Long-Term Biosolids Application on Land: Beneficial Recycling of Nutrients or Eutrophication of Agroecosystems?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Soils from Farm Fields with and without Long-Term Biosolids Application
2.2. Radish Bioassay
3. Results
3.1. Biosolids Composition
3.2. Soil Composition
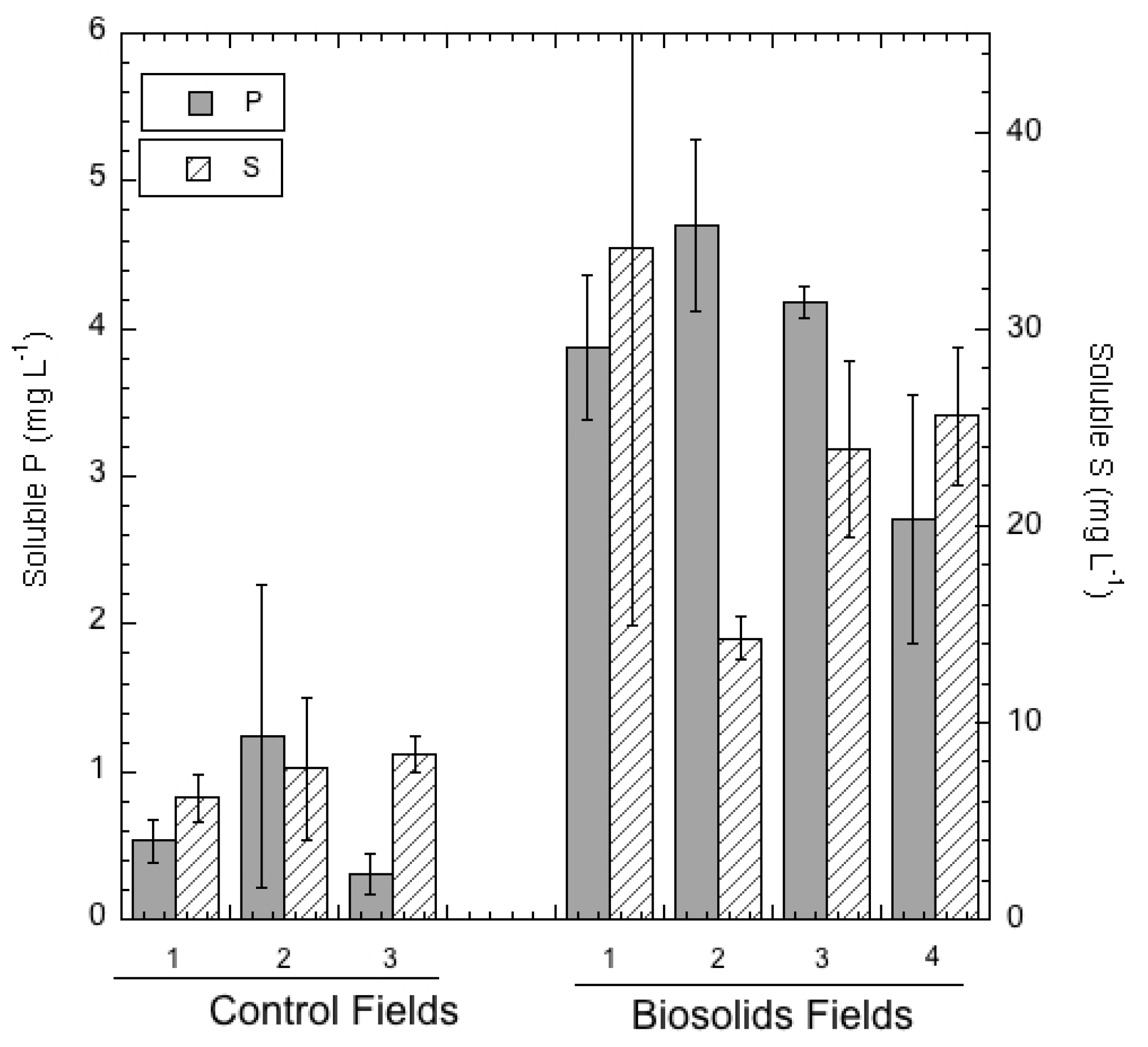
