Abstract
Soil calcium depletion has been strongly linked to acidic deposition in eastern North America and recent studies have begun to document the recovery of soils in response to large decreases in acidic deposition. However, increased calcium availability has not yet been seen in the B horizon, where calcium depletion has been most acute, but mineral weathering is critically important for resupplying ecosystem calcium. This study provides new data in seven watersheds in the Adirondack region (New York, USA), where acidic deposition impacts on soils and surface waters have been substantial and recovery remains slow. Initial sampling in 1997–1998 and 2003–2004 was repeated in 2009–2010, 2014, 2016 and 2017. Exchangeable calcium concentrations increased by an average of 43% in the Oe horizon of three watersheds where this horizon was sampled (10.7–15.3 cmolc kg−1). Changes in calcium were not seen in the individual watersheds of the Oa and B horizons, but as a group, a significant increase in calcium was measured in the upper B horizon. Liming of a calcium-depleted watershed also tripled calcium concentration in the upper B horizon in 5 years. However, stream calcium in unlimed watersheds decreased over the study period. Small increases in B-horizon calcium may be underway.
1. Introduction
Calcium is a key element in determining the characteristics of ecosystems, through both geochemical and biological controls. Originating in rock, calcium only begins to cycle in the environment once it is released from rocks and minerals in a dissolvable form through weathering. The process of weathering is an important means of neutralizing acidity, so the abundance of calcium in geologic materials usually determines whether a landscape is prone to acidification or well buffered. By adsorbing to soil surfaces, calcium can substitute for hydrogen ions, thereby buffering against soil acidification. In plants, calcium plays an essential role in regulating many physiological processes that influence both growth and responses to environmental stresses, as well as in structural components [1]. In animals, calcium is also critical for structural components and is involved in many physiological processes [2,3]. Biologically assimilated calcium is recycled through mineralization of organic matter that facilitates uptake by plant roots.
Depletion of soil calcium and other base cations has been recognized as a key factor in slowing the recovery of surface waters from acidification [4,5,6]. After peaking in the northeastern United States in the early 1980s, substantial declines in acidic deposition rates have been achieved and levels now approach those of the early 1900s [7]. Calcium, typically the most abundant base cation in soil, is the focus of this paper, although much of the analysis also applies to magnesium, sodium and potassium. Acidic deposition accelerated the leaching of soil calcium (hereafter Ca) and lowered the acid-buffering capacity of acid-sensitive landscapes where soils were already naturally low in Ca. This effect of acidic deposition has been well documented through long-term soil monitoring, such as the repeated sampling in the Calhoun Experimental Forest, where loss of soil Ca was tied to ambient acidic deposition [8], and the Bear Brook and Fernow whole-watershed manipulation studies where addition of (NH4)2SO4 led to depletion of soil calcium and other base cations [9,10]. This decrease in Ca availability increased the vulnerability of surface waters to acidification during the era of high acidic deposition levels and reduced their capacity for recovery as acidic deposition declined. Because Ca is a key nutrient needed for a range of physiological functions in trees [1], depletion of soil Ca has also impaired the health of tree species with a high Ca demand such as sugar maple (Acer saccharum Marsh) [11] and basswood (Tilia americana L.) [12]. Understory plant communities are also strongly influenced by Ca availability [13,14]. As a result, depletion of soil Ca can alter the composition of forest communities and the plant demand for Ca within forest ecosystems can be intensified [15]. The availability of Ca in both terrestrial and aquatic ecosystems also effects a host of fauna at multiple trophic levels [2,3,16,17].
Soil Ca depletion has been linked to acidic deposition in multiple regions of North America (e.g., [8,18,19,20]), Europe (e.g., [21,22,23]) and China [24]. Only recently have studies begun to document the recovery of soils in response to the decreases in acidic deposition that have been underway for decades. European soil monitoring identified increases in base saturation in the most acidic soils (initial base saturation <50 percent in the forest floor; <20 percent in the mineral soil) between 1985 and 1996 and 2004 and 2008 but decreases in less acidic soils [25]. Berger et al. [26] measured increases in base cations in the upper soil, although changes occurred largely near trees where stemflow was important. Repeated decadal sampling of two German sites provided evidence that by the mid-2000s, acidification of the forest floor and upper mineral soil was shifting to alkalinization, as evidenced by increases in base saturation and pH, and possibly also depletion of soluble organic matter [27].
The first widespread indications of soil recovery in North America were documented through resampling of 27 sites located throughout the northeastern US and eastern Canada [28]. This study included sites with sampling intervals of 8 to 24 years, with the most recent samplings falling between 2003 and 2014. Decreasing concentrations of aluminum (Al) in O horizons were observed at most sites, but this study found minimal evidence of either depletion or recovery of Ca in either the O or upper B horizon. A more recent sampling window based on collections in 2001–2002 and 2014 at 40 sites throughout the White Mountains of New Hampshire (USA) documented an increase in exchangeable Ca in the Oa horizon but a decrease in the B horizon [29]. To our knowledge, increases in B horizon Ca have not been reported in regions of North America that have experienced soil Ca depletion from acidic deposition.
