 | 30.9 | 31.2 | 30.9 | 35.7 | 35.0 | 35.4 |
| St6 | 29.0 | 25.7 | 28.7 | 30.9 | 28.5 | 30.5 | 34.1 | 34.8 | 34.8 |
| Mt6 | 28.7 | 29.6 | 28.9 | 30.8 | 30.9 | 30.8 | 34.5 | 33.5 | 34.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knicker, H.; Velasco-Molina, M. Biodegradability of Disposable Surgical Face Masks Littered into Soil Systems during the COVID 19 Pandemic—A First Approach Using Microcosms. Soil Syst. 2022, 6, 39. https://doi.org/10.3390/soilsystems6020039
Knicker H, Velasco-Molina M. Biodegradability of Disposable Surgical Face Masks Littered into Soil Systems during the COVID 19 Pandemic—A First Approach Using Microcosms. Soil Systems. 2022; 6(2):39. https://doi.org/10.3390/soilsystems6020039
Chicago/Turabian StyleKnicker, Heike, and Marta Velasco-Molina. 2022. "Biodegradability of Disposable Surgical Face Masks Littered into Soil Systems during the COVID 19 Pandemic—A First Approach Using Microcosms" Soil Systems 6, no. 2: 39. https://doi.org/10.3390/soilsystems6020039
APA StyleKnicker, H., & Velasco-Molina, M. (2022). Biodegradability of Disposable Surgical Face Masks Littered into Soil Systems during the COVID 19 Pandemic—A First Approach Using Microcosms. Soil Systems, 6(2), 39. https://doi.org/10.3390/soilsystems6020039