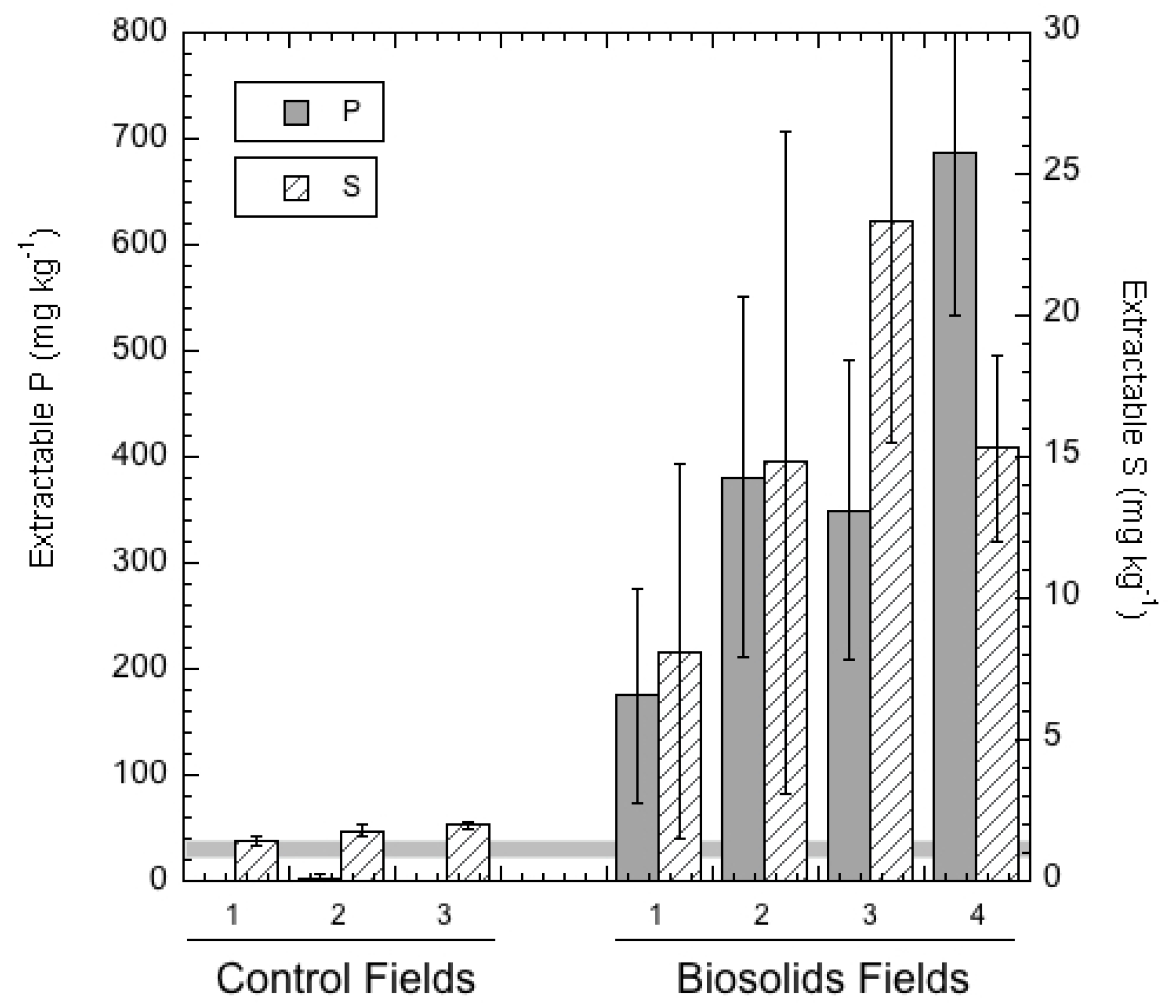
3.3. Extractable and Leachable Soil Elements
3.4. Radish Assay
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USEPA. Biosolids Recycling: Beneficial Technology for a Better Environment; Publication 832-R-94-009; United States Environmental Protection Agency, Office of Water: Washington, DC, USA, 1994.
