Perchlorate and Agriculture on Mars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regolith, Control Soil, and Additions
2.2. Species Selection
2.3. Germination and Growth Experiment
2.4. Biogeochemical Analysis
3. Results and Discussion
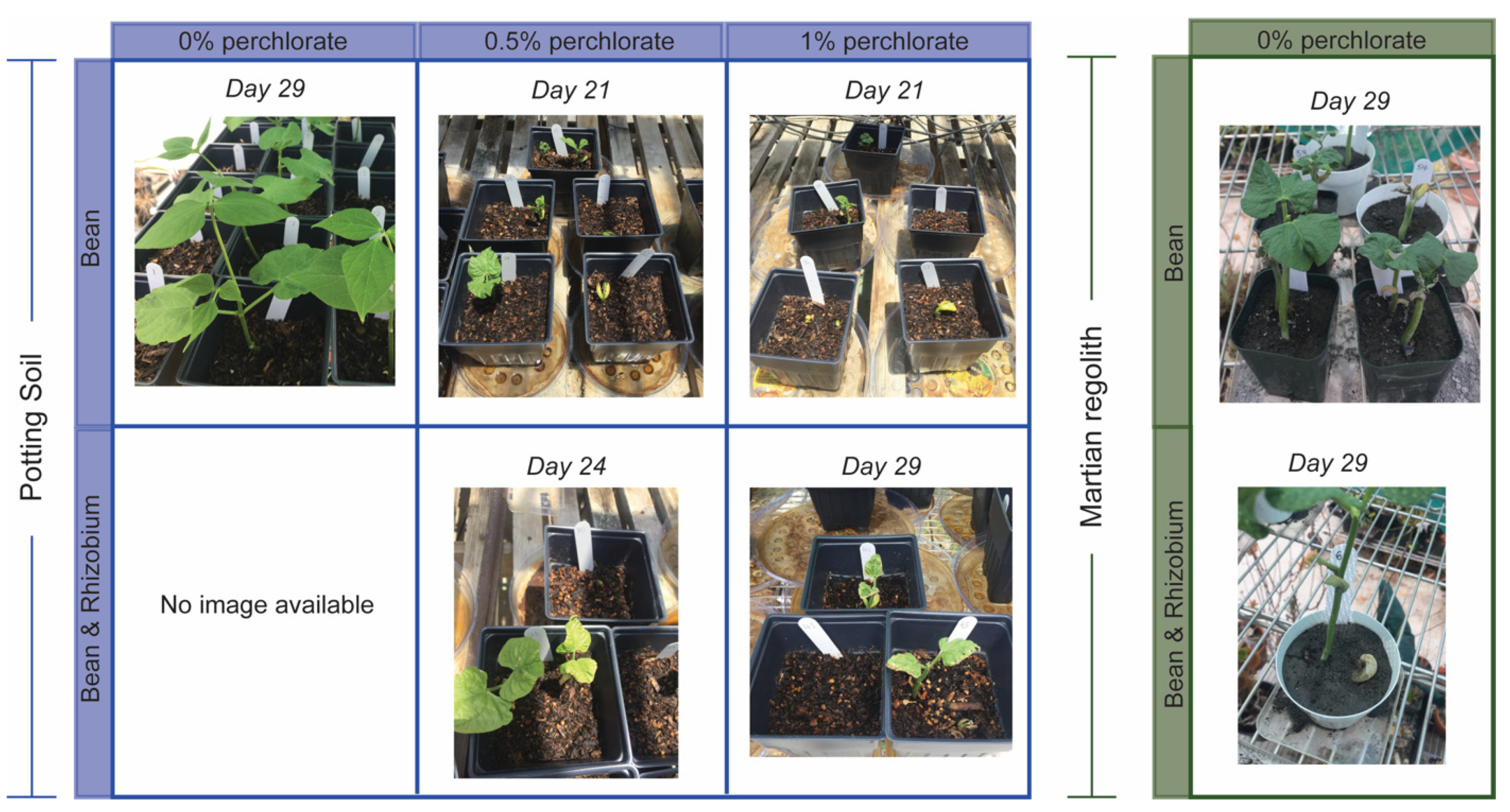
3.1. Germination and Plant Growth
3.2. Perchlorate and Metal Release in Martian Regolith Simulant
3.3. Implications for Regolith and Agriculture on Mars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelis, D.G.; Wilson, J.W.; Clowdsley, M.S.; Nealy, J.E.; Humes, D.H.; Clem, J.M. Lunar Lava Tube Radiation Safety Analysis. J. Radiat. Res. 2002, 43, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Boston, P.J.; Frederick, R.D.; Welch, S.M.; Werker, J.; Meyer, T.R.; Sprungman, B.; Hildreth-Werker, V.; Thompson, S.L. Extraterrestrial Subsurface Technology Test Bed: Human Use and Scientific Value of Martian Caves. AIP Conf. Proc. 2004, 699, 1007–1018. [Google Scholar] [CrossRef]
- Blamont, J. A Roadmap to Cave Dwelling on the Moon and Mars. Adv. Space Res. 2014, 54, 2140–2149. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cull, S.C.; Arvidson, R.E.; Catalano, J.G.; Ming, D.W.; Morris, R.V.; Mellon, M.T.; Lemmon, M. Concentrated Perchlorate at the Mars Phoenix Landing Site: Evidence for Thin Film Liquid Water on Mars. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.N.; Oze, C.; Tang, Y.; O’Loughlin, A. Development of a Martian Regolith Simulant for In-Situ Resource Utilization Testing. Acta Astronaut. 2017, 131, 45–49. [Google Scholar] [CrossRef]
- Parker, D.R.; Seyfferth, A.L.; Kiel Reese, B. Perchlorate in Groundwater: A Synoptic Survey of “Pristine” Sites in the Coterminous United States. Environ. Sci. Technol. 2008, 42, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, A.L.; Henderson, M.K.; Parker, D.R. Effects of Common Soil Anions and PH on the Uptake and Accumulation of Perchlorate in Lettuce. Plant Soil 2008, 302, 139–148. [Google Scholar] [CrossRef]
- Sijimol, M.R.; Gopikrishna, V.G.; Dineep, D.; Mohan, M. Perchlorate in Drinking Water around Rocket Manufacturing and Testing Facilities and Firework Manufacturing Sites in Kerala, India. Energy Ecol. Environ. 2017, 2, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Kumarathilaka, P.; Oze, C.; Indraratne, S.; Vithanage, M. Perchlorate as an Emerging Contaminant in Soil, Water and Food. Chemosphere 2016, 150, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Catling, D.C.; Claire, M.W.; Zahnle, K.J.; Quinn, R.C.; Clark, B.C.; Hecht, M.H.; Kounaves, S. Atmospheric Origins of Perchlorate on Mars and in the Atacama. J. Geophys. Res. Planets 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Wilson, E.H.; Atreya, S.K.; Kaiser, R.I.; Mahaffy, P.R. Perchlorate Formation on Mars through Surface Radiolysis-Initiated Atmospheric Chemistry: A Potential Mechanism. J. Geophys. Res. Planets 2016, 121, 1472–1487. