Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems
Abstract
1. Introduction
2. Materials and Methods
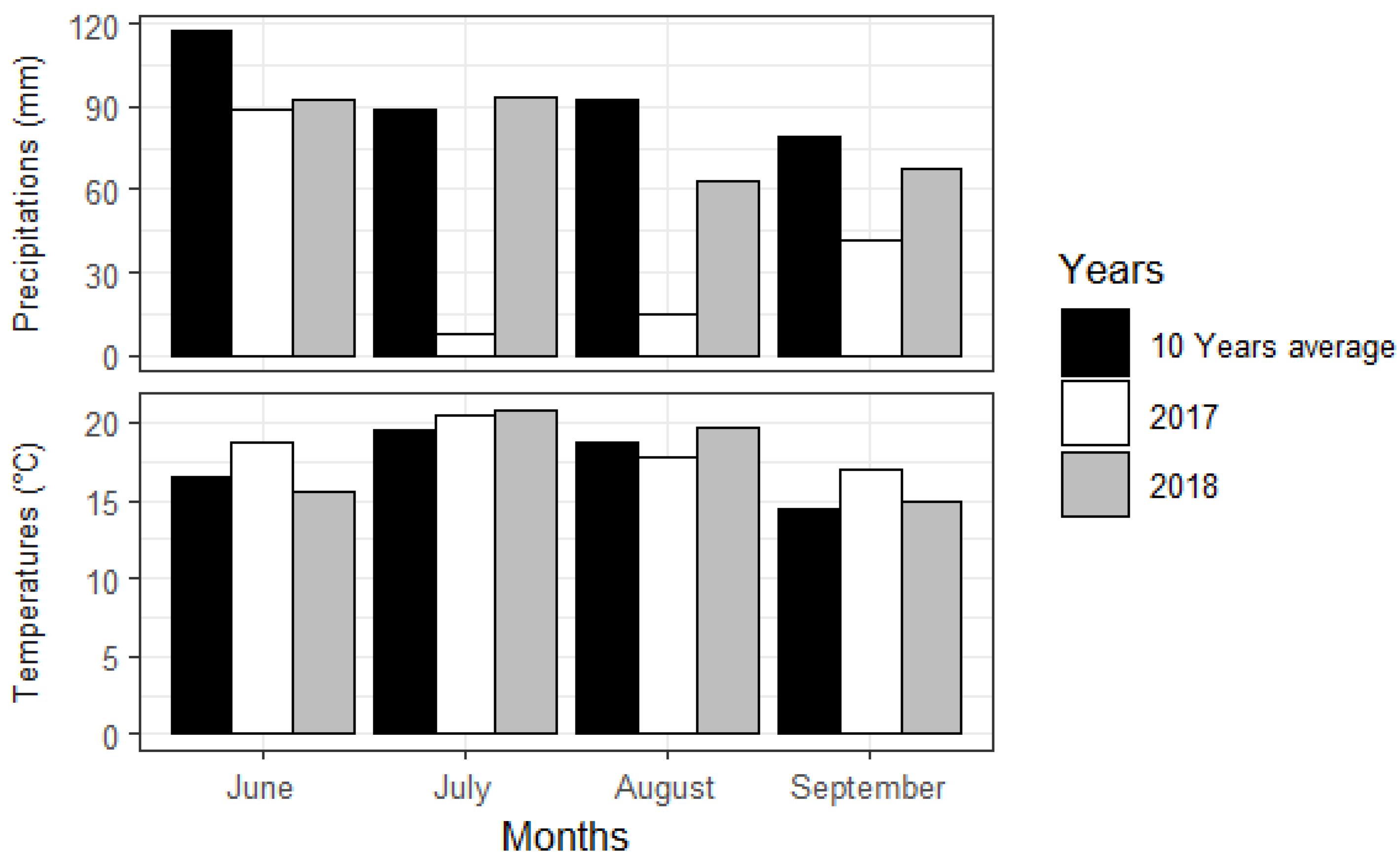
2.1. Study Area
2.2. Soil Analysis
2.3. Litter Bags
2.4. Experimental Designs
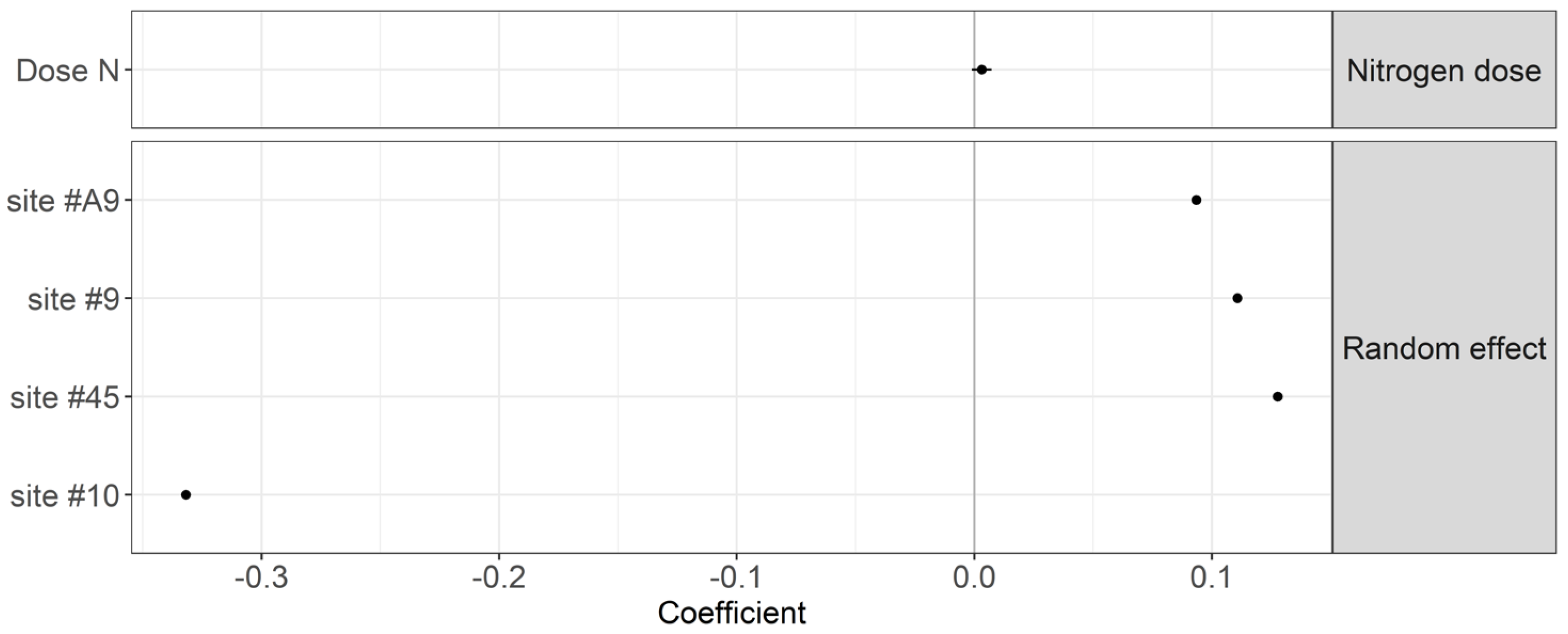
2.4.1. Year 1 Experiment to Test TBI Stability across Fertilization Regimes
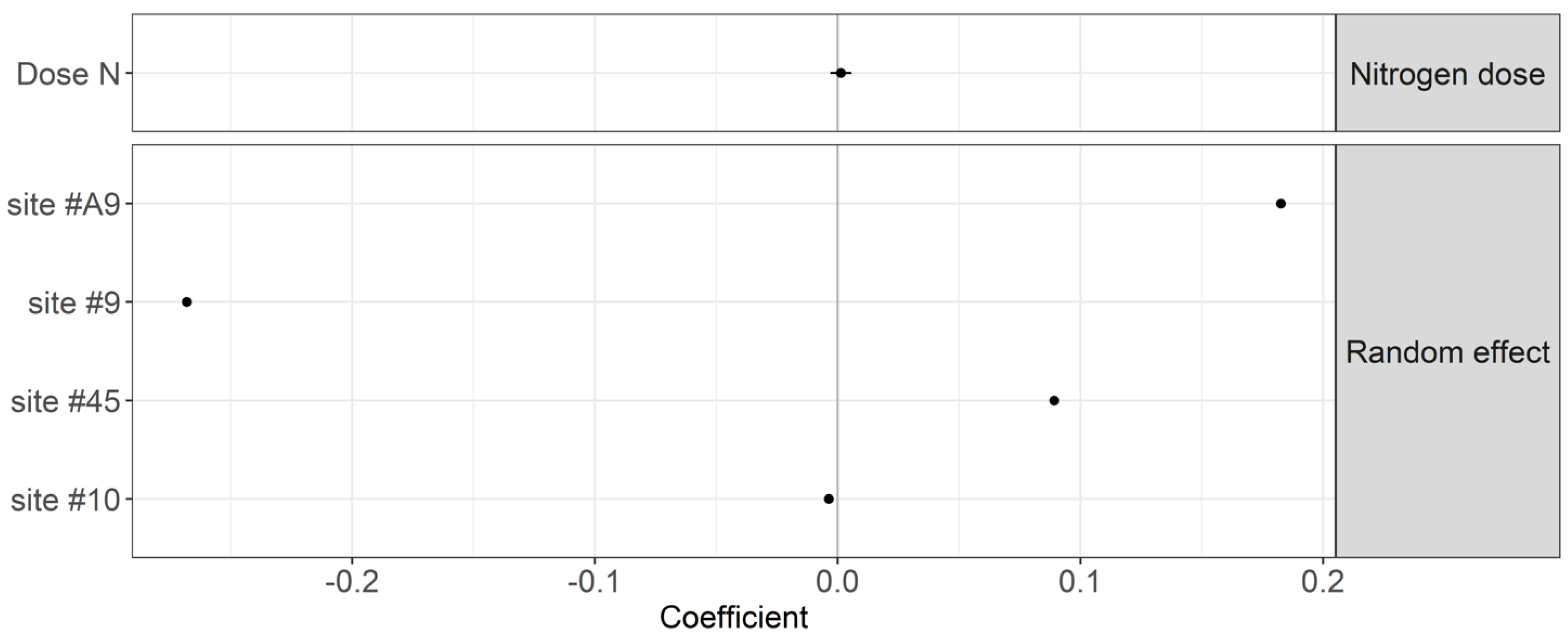
2.4.2. Year 2 Experiment to Validate TBI across Years and Test Fractal Behavior
2.4.3. Reaction Kinetics
2.4.4. TBI
2.4.5. Statistical Analysis
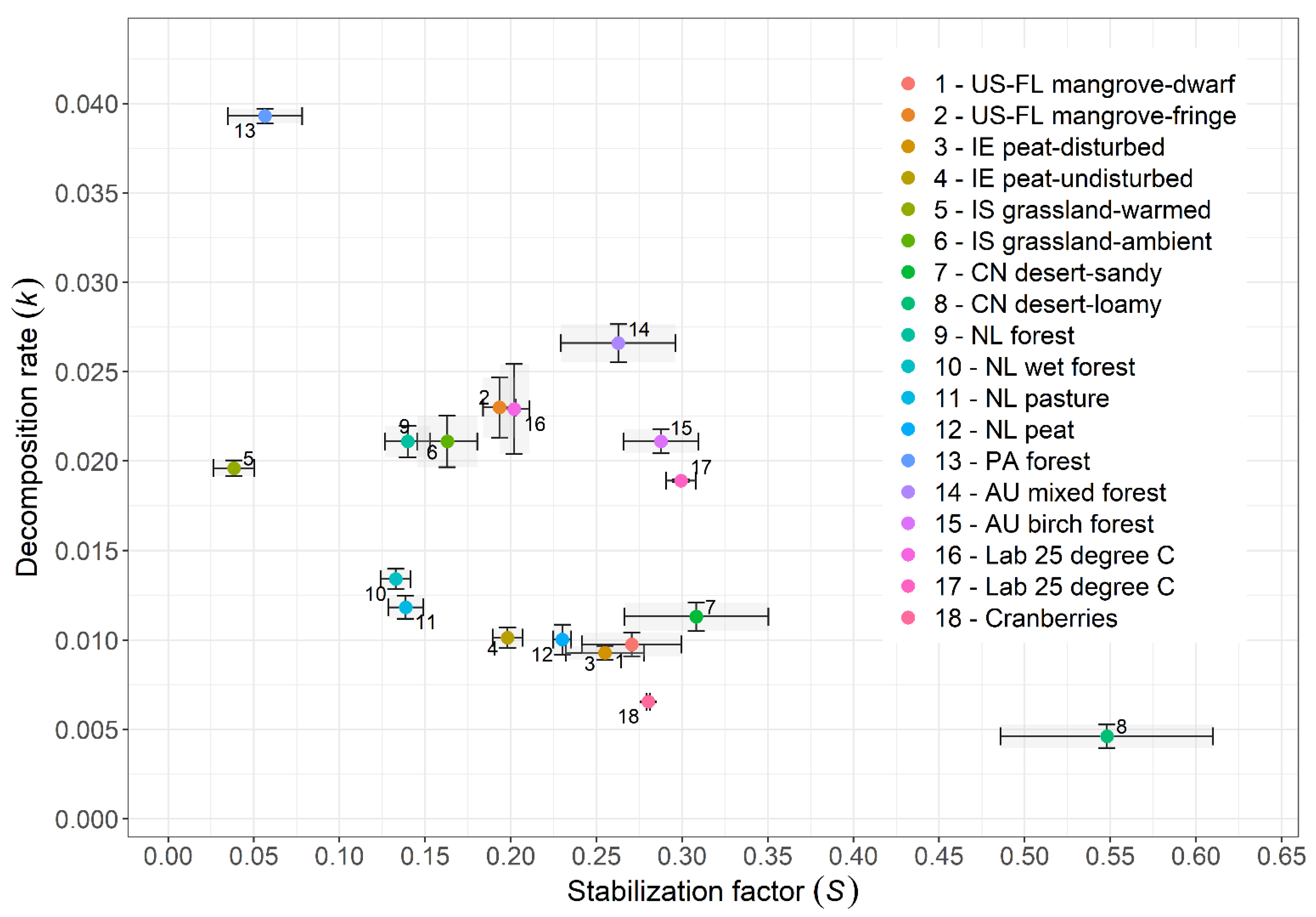
3. Results
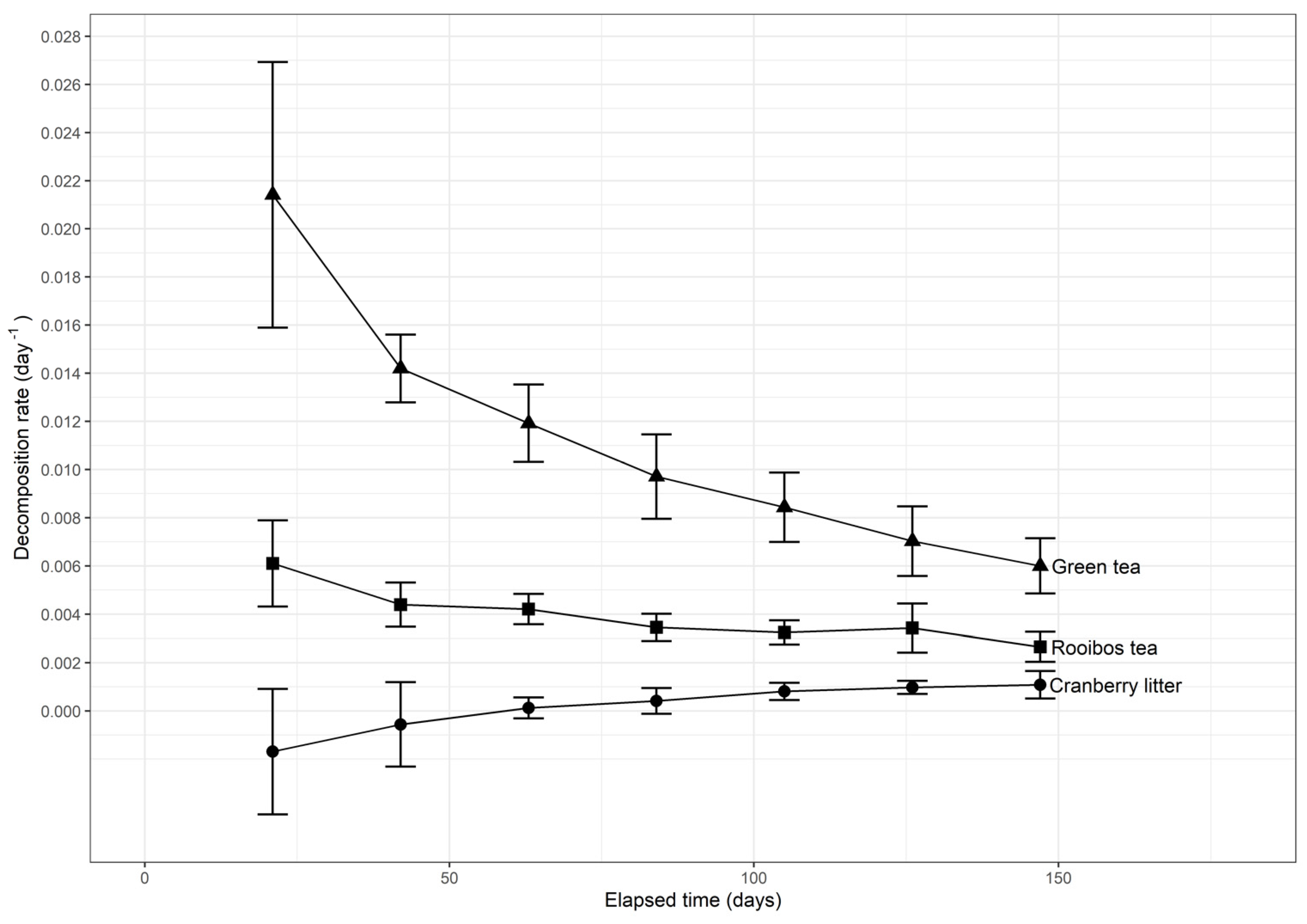
3.1. Organic Matter Decomposition
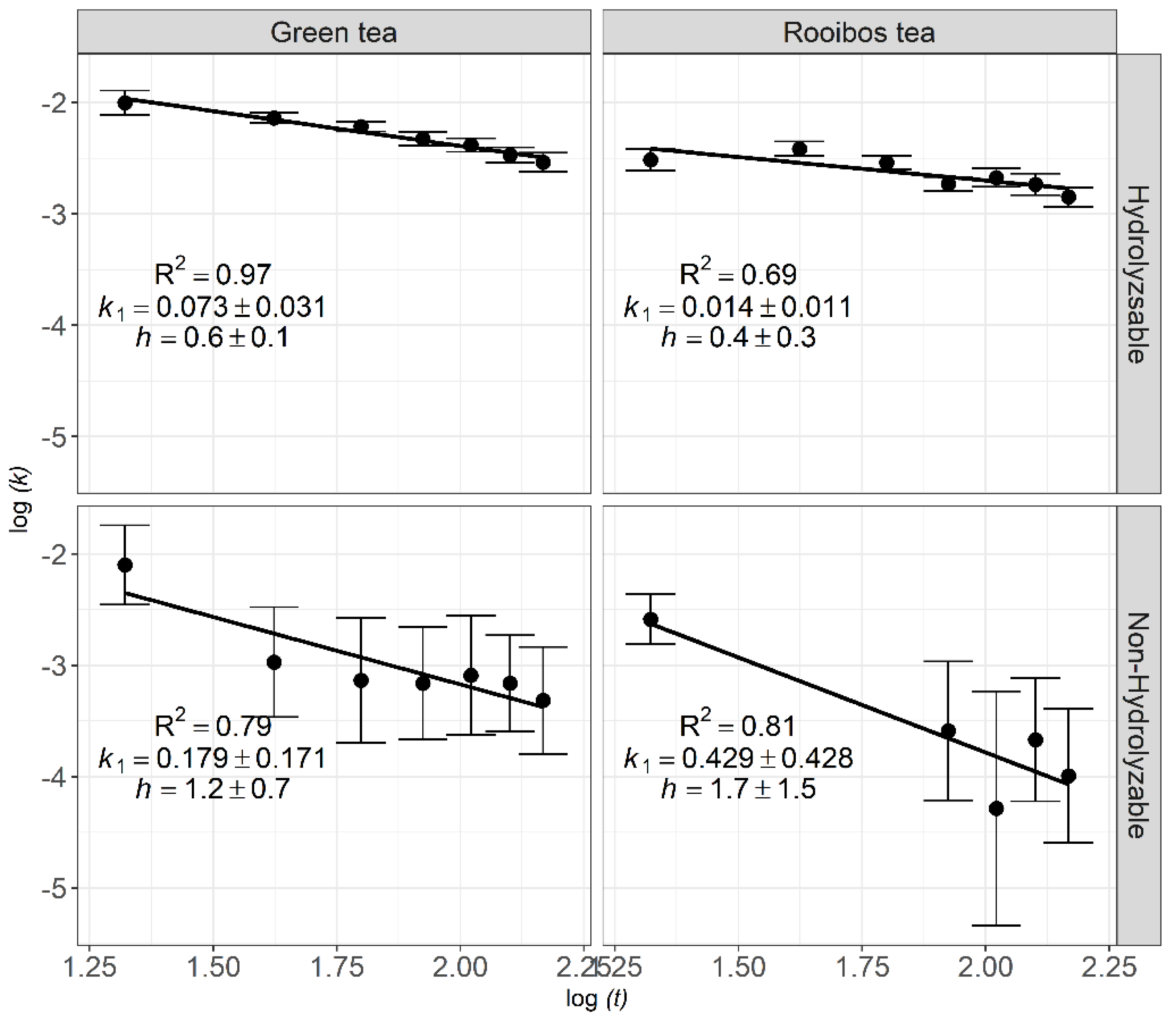
3.2. Fractal Kinetics of Litter Decomposition
4. Discussion
4.1. Effect of Nitrogen Fertilization Regime on Organic Matter Decomposition
4.2. Tea Bag Index
4.3. Fractal Kinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Parent, L.E. Fractal kinetics parameters regulating carbon decomposition rate under contrasting soil management systems. Open J. Soil Sci. 2017, 7, 111–117. [Google Scholar] [CrossRef][Green Version]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1628. [Google Scholar] [CrossRef]
- Barré, P.; Angers, D.A.; Basile-doelsch, I.; Bispo, A.; Cécillon, L.; Chevallier, T.; Derrien, D.; Eglin, T.K.; Pellerin, S. Ideas and perspectives: Can we use the soil carbon saturation deficit to quantitatively assess the soil carbon storage potential, or should we explore other strategies? Biogeosci. Discuss. 2017, 1–12. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil. 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Hubner, R.; Sporlein, P.; Geu, U.; Hangen, E.; Reischl, A.; Schilling, B.; von Lutzow, M.; Kogel-knabner, I. Carbon sequestration potential of soils in southeast Germany derived from stable soil organic carbon saturation. Glob. Chang. Biol. 2014, 20, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Balesdent, J.; Besnard, E.; Arrouays, D.; Chenu, C. The dynamics of carbon in particle-size fractions of soil in a forest-cultivation sequence. Plant Soil 1998, 201, 49–57. [Google Scholar] [CrossRef]