- Sumner, M.E. Beneficial use of effluents, wastes, and biosolids. Commun. Soil Sci. Plant Anal. 2008, 31, 1701–1715. [Google Scholar] [CrossRef]
- Basta, N.T.; WRRaun, W.R.; Johnson, G.V.; Lusby, K.S.; Turton, D.J.; Smolen, M.D.; Gillen, R.L.; Sloan, J.J.; Allen, E.R. Land Application of Biosolids: A Review of Research Concerning Benefits, Environmental Impacts, and Regulations of Applying Treated Sewage Sludge. Extension Publication B-808; Oklahoma State University: Stillwater, OK, USA, 2017. [Google Scholar]
- Brown, S.; Kurtz, K.; Bary, A.; Cogger, C. Quantifying Benefits Associated with Land Application of Organic Residuals in Washington State. Environ. Sci. Technol. 2011, 45, 7451–7458. [Google Scholar] [CrossRef]
- Harrison, E.Z.; Oakes, S.R.; Hysell, M.; Hay, A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006, 367, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, C.A.; Furlong, E.T.; Zaugg, S.D.; Burkhardt, M.R.; Werner, S.L.; Cahill, J.D.; Jorgensen, G.R. Survey of Organic Wastewater Contaminants in Biosolids Destined for Land Application. Environ. Sci. Technol. 2006, 40, 7207–7215. [Google Scholar] [CrossRef] [PubMed]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- McBride, M.B. Toxic metal accumulation from agricultural use of sludge: Are USEPA regulations protective? J. Environ. Qual. 1995, 24, 5–18. [Google Scholar] [CrossRef]
- Smith, S.R.; Cockayne, D.; Kirkland, A.I.; Nellist, P.D.; Bleloch, A. Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2009, 367, 4005–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, E.Z.; McBride, M.B.; Bouldin, D.R. Land application of sewage sludges: An appraisal of the US regulations. Int. J. Environ. Pollut. 1999, 11, 1–36. [Google Scholar] [CrossRef]
- USEPA. EPA Unable to Assess the Impact of Hundreds of Unregulated Pollutants in Land-Applied Biosolids on Human Health and the Environment; Report No. 19-P-0002; Office of Inspector General, USEPA: Washington, DC, USA, 2018.
- Stehouwer, R.C.; Wolf, A.M.; Doty, W.T. Chemical Monitoring of Sewage Sludge in Pennsylvania: Variability and Application Uncertainty. J. Environ. Qual. 2000, 29, 1686–1695. [Google Scholar] [CrossRef]
- Bashar, R.; Gungor, K.; Karthikeyan, K.; Barak, P. Cost effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Stoffella, P.J. Land Application of Biosolids in the USA: A Review. Appl. Environ. Soil Sci. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shober, A.L.; Sims, J.T. Phosphorus Restrictions for Land Application of Biosolids. J. Environ. Qual. 2003, 32, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective. BioScience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Kidd, P.; Domínguez-Rodríguez, M.; Díez, J.; Monterroso, C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 2007, 66, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J. Land application of sewage sludge: Phosphorus considerations. S. Afr. J. Plant Soil 1984, 1, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Doydora, S.; Gatiboni, L.; Grieger, K.; Hesterberg, D.; Jones, J.L.; McLamore, E.S.; Peters, R.; Sozzani, R.; Broeck, L.V.D.; Duckworth, O.W. Accessing Legacy Phosphorus in Soils. Soil Syst. 2020, 4, 74. [Google Scholar] [CrossRef]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef] [Green Version]