Where soils have been Ca depleted by acidic deposition, dissolved Ca in soil solutions and surface waters is largely balanced by SO42− [30] and NO3− [31]. Recovery towards pre-acidification conditions necessitates a shift away from SO42− and NO3− back to HCO3− and CO32− as the primary anionic balance to Ca2+. This shift is underway in watersheds where mineral weathering and other processes provide sufficient neutralization through release of Ca2+ into solution [30], but where replenishment of depleted soil Ca is not occurring, surface waters are diluting, with only limited increases in pH and acid-neutralizing capacity [32]. In regions impacted by acidic deposition, widespread decreases in surface water Ca2+ concentrations have accompanied trends of decreasing acidic deposition rates [30]. Because soil Ca depletion has been most acute in the B horizon, modeling of soil and surface water recovery from acidic deposition is often based on base saturation of the B horizon, which is largely a function of Ca [7,33]. Exchangeable Ca concentration in the B horizon is therefore a key indicator of recovery from acidic deposition for both soils and surface waters.
Modeling of soil responses to decreases in acidic deposition suggests that where soil Ca depletion is severe, recovery targets may not be reached for decades to centuries [7]. This has led to the suggestion that the addition of Ca as lime or in other forms might be warranted to accelerate recovery in high-value watersheds [34]. Although a considerable amount of liming has been done to reduce ecosystem damage from ongoing acidic deposition, experimental liming to aid ecosystem recovery under low deposition levels has not received large attention. In this approach, the goal would be to increase calcium availability without large effects on soil acidity because forests where Ca depletion has occurred tended to be naturally acidic before acidic deposition [18]. Information on the effectiveness of liming in comparison to natural recovery in differing soils would be of use in determining the applicability of this approach.
Indications of recovery in the O horizon, coupled with the substantial achievements in lowering acidic deposition rates, suggest that increases in Ca availability in the B horizon may be occurring but have not been detected because the most recent resampling intervals do not extend past 2014. Resampling of soils to assess recovery also remains limited in the US and Canada. We have therefore undertaken this study to provide new data on soil change by resampling soils in the Adirondack region of New York, where acidic deposition impacts on soils and surface waters have been substantial and recovery remains slow [7,35]. As one of the most severely impacted areas of the US, the Adirondack region provides a wealth of information on the severity of effects experienced by soils and surface waters from past studies, as well as ongoing monitoring data to assess recovery trajectories and processes. In this work, we have also included an experiment to determine the effectiveness of liming in accelerating the recovery of soil Ca in a watershed with Ca-depleted soils. The following questions are addressed in this paper:
- Are concentrations of Ca in Adirondack streams continuing to decrease in the most recent data?
- What is the recovery status of Ca-depleted Adirondack soils?
- What does experimental addition of Ca tell us about the soil processes involved in soil Ca recovery?
2. Materials and Methods
2.1. Study Region
The study was conducted in the Adirondack Ecological Region [36], which roughly corresponds to the area that comprises the Adirondack State Park (Figure 1) and is generally referred to as the Adirondack region. Sample collection occurred throughout the 24,243-km2 region, which is characterized by rugged terrain and is almost entirely forested with northern hardwood and coniferous tree species [37]. The bedrock geology is a complex mixture of granitic and gneissic rocks with low acid-neutralizing capacity and a variety of less common metasedimentary formations scattered throughout the region [37]. Repeated glaciations that ceased approximately 10,000 years ago left surficial deposits throughout the region that reflect the complex bedrock mineralogy [38] and include some areas with highly weatherable calcareous minerals [37]. Soils covering most of the region are classified as Spodosols. Mean annual precipitation can range from approximately 800 to 1600 mm across the region [39]. Below-freezing temperatures occur throughout most of the winter, resulting in accumulation of snow that melts over several weeks in March and April [40]. Atmospheric deposition of SO42− and inorganic N in the Adirondack region has been among the highest in the northeast, likely peaking in the late 1970s but decreasing substantially since then [7]. Atmospheric deposition data were obtained from the National Atmospheric Deposition Program (NADP) site NY20, located near the center of the Adirondack region (http://nadp.slh.wisc.edu/; accessed on 1 May 2020).
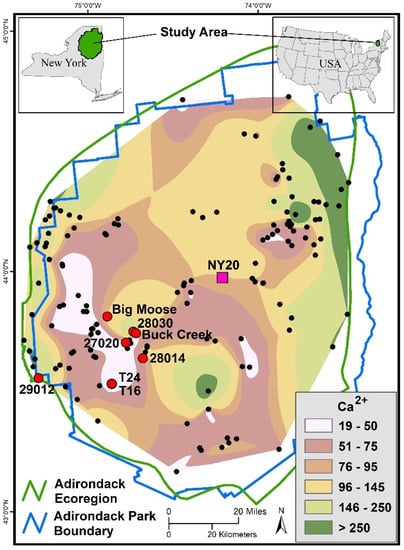
Figure 1.