- Barthès, B.G.; Kouakoua, E.; Larré-larrouy, M.; Razafimbelo, T.M.; De Luca, E.F.; Azontonde, A.; Neves, C.S.V.J.; De Freitas, P.L.; Feller, C.L. Texture and sesquioxide effects on water-stable aggregates and organic matter in some tropical soils. Geoderma 2008, 143, 14–25. [Google Scholar] [CrossRef]
- Gelaw, A.M.; Singh, B.R.; Lal, R. Organic carbon and nitrogen associated with soil aggregates and particle sizes under different land uses in tigray. Land Degrad. Dev. 2015, 26, 690–700. [Google Scholar] [CrossRef]
- Stewart, C.E.; Plante, A.F.; Conant, R.T.; Six, J. Soil carbon saturation: Linking concept and measurable carbon pools. Soil Sci. Soc. Am. 2008, 72, 379–392. [Google Scholar] [CrossRef]
- Angers, D.A.; Eriksen-Hamel, N.S. Full-inversion tillage and organic carbon distribution in soil profiles: A meta-analysis. Soil Sci. Soc. Am. J. 2008, 72, 1370–1374. [Google Scholar] [CrossRef]
- Stewart, C.E.; Follett, R.F.; Pruessner, E.G. Nitrogen and harvest effects on soil properties under rainfed switchgrass and no-till corn over 9 years: Implications for soil quality. GCB Bioenergy 2015, 7, 288–301. [Google Scholar] [CrossRef]
- Kenney, I.A.N.; Blanco-canqui, H.; Presley, D.R. Soil and crop response to stover removal from rainfed and irrigated corn. Glob. Chang. Biol. Bioenergy 2015, 7, 219–230. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Parrish, D.J. Potential soil carbon sequestration and CO2 offset by dedicated energy crops in the USA. Crit. Rev. Plant Sci. 2017, 25, 441–472. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Fitzpatrick, S. Cranberry: The Canadian Encyclopedia, Historica Canada. 2015. Available online: https://www.thecanadianencyclopedia.ca/en/article/cranberry (accessed on 17 February 2021).