- Provin, T.L.; Pitt, J.L. Phosphorus-Too Much and Plants May Suffer; Publication E-465, 5-08; Texas A&M University, Agrilife Extension Service: College Station, TX, USA, 2008. [Google Scholar]
- Teng, Y.; Timmer, V.R. Phosphorus-induced micronutrient disorders in hybrid poplar. Plant Soil 1990, 126, 19–29. [Google Scholar] [CrossRef]
- Pedas, P.; Husted, S.; Skytte, K.; Schjoerring, J.K. Elevated Phosphorus Impedes Manganese Acquisition by Barley Plants. Front. Plant Sci. 2011, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Silber, A.; Ben-Jaacov, J.; Ackerman, A.; Bar-Tal, A.; Levkovitch, I.; Matsevitz-Yosef, T.; Swartzberg, D.; Riov, J.; Granot, D. Interrelationship between phosphorus toxicity and sugar metabolism in Verticordia plumosa L. Plant Soil 2002, 245, 249–260. [Google Scholar] [CrossRef]
- Singh, J.P.; Karamanos, R.E.; Stewart, J.W.B. THE MECHANISM OF PHOSPHORUS-INDUCED ZINC DEFICIENCY IN BEAN (Phaseolus vulgaris L.). Can. J. Soil Sci. 1988, 68, 345–358. [Google Scholar] [CrossRef]
- Parker, D.R. Responses of Six Crop Species to Solution Zinc2+ Activities Buffered with HEDTA. Soil Sci. Soc. Am. J. 1997, 61, 167–176. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants Is mail. Physiol. Plant. 1987, 70, 13–20. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Wong, J.W.; Fang, M.; Jiang, R. Persistency of bacterial indicators in biosolids stabilization with coal fly ash and lime. Water Environ. Res. 2001, 73, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Bean, C.L.; Hansen, J.J.; Margolin, A.B.; Balkin, H.; Batzer, G.; Widmer, G. Class B alkaline stabilization to achieve pathogen inactivation. Int. J. Environ. Res. Public Health 2007, 4, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewil, R.; Baeyens, J.; Roels, J.; Van De Steene, B. Distribution of Sulphur Compounds in Sewage Sludge Treatment. Environ. Eng. Sci. 2008, 25, 879–886. [Google Scholar] [CrossRef]
- Wolf, A.; Beegle, D. Recommended Soil Tests for macro and micronutrients. Chapter 5. In Recommended Soil Testing Procedures for the Northeastern United States; Northeastern Regional Publication No. 493; Agricultural Experiment Stations of Northeastern USA: Burlington, VT, USA, 2011. [Google Scholar]
- Jokela, W.E.; Magdoff, F.R.; Durieux, R.P. Improved phosphorus recommendations using modified Morgan phosphorus and aluminum soil tests. Commun. Soil Sci. Plant Anal. 1998, 29, 1739–1749. [Google Scholar] [CrossRef]