Study area that encompasses the watersheds where soils were sampled (large red circles), headwater streams were sampled (small black circles) and where the National Atmospheric Deposition Program monitoring site (pink square) measured wet deposition. Spatial color patterns reflect Ca availability across the region. The colors represent ranges of expected calcium (Ca2+) concentrations (µeq L−1) in headwater streams that occur across the landscape. Spatial patterns were developed by interpolating Ca2+ concentrations measured at each of the black circles during spring snowmelt in 2018.
2.2. Soil Sampling and Analysis
The study presented here builds upon previous studies of soil monitoring in the Adirondack region that documented widespread decreases in soil Ca with sampling up to the early 2000s (Figure 2). One study that linked sampling in the 1930s to resampling in 1984 and 2004 documented depletion of Oa horizon Ca throughout much of the Adirondack region [18]. Two additional studies that compared exchangeable Ca concentrations across the Adirondack region in the mid-1980s to measurements in the early 2000s found decreases in Ca in either the Oa [20] or upper B horizon [41]. Additional Adirondack studies for individual watersheds (Figure 2) showed no changes in exchangeable Ca concentrations in either the Oa or upper B horizon in the Big Moose watershed for the period 1992–1993 to 2003 [42], the North Buck watershed from 1997 to 2009–2010 or the South Buck watershed from 1998 to 2014 [28].
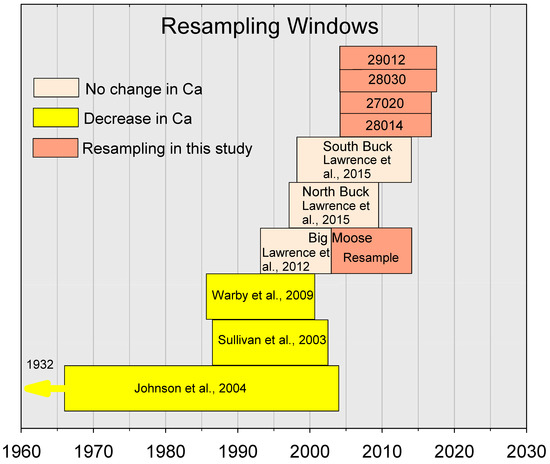
Figure 2.
Yellow and tan boxes indicate years of initial sampling and repeated sampling (indicated by the left and right vertical sides of boxes) for primary soil monitoring studies conducted up to 2014 in the Adirondack region of New York (USA). Salmon-colored boxes indicate intervals of sampling and resampling presented in this study. The numbered boxes represent the resampled watersheds from the Western Adirondack Stream Survey (WASS). References for the citations of completed studies appear at the end of the paper. Results reported in Johnson et al. (2008) extend back to the early 1930s.
Soil sampling data in this paper are presented for seven watersheds in the southwestern Adirondack region that had been previously sampled, including data for North and South Buck (Figure 1). In both Buck watersheds, sampling and resampling were performed at 28 locations distributed along transects perpendicular to the primary stream channel. At each location, samples were collected from the Oe and Oa horizons and the upper 10 cm of the B horizon. Results of the North and South Buck samplings that have been reported previously [28,31] are included here to support a regional assessment of Adirondack soils.
For this study, a third sampling was conducted at the Big Moose site in 2014, following the same design and methods used in 2003. Samples were obtained from the Oe and Oa horizons and the upper 10 cm of the B horizon from 12 pits distributed in three groups of four within the 3-ha study area. Further information on the characteristics of the Big Moose site is available in Minocha et al. [43].
Four additional watersheds (identified as 27020, 28011, 28030 and 29012) were initially sampled in 2004 as a component of the Western Adirondack Stream Survey (WASS), a project designed to assess acidic deposition effects on streams and soils in the western Adirondack region [44]. Watershed 27020 (area, 20 ha) was forested primarily with red maple (Acer rubrum L.), beech (Fagus grandifolia Ehrh.) and red spruce (Picea rubens Sarg.); watersheds 28014 (area, 67 ha), 28030 (area, 19 ha) and 29012 (area, 86 ha) were forested by a mix of sugar maple, beech, yellow birch (Betula allegheniensis Britton) and red spruce. In each watershed, five sampling areas were located to include the primary hillslope positions. Three soil pits were dug in each sampling area, from which equal volumes were collected from the Oa horizon and the upper 10 cm of B horizon. Samples from each pit were combined by horizon and area to yield one sample from each horizon, per area, yielding a total of 5 samples from each horizon to represent the watershed.
WASS watersheds 27020 and 28011 were resampled in 2016 and watersheds 28030 and 29012 were resampled in 2017 using a collection design that differed from that used in 2004. In each of three areas identified as the primary landscape types within the watershed, samples were collected from the Oe and Oa horizons and the upper 10 cm of the B horizon of a main pit and 5 associated satellite pits, yielding a total of 18 samples from each horizon to represent the watershed.