- Parent, L.E. Classification, pédogénèse et dégradation des sols organiques. In Écologie des Tourbières du Québec-Labrador; Presses de l’Université Laval: Québec, QC, Canada, 2001; pp. 241–255. [Google Scholar]
- Kennedy, C.D.; Wilderotter, S.; Payne, M.; Buda, A.R.; Kleinman, P.J.A.; Bryant, R.B. A geospatial model to quantify mean thickness of peat in cranberry bogs. Geoderma 2018, 319, 122–131. [Google Scholar] [CrossRef]
- Sandler, H.; DeMoranville, C. Cranberry Production: A Guide for Massachusetts, Summary Edition; University of Massachusetts Cranberry Station: Amherst, MA, USA, 2008; pp. 1–198. [Google Scholar]
- Jamaly, R.; Parent, S.-É.; Parent, L.E. Fertilization and soil nutrients impact differentially cranberry yield and quality in eastern Canada. Horticulturae 2021, 7, 191. [Google Scholar] [CrossRef]
- Kosola, K.R.; Workmaster, B.A.A. Mycorrhizal colonization of cranberry: Effects of cultivar, soil type, and leaf litter composition. J. Am. Soc. Hortic. Sci. 2007, 132, 134–141. [Google Scholar] [CrossRef]
- Atucha, A.; Workmaster, B. Root growth patterns in cranberries: Why is it important and how can it affect yield and production efficiency. Wis. Cranberry Sch. 2016, 24, 35–40. [Google Scholar]
- Chaopricha, N.T.; Marín-Spiotta, E. Soil burial contributes to deep soil organic carbon storage. Soil Biol. Biochem. 2014, 69, 251–264. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, H.C.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Van Bodegom, P. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Duboc, O.; Zehetner, F.; Djukic, I.; Tatzber, M.; Berger, T.W.; Gerzabek, M.H. Decomposition of European beech and black pine foliar litter along an Alpine elevation gradient: Mass loss and molecular characteristics. Geoderma 2012, 189, 522–531. [Google Scholar] [CrossRef]