- McBride, M.B.; Pitiranggon, M.; Kim, B. A comparison of TESTS for extractable copper and zinc in metal-spiked and field-contaminated soil. Soil Sci. 2009, 174, 439–444. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Czymmek, K.J.; Reid, W.S.; Wildman, R.F. Conversion of modified morgan and mehlich-III soil tests to morgan soil test values. Soil Sci. 2002, 167, 830–837. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Tiberg, C.; Edkymish, A.; Kleja, D.B. Modelling lead(II) sorption to ferrihydrite and soil organic matter. Environ. Chem. 2011, 8, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Landrot, G.; Khaokaew, S. Lead Speciation and Association with Organic Matter in Various Particle-Size Fractions of Contaminated Soils. Environ. Sci. Technol. 2018, 52, 6780–6788. [Google Scholar] [CrossRef]
- Ketterings, Q.; Czymmek, K.; Albrecht, G.; Barney, P. Starter Phosphorus Fertilizer for Corn; Agronomy Fact Sheet #8, Cornell University Cooperative Extension Publication; Cornell University: Ithaca, NY, USA, 2014. [Google Scholar]
- McBride, M.B.; Sauve, S.; Hendershot, W.H. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- McBride, M.B. Molybdenum and Copper Uptake by Forage Grasses and Legumes Grown on a Metal-Contaminated Sludge Site. Commun. Soil Sci. Plant Anal. 2005, 36, 2489–2501. [Google Scholar] [CrossRef]
- McBride, M.B.; Cherney, J. Molybdenum, Sulfur, and Other Trace Elements in Farm Soils and Forages After Sewage Sludge Application. Commun. Soil Sci. Plant Anal. 2004, 35, 517–535. [Google Scholar] [CrossRef]
- McBride, M.; Hale, B. Molybdenum extractability in soils and uptake by alfalfa 20 years after sewage sludge application. Soil Sci. 2004, 169, 505–514. [Google Scholar] [CrossRef]
- Tiffany, M.E.; McDowell, L.R.; O’Connor, G.A.; Martin, F.G.; Wilkinson, N.S.; Percival, S.S.; Rabiansky, P.A. Effects of residual and reapplied biosolids on performance and mineral status of grazing beef steers. J. Anim. Sci. 2002, 80, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.T. Fertilizers and the environment. Nutr. Cycl. Agroecosyst. 1999, 55, 117–121. [Google Scholar] [CrossRef]
- Pöyry, J.; Carvalheiro, L.G.; Heikkinen, R.K.; Kühn, I.; Kuussaari, M.; Schweiger, O.; Valtonen, A.; van Bodegom, P.; Franzén, M. The effects of soil eutrophication propagate to higher trophic levels. Glob. Ecol. Biogeogr. 2016, 26, 18–30. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Phosphorus toxicity in tomato plants: When and how does it occur? Commun. Soil Sci. Plant Anal. 1998, 29, 1779–1784. [Google Scholar] [CrossRef]
- Lim, M.P.; McBride, M.B. Arsenic and lead uptake by Brassicas grown on an old orchard site. J. Hazard. Mater. 2015, 299, 656–663. [Google Scholar] [CrossRef]
- McBride, M.; Richards, B.; Steenhuis, T. Bioavailability and crop uptake of trace elements in soil columns amended with sewage sludge products. Plant Soil 2004, 262, 71–84. [Google Scholar] [CrossRef]
- Gooneratne, S.R.; Buckley, W.T.; Christensen, D.A. Review of copper deficiency and metabolism in ruminants. Can. J. Anim. Sci. 1989, 69, 819–845. [Google Scholar] [CrossRef]