In addition to soil resampling to evaluate recovery from acidic deposition under ambient conditions, repeated soil sampling was also conducted to assess the effects of adding lime (CaCO3 with 3.3% magnesium) to a 30-ha watershed labeled T16 in October 2013. The pelletized limestone was distributed by helicopter uniformly over the watershed using global positioning system (GPS) guidance to provide a Ca dose of 1.4 Mg ha−1. This dose was selected to increase Ca availability without causing large changes in soil acidity. The amount is double that of the only previous Adirondack liming experiment conducted in 1989 [45], when acidic deposition was twice that in 2013. Soil samples were collected from the Oe and Oa horizons and the upper 10 cm of the B horizon, once per year, from September 2013 (pretreatment) through 2018 (omitting 2016). Within T16, sampling was performed in three pits located in five plots, 50 m by 20 m, yielding a watershed total of 15 samples from each horizon in each sampling year. In addition, one pit was excavated within each plot to enable collection of samples from the mid and lower B horizons. A nearby (<1.0 km) reference watershed labeled T24 was sampled using the same collection design, once in 2013 and once in 2018. Watershed T24 served as a reference in this study because it was similar in size (20 ha) with highly similar slope, aspect, vegetation and soils. Because each of these watersheds drain directly into Honnedaga Lake, this project was named the Honnedaga Liming Study.
All soil data used to produce this manuscript along with further details on each of the soil sampling designs used in this study are available elsewhere [46,47]. General methods of soil collection used in these studies are available in Lawrence et al. [48].
Measurements of pH (0.01 CaCl2 extraction), exchangeable acidity and Al (1.0 M KCl extraction) and exchangeable Ca, Mg, Na and K (1.0 M NH4Cl extraction) were made on all samples. Only exchangeable forms of Ca and Al are reported in this manuscript. All chemical analyses were performed in the U.S. Geological Survey (USGS) New York Water Science Center Soil and Low-Ionic Strength Water Quality Laboratory (hereafter the USGS Troy Laboratory). Full documentation of the analysis methods appears elsewhere [46]. Cation exchange capacity (CEC) was determined from the laboratory measurements by summing exchangeable concentrations of the primary cations in these soils (Ca, Mg, Na, K, Al and hydrogen (H)). Base saturation was determined by dividing the sum of base cations (Ca, Mg, Na and K) by the CEC.
2.3. Stream Sampling and Analysis
To evaluate trends in stream water Ca2+ (note charge is included when referencing solution concentrations) concentrations, monitoring data from fixed-interval manual sampling were included for five of the watersheds where soil sampling occurred. Data are presented for North and South Buck samples collected biweekly from January 1999 to December 2019, West Pond outlet samples from the Big Moose watershed (approximately 300 m upstream of the soil sampling area) collected monthly from January 1999 to December 2019 and T16 and T24 samples collected from October 2011 to September 2019.
Stream water Ca2+ concentrations for 27020, 28011, 29030 and 29012 were determined on samples collected in the WASS during spring snowmelt in 2004, 2005, 2014, 2015, 2018 and 2019. To provide information on Ca availability throughout the Adirondack region, data from 55 streams, collected in 2018 during spring snowmelt through the WASS, were combined with 59 samples collected in 2018 during snowmelt through the East-Central Adirondack Stream Survey (ECASS). The locations of all of the sampled streams are shown in Figure 1. Full details are available elsewhere for the WASS [44] and ECASS [49]. Concentrations of Ca2+ are also presented for samples collected approximately monthly from streams draining watersheds T16 and T24 in the Honnedaga Liming Study. Stream sampling data extend from October 2011 through September 2019.
Stream samples were analyzed through a collaboration between the Adirondack Lake Survey Corporation (ALSC) laboratory and the USGS Troy Laboratory. Both laboratories followed the same U.S. Environmental Protection Agency (USEPA)-approved methods (https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=30000TA0.PDF). An interlaboratory comparison of chemical analyses was conducted on 155 samples collected biweekly from Buck Creek and the North and South tributaries of Buck Creek from September 2006 through August 2008. Full results of the interlaboratory comparison are available elsewhere [50]. All stream chemistry data used in this paper, with the exception of the West Pond data, are available through the USGS National Water Information System (NWIS) database [51]. NWIS identification codes and coordinates are available in Supplemental Table S1. West Pond data were obtained from the Adirondack Lake Survey Corporation online site (http://www.adirondacklakessurvey.org/; accessed on 26 August 2020).
2.4. Statistical and Spatial Analysis Methods
This study focused on differences in soil chemistry between two points in time, and therefore, it relied on data comparison between the paired samplings. All statistics that involved means comparison tests used unweighted arithmetic means. Paired t-tests were used to identify differences between initial soil sampling and resampling unless the assumption of normality was rejected; then, a signed rank test was used. Differences among years of T16 soil data were assessed with a repeated measures one-way analysis of variance unless the assumption of normality was rejected; then, the Bonferroni t-test was used. Trends in atmospheric deposition and stream Ca2+ concentrations were assessed with linear regression. All statistical tests were conducted with SigmaPlot 14. Values of mean pH were computed from individual pH values.