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-Y.S.; McLennan, S.M.; Jackson, W.A.; Karunatillake, S. Photochemical Controls on Chlorine and Bromine Geochemistry at the Martian Surface. Earth Planet. Sci. Lett. 2018, 497, 102–112. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Oberlin, E.A. Volatiles Measured by the Phoenix Lander at the Northern Plains of Mars. In Volatiles in the Martian Crust; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–283. ISBN 978-0-12-804191-8. [Google Scholar]
- Smith, M.L.; Claire, M.W.; Catling, D.C.; Zahnle, K.J. The Formation of Sulfate, Nitrate and Perchlorate Salts in the Martian Atmosphere. Icarus 2014, 231, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Rieder, R.; Economou, T.; Wänke, H.; Turkevich, A.; Crisp, J.; Brückner, J.; Dreibus, G.; McSween, H.Y., Jr. The Chemical Composition of Martian Soil and Rocks Returned by the Mobile Alpha Proton X-Ray Spectrometer: Preliminary Results from the X-Ray Mode. Science 1997, 278, 1771–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, B.L.; Kounaves, S.P. The Origins of Perchlorate in the Martian Soil. Geophys. Res. Lett. 2015, 42, 3739–3745. [Google Scholar] [CrossRef]
- Clark, B.C.; Kounaves, S.P. Evidence for the Distribution of Perchlorates on Mars. Int. J. Astrobiol. 2016, 15, 311–318. [Google Scholar] [CrossRef]
- Eichler, A.; Hadland, N.; Pickett, D.; Masaitis, D.; Handy, D.; Perez, A.; Batcheldor, D.; Wheeler, B.; Palmer, A. Challenging the Agricultural Viability of Martian Regolith Simulants. Icarus 2021, 354, 114022. [Google Scholar] [CrossRef]
- Wamelink, G.W.W.; Frissel, J.Y.; Krijnen, W.H.J.; Verwoert, M.R.; Goedhart, P.W. Can Plants Grow on Mars and the Moon: A Growth Experiment on Mars and Moon Soil Simulants. PLoS ONE 2014, 9, e103138. [Google Scholar] [CrossRef]
- Duri, L.G.; El-Nakhel, C.; Caporale, A.G.; Ciriello, M.; Graziani, G.; Pannico, A.; Palladino, M.; Ritieni, A.; De Pascale, S.; Vingiani, S.; et al. Mars Regolith Simulant Ameliorated by Compost as In Situ Cultivation Substrate Improves Lettuce Growth and Nutritional Aspects. Plants 2020, 9, 628. [Google Scholar] [CrossRef]
- Chevrier, V.F.; Hanley, J.; Altheide, T.S. Stability of Perchlorate Hydrates and Their Liquid Solutions at the Phoenix Landing Site, Mars. Geophys. Res. Lett. 2009, 36, L10202. [Google Scholar] [CrossRef] [Green Version]
- Oze, C.; Sleep, N.H.; Coleman, R.G.; Fendorf, S. Anoxic Oxidation of Chromium. Geology 2016, 44, 543–546. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Seven, K.; Germann, P. Water Flow in Soil Macropores II. A Combined Flow Model. J. Soil Sci. 1981, 32, 15–29. [Google Scholar] [CrossRef]
- Esechie, H.A. Interaction of Salinity and Temperature on the Germination of Sorghum. J Agron. Crop Sci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Yu, L.; Cañas, J.E.; Cobb, G.P.; Jackson, W.A.; Anderson, T.A. Uptake of Perchlorate in Terrestrial Plants. Ecotoxicol. Environ. Saf. 2004, 58, 44–49. [Google Scholar] [CrossRef]