- McBride, M.B.; Richards, B.K.; Steenhuis, T.; Spiers, G. Molybdenum Uptake by Forage Crops Grown on Sewage Sludge-Amended Soils in the Field and Greenhouse. J. Environ. Qual. 2000, 29, 848–854. [Google Scholar] [CrossRef]
- Tiffany, M.E.; McDowell, L.R.; O’Connor, G.A.; Nguyen, H.; Martin, F.G.; Wilkinson, N.S.; Cardoso, E.C. Effects of pasture-applied biosolids on forage and soil concentrations over a grazing season in North Florida. II. Microminerals. Commun. Soil Sci. Plant Anal. 2000, 31, 215–227. [Google Scholar] [CrossRef]
- Villa, Y.B.; Ryals, R. Soil carbon response to long-term biosolids application. J. Environ. Qual. 2021, 50, 1084–1096. [Google Scholar] [CrossRef]
- Tian, G.; Granato, T.C.; Cox, A.E.; Pietz, R.I.; Carlson, C.R., Jr.; Abedin, Z. Soil Carbon Sequestration Resulting from Long-Term Application of Biosolids for Land Reclamation. J. Environ. Qual. 2009, 38, 61–74. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.M.B.; Sui, X.; Sollenberger, L.E.; O’Connor, G.A. Use of Warm-Season Grasses Managed as Bioenergy Crops for Phytoremediation of Excess Soil Phosphorus. Agron. J. 2013, 105, 95–100. [Google Scholar] [CrossRef]
- Shober, A.L.; Stehouwer, R.C.; MacNeal, K.E. On-Farm Assessment of Biosolids Effects on Soil and Crop Tissue Quality. J. Environ. Qual. 2003, 32, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Jian, J.; Gyawali, A.J.; Thomason, W.E.; Badgley, B.D.; Reiter, M.S.; Strickland, M.S. What We Talk about When We Talk about Soil Health. Agric. Environ. Lett. 2018, 3, 180033. [Google Scholar] [CrossRef] [Green Version]
- Norris, C.E.; Mac Bean, G.; Cappellazzi, S.B.; Cope, M.; Greub, K.L.; Liptzin, D.; Rieke, E.L.; Tracy, P.W.; Morgan, C.L.; Honeycutt, C.W. Introducing the North American project to evaluate soil health measurements. Agron. J. 2020, 112, 3195–3215. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Johnson, M.S.; Leeper, G.W. Managing the Heavy Metals on the Land. J. Appl. Ecol. 1979, 16, 336. [Google Scholar] [CrossRef]
- Trocme, S.; Barbier, G.; Chabannes, J. Chlorosis, caused by lack of manganese, of crops irrigated with filtered water from Paris sewers. Ann. Agron. 1950, 1, 663–685. [Google Scholar]
- Shukla, D.; Rinehart, C.A.; Sahi, S.V. Comprehensive study of excess phosphate response reveals ethylene mediated signaling that negatively regulates plant growth and development. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves. Plant Cell Environ. 2020, 43, 2033–2053. [Google Scholar] [CrossRef]
- Marolt, G.; Gričar, E.; Pihlar, B.; Kolar, M. Complex Formation of Phytic Acid with Selected Monovalent and Divalent Metals. Front. Chem. 2020, 8, 582746. [Google Scholar] [CrossRef]
- Frossard, E.; Bucher, M.; Machler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Kaminsky, L.M.; Thompson, G.L.; Trexler, R.V.; Bell, T.H.; Kao-Kniffin, J. Medicago sativa has Reduced Biomass and Nodulation When Grown with Soil Microbiomes Conditioned to High Phosphorus Inputs. Phytobiomes J. 2018, 2, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Erich, M.S. Inhibitory Effects of Crop Residue-Derived Organic Ligands on Phosphate Adsorption Kinetics. J. Environ. Qual. 1997, 26, 889–895. [Google Scholar] [CrossRef]