- Bonan, G.B.; Hartman, M.D.; Parton, W.J.; Wieder, W.R. Evaluating litter decomposition in earth system models with long-term litterbag experiments: An example using the Community Land Model version 4 (CLM4). Glob. Chang. Biol. 2013, 19, 957–974. [Google Scholar] [CrossRef]
- Janssens, I.A.; Luyssaert, S. Nitrogen’s carbon bonus. Nat. Geosci. 2009, 2, 318–319. [Google Scholar] [CrossRef]
- Gagnon, B.; Parent, S.-É.; Abdi, D.; Ziadi, N.; Parent, L.-É. The use of isometric log ratios to classify phosphorus attributes in composts. Can. J. Soil Sci. 2018, 98, 448–457. [Google Scholar] [CrossRef]
- Davenport, J. The Effect of Nitrogen Fertilizer Rates and Timing on Cranberry Yield and Fruit Quality. J. Am. Soc. Hortic. Sci. 1996, 121, 1089–1094. [Google Scholar] [CrossRef]
- Davenport, J.; Vorsa, N. Cultivar fruiting and vegetative response to nitrogen fertilizer in cranberry. J. Am. Soc. Hortic. Sci. 1999, 124, 90–93. [Google Scholar] [CrossRef]
- Davenport, J.; Demoranville, C.; Hart, J.; Patten, K.; Peterson, L.; Planer, T.; Poole, A.; Roper, T.; Smith, J. Cranberry Tissue Testing for Producing Beds in North America; Oregon State University: Corvallis, OR, USA, 1995. [Google Scholar]
- Vanden Heuvel, J.E.; Davenport, J.R. Growth and carbon partitioning in cranberry uprights as influenced by nitrogen supply. HortScience 2006, 41, 1552–1558. [Google Scholar] [CrossRef]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; De Anta, R.C.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Trofymow, J.A.; Moore, T.R.; Titus, B.; Prescott, C.; Morrison, I.; Siltanen, M.; Smith, S.; Fyles, J.; Wein, R.; Camiré, C.; et al. Rates of litter decomposition over 6 years in Canadian forests: Influence of litter quality and climate. Can. J. For. Res. 2002, 32, 789–804. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361. [Google Scholar] [CrossRef]