The natural neighbor algorithm in ArcGIS software was used for interpolation with 100 m cells to produce Figure 1, as described here: http://desktop.arcgis.com/en/arcmap/10.3/tools/spatial-analyst-toolbox/how-natural-neighbor-works.htm [52]. The geographic information system (GIS) was used to compute the areas of each category of Ca2+ concentrations.
3. Results
3.1. Atmospheric Deposition Trends and Changes in Adirondack Soil Chemistry
Annual wet deposition of sulfur (S) measured near the center of the Adirondack region (NY20; Figure 1) decreased substantially from approximately 8 kg S ha−1 y−1 in 1980 to approximately 1 kg S ha−1 y−1 in 2019 (Figure 3). A less-pronounced decrease in wet deposition of NO3− plus NH4+ (inorganic N) from approximately 5.5 to 3 kg N ha−1 y−1 occurred over the same period. The rate of decrease in S and inorganic N somewhat accelerated after the mid-2000s. Atmospheric deposition of Ca also decreased by approximately 45% over this period, although annual deposition rarely exceeded 1.0 kg ha−1 y−1. Based on the linear regression, all trends in Figure 3 were significant at p < 0.01.
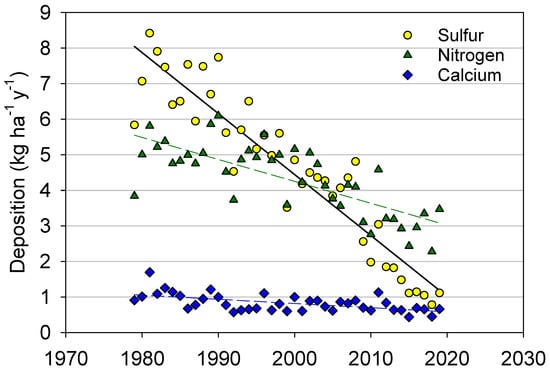
Figure 3.
Annual values of total atmospheric wet deposition of sulfur, inorganic nitrogen (sum of NO3− and NH4+) and calcium at the National Atmospheric Deposition Program monitoring site NY20. The solid black line represents the best linear fit of the sulfur trend; the dashed green line represents the best linear fit of the nitrogen trend; the dashed blue line represents the best linear fit of the calcium trend.
The North and South Buck results showed strong indications of improved Ca availability through increases in the Oe horizon. The Oe horizon data for North and South Buck, however, showed strong indications of improved Ca availability (Table 1). In both watersheds, concentrations of Ca increased and concentrations of Al decreased substantially (p < 0.01). Base saturation showed a small, non-significant increase (p > 0.10) at North Buck and a large significant increase (p < 0.01) from 50.2 percent to 83.1 percent at South Buck. A small, significant (p < 0.01) increase in pH occurred at North Buck, but pH values at South Buck were nearly identical in the two samplings (p > 0.10). The Big Moose samplings in 2003 and 2014 showed similar results to the Buck watersheds. Concentrations of Ca increased (0.01 < p < 0.05) and concentrations of Al decreased substantially (p < 0.01). Like South Buck, Big Moose showed a large increase in base saturation (p < 0.01) but, in contrast to South Buck, showed no change in pH (p > 0.10).

Table 1.
Concentrations of exchangeable Ca and Al (cmolc kg−1), measurements of base saturation (base sat.; percent), pH and cation exchange capacity (CEC; cmolc kg−1) of Oe horizon samples in North Buck, South Buck and Big Moose watersheds. Number of samples is indicated by n; sig. represents significance levels for differences between sampling years at individual watersheds abbreviated as a: p < 0.01; b: 0.01 < p < 0.05; and ns: p > 0.10.
Results for the Oa horizon in North Buck, South Buck and Big Moose differed considerably from the Oe horizon results for the same sampling windows (Table 2). There were no significant changes in Ca concentrations except for a decrease (0.05 < p < 0.1) at South Buck. Concentrations of Al decreased in North Buck (0.01 < p < 0.05) and South Buck (p < 0.01), but no change (p > 0.10) was observed in Big Moose. No change in Ca (p > 0.10) was observed in any of the WASS watersheds (Table 2). Concentrations of Al did not change (p > 0.10) in the four WASS watersheds, although non-significant decreases occurred in each. Base saturation showed a decrease (0.01 < p < 0.05) in watershed 29012, but no changes (p > 0.10) in any of the other watersheds. Increases in pH were observed in Big Moose, 27020 and 28030, (p < 0.01; 0.05 < p < 0.10; 0.05 < p < 0.10, respectively), but no changes (p > 0.10) were seen in the other watersheds.

Table 2.
Concentrations of exchangeable Ca and Al (cmolc kg−1), measurements of base saturation (base sat.; percent), pH and cation exchange capacity (CEC; cmolc kg−1) of Oa horizon samples in North Buck, South Buck, Big Moose and WASS watersheds. Number of samples indicated by n; sig. represent significance levels for differences between sampling years at individual watersheds abbreviated as a: p < 0.01; b: 0.01 < p < 0.05; c: 0.05 < p < 0.10; and ns: p > 0.10.