| Element | 2016 Sample | 2019 Sample |
|---|---|---|
| Ba | 374 | 348 |
| Ca | 70,100 | 45,100 |
| Cd | 1.3 | 1.3 |
| Cr | 57.7 | 80.7 |
| Cu | 160 | 143 |
| Fe | 21,700 | 11,400 |
| K | 8910 | 20,930 |
| Mg | 2840 | 2130 |
| Mn | 160 | 325 |
| Mo | 9.40 | 5.40 |
| Ni | 24.5 | 31.5 |
| P | 8990 | 7680 |
| Pb | 25.7 | 35.3 |
| S | 9730 | 7490 |
| Zn | 1030 | 597 |
| Field Soil | Carbon (%) | Nitrogen (%) | pH | Total P | Total K | Total S | Total Fe | Total Ca | Total Mg |
|---|---|---|---|---|---|---|---|---|---|
| mg g−1 | |||||||||
| Control Soils | |||||||||
| Control A | 0.75 ± 0.13 | 0.07 ± 0.01 | 6.08 ± 0.07 | 0.110 ± 0.011 | 0.54 ± 0.046 | 0.196 ± 0.030 | 3.55 ± 0.57 | 0.83 ± 0.08 | 0.51 ± 0.08 |
| Control B | 1.11 ± 0.65 | 0.11 ± 0.05 | 5.52 ± 0.55 | 0.174 ± 0.059 | 1.05 ± 0.62 | 0.091 ± 0.046 | 7.0 ± 3.0 | 1.28 ± 1.20 | 1.58 ± 1.29 |
| Control C | 0.73 ± 0.11 | 0.077± 0.011 | 4.83 ± 0.09 | 0.180 ± 0.022 | 0.69 ± 0.087 | 0.074 ± 0.006 | 5.13 ± 1.12 | 0.91 ± 0.09 | 1.65 ± 0.30 |
| Biosolids-Amended Soils | |||||||||
| Biosolid A | 1.71 ± 0.29 | 0.18 ± 0.04 | 6.88 ± 0.09 | 0.837 ± 0.655 | 2.02 ± 0.24 | 0.363 ± 0.101 | 8.67 ± 3.64 | 5.06 ± 1.48 | 3.39 ± 0.50 |
| Biosolid B | 1.57 ± 0.38 | 0.16 ± 0.04 | 6.88 ± 0.17 | 1.46 ± 0.56 | 0.95 ± 0.43 | 0.340 ± 0.051 | 9.18 ± 1.69 | 5.75 ± 2.51 | 0.99 ± 0.27 |
| BiosolidC | 3.00 ± 0.13 | 0.31 ± 0.01 | 6.08 ± 0.23 | 2.31 ± 0.55 | 3.39 ± 0.66 | 0.675 ± 0.159 | 18.9 ± 3.4 | 15.8 ± 3.8 | 5.96 ± 1.52 |
| Biosolid D | 3.67 ± 0.76 | 0.36 ± 0.06 | 6.52 ± 0.16 | 2.38 ± 0.05 | 1.45 ± 0.14 | 0.504 ± 0.005 | 10.9 ± 0.68 | 9.89 ± 0.53 | 2.56 ± 0.16 |
| Soil | Cr | Cu | Mn | Ni | Pb | Sr | Zn |
|---|---|---|---|---|---|---|---|
| Control Soils | |||||||
| Control A | 6.6 ± 0.4 | 3.3 ± 0.2 | 134 ± 35 | 5.3 ± 0.9 | 12.1 ± 1.1 | 4.9 ± 0.9 | 16.0 ± 1.3 |
| Control B | 11.5 ± 2.5 | 8.2 ± 3.3 | 178 ± 55 | 12.2 ± 10.5 | 9.5 ± 3.5 | 7.0 ± 3.9 | 16.1 ± 7.2 |
| Control C | 6.6 ± 0.9 | 3.5 ± 0.8 | 92 ± 11 | 5.3 ± 1.0 | 7.0 ± 1.1 | 7.1 ± 0.9 | 14.9 ± 2.0 |
| Biosolids-Amended Soils | |||||||
| Biosolid A | 15.8 ± 4.3 | 18.2 ± 7.3 | 261 ± 36 | 13.1 ± 2.1 | 16.7 ± 4.4 | 27.9 ± 7.2 | 71.1 ± 20.3 |
| Biosolid B | 15.9 ± 2.8 | 28.0 ± 10.2 | 85.1 ± 4.7 | 11.5 ± 2.2 | 23.3 ± 3.0 | 25.0 ± 8.6 | 87.9 ± 31.7 |
| Biosolid C | 22.9 ± 3.6 | 30.7 ± 7.5 | 414 ± 54 | 17.9 ± 1.1 | 22.3 ± 2.4 | ND * | 166 ± 47 |
| Biosolid D | 17.8 ± 1.0 | 35.4 ± 0.8 | 189 ± 4 | 9.3 ± 0.5 | 20.5 ± 1.7 | ND * | 150 ± 3.8 |
| Soil | Ca | Cd | Cu | Zn | Fe | Mn | P | S |
|---|---|---|---|---|---|---|---|---|
| Control Soils | ||||||||
| Control A | 279 ± 55 | 0.017 ± 0.006 | 0.03 ± 0.03 | 0.63 ± 0.10 | 0.18 ± 0.06 | 6.98 ± 2.00 | 0.81 ± 0.14 | 1.43 ± 0.17 |
| Control B | 845 ± 994 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.11 ± 0.09 | 0.25 ± 0.19 | 5.00 ± 2.75 | 2.94 ± 3.38 | 1.79 ± 0.22 |