- Hildrew, A.; Townsend, C.; Francis, J.; Finch, K. Cellulolytic decomposition in streams of contrasting pH and its relationship with invertebrate community structure. Freshw. Biol. 1984, 14, 323–328. [Google Scholar] [CrossRef]
- Boulton, A.J.; Quinn, J.M. A simple and versatile technique for assessing cellulose decomposition potential in floodplain and riverine sediments. Arch. Für Hydrobiol. 2000, 150, 133–151. [Google Scholar] [CrossRef]
- Claret, A.; Boulton, J.; Marmonier, P. Functional processes versus state variables: Interstitial organic matter pathways in floodplain habitats. Can. J. Fish. Aquat. Sci. 2001, 58, 1594–1602. [Google Scholar] [CrossRef]
- Tiegs, S.; Langhans, S.; Tockner, K.; Gessner, M. Cotton strips as a leaf surrogate to measure decomposition in river floodplain habitats. J. N. Am. Benthol. Soc. 2007, 26, 70–77. [Google Scholar] [CrossRef]
- Harrison, A.F.; Latter, P.M.; Walton, D.W.H. Cotton strip assay: An index of decomposition in soil. ITE Symp. Ed. 1988, 24, 100–108. [Google Scholar]
- Correll, R.L.; Harch, B.D.; Kirkby, C.A.; Brien, K.O.; Pankhurst, C.E. Methods Statistical analysis of reduction in tensile strength of cotton strips as a measure of soil microbial activity. J. Microbiol. Methods 1997, 31, 9–17. [Google Scholar] [CrossRef]
- Slocum, M.G.; Roberts, J.; Mendelssohn, I.A. Artist canvas as a new standard for the cotton-strip assay. J. Plant Nutr. Soil Sci. 2009, 172, 71–74. [Google Scholar] [CrossRef]
- Fritz, K.M.; Fulton, S.; Johnson, B.R.; Barton, C.D.; Jack, J.D.; Word, D.A.; Burke, R.A. An assessment of cellulose filters as a standardized material for measuring litter breakdown in headwater streams. Ecohydrology 2011, 4, 469–476. [Google Scholar] [CrossRef]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Didion, M.; Repo, A.; Liski, J.; Forsius, M.; Bierbaumer, M.; Djukic, I. Towards harmonizing leaf litter decomposition studies using standard Tea Bags—A field study and model application. Forests 2016, 7, 167. [Google Scholar] [CrossRef]