In the upper B horizon, the only watershed to show a change in Ca concentrations was South Buck (Table 3), where concentrations decreased (0.05 < p < 0.10). Note that in Lawrence et al. [28], the B horizon Ca change at South Buck was reported as non-significant (p = 0.28) based on the Mann–Whitney rank sum test that assumes that data from the two periods are independent, whereas in this analysis, using the same data, the Wilcoxon signed rank test identified a difference that is reported here as significant (p = 0.056). Because the sampling was performed at the same locations in both samplings, the Wilcoxon test is most appropriate. Non-significant increases (p > 0.10) in Ca were measured in each of the other six watersheds.

Table 3.
Concentrations of exchangeable Ca and Al (cmolc kg−1), measurements of base saturation (base sat.; percent), pH and cation exchange capacity (CEC; cmolc kg−1) of B horizon samples (upper 10 cm) in North Buck, South Buck, Big Moose and WASS watersheds. Number of samples indicated by n; sig. represent significance levels for differences between sampling years at individual watersheds abbreviated as a: p < 0.01; b: 0.01 < p < 0.05; c: 0.05 < p < 0.10; and ns: p > 0.10.
In contrast to the Oe and Oa horizons, the upper B horizon showed increases in Al in North Buck, South Buck and Big Moose (p < 0.01). No changes (p > 0.01) in Al were observed in the WASS watersheds. Decreases in base saturation were observed in North Buck (0.05 < p < 0.10) and South Buck (p < 0.01), but an increase was also seen in 28014 (0.01 < p < 0.05). Increases in pH were seen in North Buck (0.05 < p < 0.10), 27020 (0.05 < p < 0.10) and 28030 (0.01 < p < 0.05). Although none of the individual watersheds showed a significant increase in Ca in the upper B horizon, when taken as a group (n = 7), a significant increase in Ca (p = 0.03) was seen in a t-test when the data were normalized to percent change per year and the value of each site was compared to a value of 0, which would be the value if no change had occurred (Figure 4). Excluding the two Buck watersheds, where the initial sampling occurred before 2000, an increase in Ca (percent change per year) was observed at p = 0.01 for the five watersheds with the most recent sampling windows.
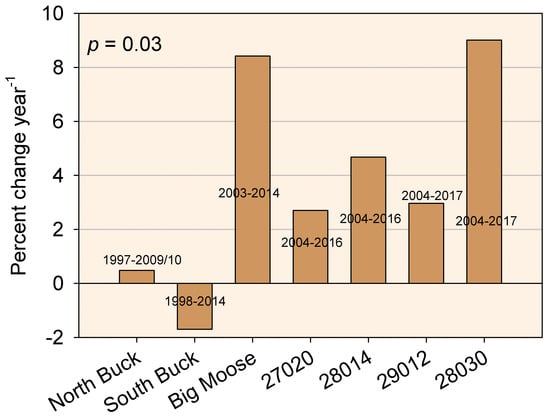
Figure 4.
The percent change year−1 for exchangeable calcium concentrations in the upper B horizons of each study watershed based on the date of initial and final sampling. Years of initial and final sampling are listed on the bars for each watershed. The significance level is indicated by p for an increase in values for the group of watersheds as a whole.
3.2. Trends in Headwater Stream Chemistry
In continuously monitored streams, annual mean concentrations of Ca2+ decreased by approximately 20 percent and 30 percent in South and North Buck, respectively, from 1999 to 2019 (Figure 5). The slope and R2 of South Buck equaled −0.88 µeq y−1 and 0.35, respectively, and the slope and R2 of North Buck equaled −1.26 µeq y−1 and 0.83, respectively. Annual mean concentrations of Ca2+ for the outlet stream of West Pond showed concentrations and a rate of decrease highly similar to those measured in North Buck (Figure 5) with a slope and R2 of −1.31 µeq y−1 and 0.84, respectively. The trends in each of these streams were approximately linear and were significant at p < 0.01. Although the North and South Buck watersheds lie in close proximity, Ca2+ concentrations at North Buck were consistently lower than at South Buck, and the difference in stream concentrations between the watersheds increased somewhat over the 20 years.
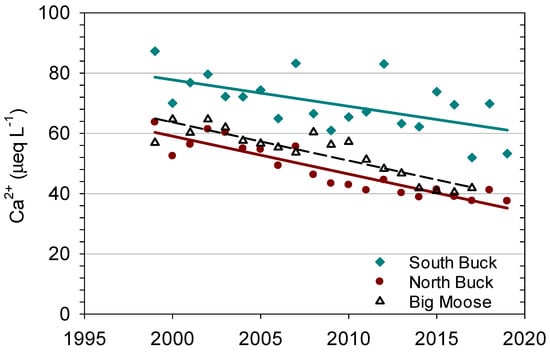
Figure 5.
Mean annual concentrations of calcium (Ca2+) in the streams of South Buck, North Buck and Big Moose during the study period.