| Control C | 260 ± 28 | 0.017 ± 0.003 | 0.05 ± 0.05 | 0.12 ± 0.05 | 2.9 ± 1.2 | 2.33 ± 1.24 | 0.74 ± 0.33 | 1.98 ± 0.11 |
| Biosolids-Amended Soils | ||||||||
| Biosolid A | 2380 ± 904 | 0.03 ± 0.00 | 0.073 ± 0.035 | 2.15 ± 1.35 | 0.53 ± 0.06 | 5.47 ± 0.35 | 175 ± 101 | 8.12 ± 6.61 |
| Biosolid B | 3700 ± 2150 | 0.043 ± 0.015 | 0.46 ± 0.20 | 9.37 ± 5.29 | 1.62 ± 0.41 | 3.01 ± 0.67 | 381 ± 170 | 14.8 ± 11.7 |
| Biosolid C | 7790 ± 2740 | 0.045 ± 0.010 | 0.20 ± 0.07 | 7.30 ± 3.74 | 0.77 ± 0.37 | 6.36 ± 4.50 | 350 ± 142 | 23.3 ± 7.76 |
| Biosolid D | 4430 ± 871 | 0.077 ± 0.003 | 0.41 ± 0.01 | 12.3 ± 1.4 | 1.48 ± 0.18 | 7.23 ± 1.60 | 687 ± 154 | 15.3 ± 3.26 |
| Soil | Ca | Cu | Zn | Mo | P | S |
|---|---|---|---|---|---|---|
| Control Soils | ||||||
| Control A | 46.5 ± 16.8 | 0.04 ± 0.03 | 0.093 ± 0.076 | 0.00 ± 0.00 | 0.53 ± 0.14 | 6.16 ± 1.24 |
| Control B | 91.6 ± 34.3 | 0.03 ± 0.006 | 0.077 ± 0.047 | 0.00 ± 0.00 | 1.24 ± 1.02 | 7.65 ± 3.65 |
| Control C | 21.1 ± 4.3 | 0.02 ± 0.006 | 0.057 ± 0.015 | 0.00 ± 0.00 | 0.31 ± 0.14 | 8.37 ± 0.92 |
| Biosolids-Amended Soils | ||||||
| Biosolid A | 188 ± 110 | 0.06 ± 0.01 | 0.043 ± 0.006 | 0.05 ± 0.03 | 3.87 ± 0.49 | 34.1 ± 19.2 |
| Biosolid B | 133 ± 20.5 | 0.05 ± 0.006 | 0.040 ± 0.026 | 0.01 ± 0.006 | 4.70 ± 0.58 | 14.3 ± 1.1 |
| Biosolid C | 224 ± 23.2 | 0.04 ± 0.02 | 0.030 ± 0.017 | 0.06 ± 0.01 | 2.71 ± 0.84 | 25.6 ± 3.5 |
| Biosolid D | 204 ± 18.5 | 0.05 ± 0.006 | 0.043± 0.006 | 0.07 ± 0.03 | 4.18 ± 0.11 | 23.9 ± 4.5 |
| Soil | Ca (%) | Mn (mg kg−1) | Mo (mg kg−1) | P (%) | S (%) | Zn (mg kg−1) |
|---|---|---|---|---|---|---|
| Control Soils | ||||||
| Control A | 1.26 ± 0.15 | 33.3 ± 4.3 | 0.37 ± 0.21 | 0.074 ± 0.017 | 0.119 ± 0.007 | 17.2 ± 3.9 |
| Control B | 0.82 ± 0.27 | 65.2 ± 6.4 | 0.17 ± 0.24 | 0.127 ± 0.006 | 0.136 ± 0.004 | 9.35 ± 2.19 |
| Control C | 0.82 ± 0.048 | 41.5 ± 9.6 | 0.22 ± 0.19 | 0.058 ± 0.015 | 0.146 ± 0.054 | 11.3 ± 4.4 |
| Biosolids-Amended Soils | ||||||
| Biosolid A | 1.23 ± 0.37 | 19.5 ± 5.5 | 3.80 ± 2.28 | 0.199 ± 0.024 | 0.273 ± 0.022 | 20.1 ± 5.4 |
| Biosolid B | 1.32 ± 0.097 | 6.53 ± 1.1 | 1.07 ± 0.44 | 0.206 ± 0.006 | 0.238 ± 0.005 | 17.2 ± 1.1 |
| Biosolid C | 1.61 ± 0.43 | 14.1 ± 4.1 | 3.61± 1.01 | 0.363 ± 0.039 | 0.198 ± 0.036 | 29.9 ± 7.9 |
| Biosolid D | 1.50 ± 0.33 | 5.15 ± 0.66 | 5.47 ± 1.97 | 0.364 ± 0.095 | 0.137 ± 0.048 | 25.0 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McBride, M.B. Long-Term Biosolids Application on Land: Beneficial Recycling of Nutrients or Eutrophication of Agroecosystems? Soil Syst. 2022, 6, 9. https://doi.org/10.3390/soilsystems6010009
McBride MB. Long-Term Biosolids Application on Land: Beneficial Recycling of Nutrients or Eutrophication of Agroecosystems? Soil Systems. 2022; 6(1):9. https://doi.org/10.3390/soilsystems6010009
Chicago/Turabian StyleMcBride, Murray B. 2022. "Long-Term Biosolids Application on Land: Beneficial Recycling of Nutrients or Eutrophication of Agroecosystems?" Soil Systems 6, no. 1: 9. https://doi.org/10.3390/soilsystems6010009