- Thuriès, L.; Pansu, M.; Larré-Larrouy, M.C.; Feller, C. Biochemical composition and mineralization kinetics of organic inputs in a sandy soil. Soil Biol. Biochem. 2002, 34, 239–250. [Google Scholar] [CrossRef]
- Bosatta, E.; Agren, G.I. Dynamics of carbon and nitrogen in the organic matter of the soil: A generic theory. Am. Nat. 1991, 138, 227–245. [Google Scholar] [CrossRef]
- Kopelman, R. Fractal reaction kinetics. Science 1988, 241, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, J.H.; Feng, H.; Qi, H. Fractal kinetic analysis of polymers/nonionic surfactants to eliminate lignin inhibition in enzymatic saccharification of cellulose. Bioresour. Technol. 2011, 102, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, H. Fractal kinetic analysis of the enzymatic saccharification of cellulose under different conditions. Bioresour. Technol. 2010, 101, 7995–8000. [Google Scholar] [CrossRef]
- Rompre, M.; Laflamme, G.; Ouellet, L.; Carrier, D.; Dubé, J.-C.; Pagé, F. Étude Pédologique du Comté d’Arthabaska; Ministère de l’agriculture, des pêcheries et de l’alimentation du Québec, Direction de la Recherche Agricole: Quebec, QC, Canada, 1984.
- Caron, J.; Pelletier, V.; Kennedy, C.D.; Gallichand, J.; Gumiere, S.; Bonin, S.; Bland, W.; Pepin, S. Guidelines of irrigation and drainage management strategies to enhance cranberry production and optimize water use in North America. Can. J. Soil Sci. 2017, 97, 82–91. [Google Scholar]
- Gouvernement du Canada. Météo, Climat et Catastrophes Naturelles. 2018. Available online: https://www.canada.ca/fr/services/environnement/meteo.html (accessed on 6 August 2021).