Values from intermittent stream sampling during spring snowmelt also revealed decreases in Ca2+ concentrations over time that were similar to the biweekly and monthly data from the three continuously monitored waters. Concentrations of Ca2+ decreased sequentially from 2004–2005 to 2018–2019 in all streams except Big Moose and 29012 (Figure 6). Consistent downward values were observed at 28030, which had the highest overall Ca2+ concentrations, as well as at North Buck and 27020, which had the lowest Ca2+ concentrations. South Buck showed the most pronounced change, decreasing by 50 percent from 2004–2005 to 2018–2019. In contrast, the stream draining 29012 showed a notable increase, although data were not available for 2014–2015. Furthermore, the value for 29012 was less than half that of 28030 in 2004–2005 but greater than 28030 in 2018–2019, which reflected a change that strongly differed from the other watersheds.
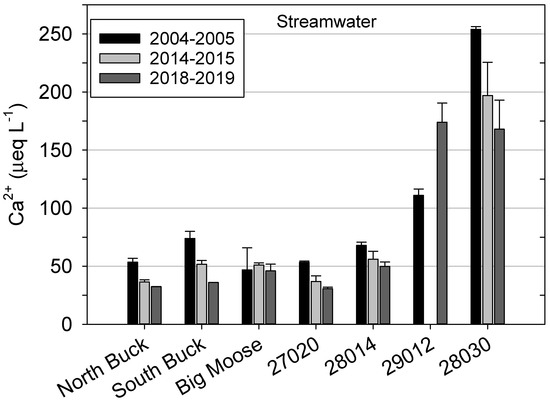
Figure 6.
Concentrations of calcium (Ca2+) averaged for samples collected in consecutive years in headwater streams sampled during spring snowmelt in the watersheds where soils were collected. The label for watershed 29012 does not appear in the review PDF.
Spatial interpolation of Ca2+ concentrations in headwater streams sampled in the WASS and the ECASS during spring snowmelt in 2018 indicated a wide range of Ca availability across the Adirondack region, although low Ca2+ concentrations were concentrated in the west (Figure 1). The spatial distribution of streams enabled interpolation of 19,766 km2 of the total 24,243 km2 that comprise the Adirondack region. All of the seven watersheds sampled for soils were in the west, and five had stream Ca2+ concentrations that fell in the category of extremely low Ca availability. The large difference in Ca2+ concentrations between the Buck Creek watersheds and the 28030 watershed, which were only 2 to 3 km apart, demonstrates that spatial variations in soil Ca availability can be high over small distances, although the region in general tends to have low Ca availability. Only six percent of the region was estimated to have extremely low Ca availability, but 29 percent of the area was estimated to have Ca2+ concentrations in streams that fell below the nutritional threshold required for many aquatic species. In nearly half of the region (48 percent), Ca availability provided only marginal acid buffering at best, which was insufficient to provide chemical conditions that would avoid aquatic ecosystem harm. Stream Ca2+ concentrations covering approximately half of the region are sufficiently high to avoid mortality of aquatic fauna from acidification and harmful Al concentrations.
3.3. Effects of Watershed Liming on Ca Availability in Soil and Stream Water
Measurements of soil chemistry prior to liming in October 2013 were generally similar in the reference (T24) and treated (T16) watersheds throughout the soil profiles (Table 4). There were no significant differences (p > 0.10) between watersheds in any of the measurements for any of the horizons. Concentrations of Ca decreased over two orders of magnitude from the Oe horizons downward to the lower B horizons, which reflected vegetative recycling in the upper profile where fine root densities were highest, as well as the higher CEC of organic horizons than mineral horizons. Concentrations of Al were lowest in the Oe and highest in the Oa, and Al comprised a much larger fraction of CEC than Ca in all of the horizons except the Oe horizon, where Al concentration was less than one tenth that of Ca concentrations. Measurements of pH indicated that some acid neutralization occurred with increasing depth in the mineral soil, but base saturation values reflected extremely acidic soil throughout the mineral profile.

Table 4.
Concentrations of exchangeable Ca and Al (cmolc kg−1), measurements of base saturation (base sat.; percent), pH and cation exchange capacity (CEC) of Oe, Oa (or A) and upper, mid and lower B horizon samples in watersheds T24 (reference) and T16 (treated) in the pretreatment year. Each value represents the average of the five pits excavated in each watershed. Base saturation and CEC were determined for individual pits prior to averaging.
No changes (p > 0.10) were observed for any of the measurements in the reference watershed between 2013 and 2018 (Table 5), except for a small decrease in Oe horizon pH (0.01 < p < 0.05). In the treated watershed, large changes were observed in the Oe horizon (p < 0.01) for each measurement (Table 5). In the Oa horizon, large increases were also observed for Ca and base saturation (p < 0.01), and a small increase was observed in pH (p < 0.05). In the upper B horizon, increases in Ca and base saturation (p < 0.01) were the only changes that were observed.

Table 5.