- Susarla, S.; Bacchus, S.T.; McCutcheon, S.C.; Wolfe, N.L. Potential Species for Phytoremediation of Perchlorate; Diane Publishing Company: Darby, PA, USA, 1999. [Google Scholar]
- Seyfferth, A.L.; Parker, D.R. Effects of Genotype and Transpiration Rate on the Uptake and Accumulation of Perchlorate (ClO4-) in Lettuce. Environ. Sci. Technol. 2007, 41, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.; Suarez, D.L.; Lesch, S.M. Perchlorate Uptake in Spinach As Related to Perchlorate, Nitrate, And Chloride Concentrations in Irrigation Water. Environ. Sci. Technol. 2011, 45, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.; Suarez, D.L.; Lesch, S.M. Predicting Perchlorate Uptake in Greenhouse Lettuce from Perchlorate, Nitrate, and Chloride Irrigation Water Concentrations. J. Environ. Qual. 2013, 42, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, W.A.; Joseph, P.; Laxman, P.; Tan, K.; Smith, P.N.; Yu, L.; Anderson, T.A. Perchlorate Accumulation in Forage and Edible Vegetation. J. Agric. Food Chem. 2005, 53, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.; Palma, P.; Eltit, K.; Arancibia-Miranda, N.; Silva-Moreno, E.; Yu, W. Field Study on the Uptake, Accumulation and Risk Assessment of Perchlorate in a Soil-Chard/Spinach System: Impact of Agronomic Practices and Fertilization. Sci. Total Environ. 2020, 719, 137411. [Google Scholar] [CrossRef] [PubMed]



| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| --------------------------------------Wt. %---------------------------- | |||||||||
| Martian regolith simulant | 57.9 | 14.7 | 8.67 | 4.9 | 3.91 | 4.12 | 3.12 | 1.48 | 1.88 |
| Martian regolith simulant(Perchlorate) | 46.9 | 15.25 | 12.55 | 7.1 | 6.11 | 4.43 | 1.99 | 2.22 | 2.85 |
| Potting soil | 38.3 | 8.17 | 2.69 | 3.9 | 1.24 | 2.04 | 2.09 | 0.36 | 42 |
| Potting soil (Perchlorate) | 34.9 | 7.26 | 2.58 | 3.68 | 1.42 | 1.79 | 2.49 | 0.33 | 45.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oze, C.; Beisel, J.; Dabsys, E.; Dall, J.; North, G.; Scott, A.; Lopez, A.M.; Holmes, R.; Fendorf, S. Perchlorate and Agriculture on Mars. Soil Syst. 2021, 5, 37. https://doi.org/10.3390/soilsystems5030037
Oze C, Beisel J, Dabsys E, Dall J, North G, Scott A, Lopez AM, Holmes R, Fendorf S. Perchlorate and Agriculture on Mars. Soil Systems. 2021; 5(3):37. https://doi.org/10.3390/soilsystems5030037
Chicago/Turabian StyleOze, Christopher, Joshua Beisel, Edward Dabsys, Jacqueline Dall, Gretchen North, Allan Scott, Alandra Marie Lopez, Randall Holmes, and Scott Fendorf. 2021. "Perchlorate and Agriculture on Mars" Soil Systems 5, no. 3: 37. https://doi.org/10.3390/soilsystems5030037
APA StyleOze, C., Beisel, J., Dabsys, E., Dall, J., North, G., Scott, A., Lopez, A. M., Holmes, R., & Fendorf, S. (2021). Perchlorate and Agriculture on Mars. Soil Systems, 5(3), 37. https://doi.org/10.3390/soilsystems5030037