- Kowalenko, C.G. Assessment of Leco CNS-2000 analyzer for simultaneously measuring total carbon, nitrogen, and sulphur in soil. Commun. Soil Sci. Plant Anal. 2001, 32, 2065–2078. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 383–411. [Google Scholar]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kroetsch, D.; Wang, C. Particle size distribution. Soil Sampl. Methods Anal. 2008, 2, 713–725. [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Tremblay, M.E.; Nduwamungu, C.; Parent, L.É.; Tremblay, M.E. Biological stability of carbon and nitrogen in organic products and crop residues using Fourier- Transform Near-Infrared Reflectance Spectroscopy. Soil Sci. Plant Anal. 2010, 41, 917–934. [Google Scholar] [CrossRef]
- MacDonald, E.; Brummell, M.E.; Bieniada, A.; Elliot, J.; Engering, A.; Gauthier, T.L.; Saraswati, S.; Touchette, S.; Tourmel-Courchesne, L.; Strack, M. Using the Tea Bag Index to characterize decomposition rates in restored peatlands. Boreal Environ. Res. 2018, 2469, 221–235. [Google Scholar]
- Saint-Laurent, L. Arsenault-Boucher, Soil properties and rate of organic matter decomposition in riparian woodlands using the TBI protocol. Geoderma 2020, 358, 113976. [Google Scholar] [CrossRef]
- Duddigan, S.; Shaw, L.J.; Alexander, P.D.; Collins, C.D. Chemical underpinning of the Tea Bag Index: An examination of the decomposition of tea leaves. Appl. Environ. Soil Sci. 2020, 2020, 6085180. [Google Scholar] [CrossRef]
- Pande, R.; Mishra, H.N. Fourier Transform Near-Infrared Spectroscopy for rapid and simple determination of phytic acid content in green gram seeds (Vigna radiata). Food Chem. 2015, 172, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Verzani, J. Using R for Introductory Statistics; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Authors, E.; Heisterkamp, S.; Van Willigen, B. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 2007, 3, 1–89. [Google Scholar]
- West, B.T.; Welch, K.B.; Galecki, A.T. Linear Mixed Models: A Practical Guide Using Statistical Software; Chapman and Hall/CRC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Amrhein, V.; Greenland, S.; Mcshane, B. Retire statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Prescott, C.E. Does Nitrogen Availability Control Rates of Litter Decomposition in Forests? Plant Soil 1995, 168, 83–88. [Google Scholar] [CrossRef]
- Thomas, D.C.; Zak, D.R.; Filley, T.R. Chronic N deposition does not apparently alter the biochemical composition of forest floor and soil organic matter. Soil Biol. Biochem. 2012, 54, 7–13. [Google Scholar] [CrossRef]
- Fog, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Tan, W.; Wang, G.; Huang, C.; Gao, R.; Xi, B.; Zhu, B. Physico-chemical protection, rather than biochemical composition, governs the responses of soil organic carbon decomposition to nitrogen addition in a temperate agroecosystem. Sci. Total Environ. 2017, 598, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Tamm, C.O. Decomposition and nutrient dynamics of litter in long-term optimum nutrition experiments. Scand. J. For. Res. 1991, 6, 305–321. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Berg, B.; Laskowski, R. Decomposers: Soil microorganisms and animals. Adv. Ecol. Res. 2005, 38, 73–100. [Google Scholar]
- Davenport, J.R.; Provost, J. Cranberry tissue nutrient levels as impacted by three levels of nitrogen fertilizer and their relationship to fruit yield and quality. J. Plant Nutr. 1994, 17, 1625–1634. [Google Scholar] [CrossRef]
- Davenport, J.R.; Demoranville, C.J.; Hart, J.; Roper, T. Nitrogen for Bearing Cranberries in North America; Oregon State University: Corvallis, OR, USA, 2000. [Google Scholar]
- Min, K.; Kang, H.; Lee, D. Effects of ammonium and nitrate additions on carbon mineralization in wetland soils. Soil Biol. Biochem. 2011, 43, 2461–2469. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S. Effects of lime and phosphate additions on changes in enzyme activities, microbial biomass and levels of extractable nitrogen, sulphur and phosphorus in an acid soil. Biol. Fertil. Soils 1988, 6, 153–158. [Google Scholar] [CrossRef]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Ryan, M.G.; Melollo, J.M.; Ricca, A. A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res. 1990, 20, 166–171. [Google Scholar] [CrossRef]
- Heim, A.; Frey, B. Early stage litter decomposition rates for Swiss forests. Biogeochemistry 2004, 70, 299–313. [Google Scholar] [CrossRef]
- Andrén, O.; Kätterer, T. ICBM: The introductory carbon balance model for exploration of soil carbon balances. Ecol. Soc. Am. 1997, 7, 1226–1236. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.S. RothC—A Model for the Turnover of Carbon in Soil. Model Description and Users Guide; Rothamsted Research: Harpenden, UK, 2014; Available online: https://www.rothamsted.ac.uk/sites/default/files/RothC_guide_WIN.pdf (accessed on 10 August 2021).
- Newton, L.S.J.; Lopes, A.; Spokas, K.; Archer, D.W.; Reicosky, D. First-order decay models to describe soil C-CO2 loss after rotary tillage. Sci. Agric. 2009, 66, 650–657. [Google Scholar]
- Thuriès, L.; Pansu, M.; Feller, C.; Herrman, P.; Rémy, J.C. Kinetics of added organic matter decomposition in a Mediterranean sandy soil. Soil Biol. Biochem. 2001, 33, 997–1010. [Google Scholar] [CrossRef]
- Perfecf, B.; Kay, D. Applications of fractals in soil and tillage research: A review. Soil Tillage Res. 1995, 36, 1–20. [Google Scholar] [CrossRef]
- Dolgonosov, B.M.; TGubernatorova, N. Kinetics of the enzymatic decomposition of macromolecules with a fractal structure. Theor. Found. Chem. Eng. 2007, 41, 868–877. [Google Scholar] [CrossRef]
- Parent, S.É.; Parent, L.É.; Parent, A.-C.; Leblanc, M.; Coulibaly, Z. Site-specific multilevel modeling of potato response to nitrogen fertilization. In Front. Environ. Sci.; 2017; Volume 5, p. 81. Available online: https://www.frontiersin.org/articles/10.3389/fenvs.2017.00081/full (accessed on 6 August 2021).
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blago-datskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effect. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]


| Site #A9 | Site #10 | Site #9 | Site #45 | |
|---|---|---|---|---|
| pHcacl2 | 4.3 | 4.3 | 4.7 | 4.6 |
| C:N | 19.0 | 7.7 | 11.0 | 21.8 |
| g kg−1 | ||||
| C | 11.4 | 5.4 | 12.1 | 13.1 |
| N | 0.6 | 0.7 | 1.1 | 0.6 |
| S | 0.2 | 0.2 | 0.1 | 0.1 |
| Sand 1–2 mm | 13 | 6 | 34 | 19 |
| Sand 0.5–1 mm | 71 | 28 | 93 | 74 |
| Sand 0.25–0.5 mm | 411 | 329 | 404 | 279 |
| Sand 0.1–0.25 mm | 375 | 461 | 261 | 475 |
| Sand 0.25–0.01 mm | 44 | 102 | 69 | 91 |
| Silt | 49 | 5 | 44 | 37 |
| Clay | 36 | 23 | 26 | 26 |
| Mehlich-3 extractable mg element kg−1 | ||||
| P | 47 | 46 | 88 | 62 |
| S | 39 | 25 | 25 | 38 |
| K | 26 | 23 | 36 | 35 |
| Ca | 28 | 26 | 170 | 135 |
| Mg | 6 | 7 | 12 | 10 |
| Zn | 0.5 | 0.7 | 2.0 | 1.1 |
| Cu | 1.5 | 2.5 | 2.1 | 1.9 |
| Mn | 0.8 | 0.9 | 3.0 | 1.0 |
| Fe | 128 | 108 | 159 | 182 |
| Al | 591 | 345 | 690 | 662 |
| Mehlich-3 P saturation ratio | ||||
| P/Al | 0.08 | 0.13 | 0.13 | 0.09 |
| Tea Type | Size Fraction (mm) | Particle Size Distribution (%) |
|---|---|---|
| Green tea | >2 | 32.1 |
| 1–2 | 26.2 | |
| 0.5–1 | 5.9 | |
| 0.25–0.50 | 1.7 | |
| <0.25 | 34.1 | |
| Rooibos tea | >2 | 64.8 |
| 1–2 | 14.4 | |
| 0.5–1 | 1.3 | |
| 0.25–0.50 | 0.5 | |
| <0.25 | 19.0 | |
| Cranberry residue | >2 | 89.2 |
| 1–2 | 0.1 | |
| 0.5–1 | - | |
| 0.25–0.50 | - | |
| <0.25 | 10.7 |
| Green Tea | Rooibos Tea | Cranberry Litter | |
|---|---|---|---|
| C:N Ratio | 11.1 ± 1.3 | 54.1 ± 9.1 | 66.7 ± 5.4 |
| % of dry organic mass (total biomass minus ash) (mean ± SD) | |||
| 75.2 ± 1.5 | 45.4 ± 1.4 | 33.9 ± 3.3 |
| 14.8 ± 0.9 | 37.5 ± 0.3 | 25.9 ± 0.9 |
| 17.1 ± 1.1 | 9.8 ± 1.4 | 40.1 ± 2.7 |
| Hydrolyzable (1 + 2) | 90.5 ± 2.4 | 82.9 ± 1.7 | 47.6 ± 4.2 |
| Biomass weight (g) (mean ± SD) | |||
| † | 1.7 ± 1.3 × 10−2 | 1.9 ± 1.1 × 10−2 | 1.9 ± 1.5 × 10−2 |
| 0.7 ± 1.2 × 10−1 | 1.4 ± 1.1 × 10−1 | 1.8 ± 1.3 × 10−1 | |
| Decomposition rate k (day−1) as (ln(M90 days/M0))/90 days (mean ± SD) | |||
| 9.7 × 10−3 ± 1.6 × 10−3 | 3.3 × 10−3 ± 0.8 × 10−3 | 0.4 × 10−3 ± 0.86 × 10−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dossou-Yovo, W.; Parent, S.-É.; Ziadi, N.; Parent, É.; Parent, L.-É. Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems. Soil Syst. 2021, 5, 44. https://doi.org/10.3390/soilsystems5030044
Dossou-Yovo W, Parent S-É, Ziadi N, Parent É, Parent L-É. Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems. Soil Systems. 2021; 5(3):44. https://doi.org/10.3390/soilsystems5030044
Chicago/Turabian StyleDossou-Yovo, Wilfried, Serge-Étienne Parent, Noura Ziadi, Élizabeth Parent, and Léon-Étienne Parent. 2021. "Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems" Soil Systems 5, no. 3: 44. https://doi.org/10.3390/soilsystems5030044
APA StyleDossou-Yovo, W., Parent, S.-É., Ziadi, N., Parent, É., & Parent, L.-É. (2021). Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems. Soil Systems, 5(3), 44. https://doi.org/10.3390/soilsystems5030044