Concentrations of exchangeable Ca and Al (cmolc kg−1), measurements of base saturation (base sat.; percent), pH and cation exchange capacity (CEC) of Oe, Oa and upper B horizon samples from watersheds T24 (reference) and T16 (treated) in 2013 (pretreatment) and 2018. All values represent the mean of 15 samples collected in each watershed in each sampling year. Base saturation and CEC were determined on individual samples prior to averaging; sig. represents significance levels for differences between sampling years at individual watersheds abbreviated as a: p < 0.01; b: 0.01 < p < 0.05; and n: p > 0.10.
As expected, increases in Oe horizon Ca were apparent after one year (p < 0.01) in the treated watershed, but values did not change in the second year (Figure 7). However, further increases (p < 0.01) in Ca relative to the pretreatment year were observed after 3 years and 5 years (Figure 7). The linear regression indicated an increase in Ca over the 5 years in the Oa horizon of the treated watershed (p = 0.02), but a significant change relative to pretreatment was not recorded until the end of the third year (p = 0.04), and then after 5 years (p < 0.01). In the B horizon, the linear regression also indicated an increase in Ca over the 5 years in the treated watershed (Figure 7). However, only the fifth year was significantly higher (p < 0.01) than the pretreatment year. Although a significant increase was not recorded until the fifth year, the Ca concentration in the fifth year was approximately three times that of the pretreatment year.
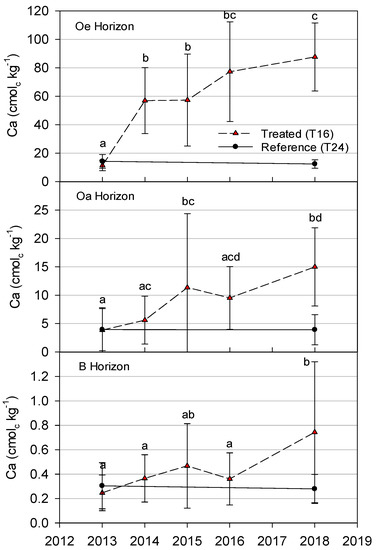
Figure 7.
Concentrations of exchangeable calcium (Ca) in Oe, Oa and upper B horizons in T16 (treated) and T24 (reference) for each year sampling was conducted. Sampling in 2013 occurred before treatment. Sampling years with the same letters are not significantly different (p > 0.10).
Concentrations of Ca2+ in stream water prior to the October 2013 treatment were somewhat higher in the watershed to be treated (T16) than in the reference watershed (T24) during this period (Figure 8). The Ca addition on 1–4 October 2013 immediately increased Ca2+ concentrations in the stream of the treated watershed from approximately 30 µeq L−1 to over 550 µeq L−1, but by October 30, concentrations had decreased to 121 µeq L−1. Concentrations continued to decrease nonlinearly through 2014–2016, reaching an apparent asymptote of approximately 70 µeq L−1 from 2017 to 2019. Although concentrations in the last 3 years plateaued, month-to-month variation increased. Over the full study period, Ca2+ concentrations in the stream of the reference watershed decreased linearly (p < 0.01) from approximately 25 to 20 µeq L−1, although a consistent seasonal pattern existed throughout the record (Figure 8). The Ca2+ values of the two watersheds prior to treatment fell in the lowest category of Ca availability in the Adirondack region (Figure 1).
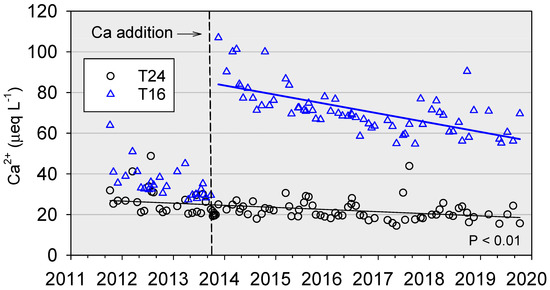
Figure 8.
Concentrations of calcium (Ca2+) in the streams of T24 (reference) and T16 (treated). The vertical line indicates the treatment date. Measurements during October 2013 are excluded for scaling, but concentrations exceeded 550 µeq L−1 shortly after treatment in early October.
4. Discussion
4.1. Possible Recovery Responses
Assessment of soil recovery from acidic deposition effects is complicated by interactions among horizons responding to the top-down forcing of decreasing acid inputs as well as the natural influences of pedogenic processes. Nevertheless, a growing number of studies are linking consequential soil changes to decreasing acidic deposition, such as increases in pH of O and upper B horizons at multiple sites in eastern North America [28]. Lower acidic deposition levels not only decrease inputs of acidity but also decrease ionic strength, which fosters disaggregation of soil particles [53] and increases organic carbon solubility [50]. Lower H2SO4 concentrations also mean lower rates of Ca leaching [54] and reduced mobility of inorganic Al [55]. Together, these changes have also led to net movement of Al out of the forest floor, thereby decreasing exchangeable Al concentrations in O horizons, and possibly increasing exchangeable Al in the upper B horizon through deposition [28]. Furthermore, forest floor increases in organic C solubility and decreases in Al can contribute to higher rates of decomposition and mineralization of organic Ca [56,57].
Changes in soils linked to decreasing acidic deposition are likely to improve overall soil fertility and enhance conditions for the biocycling of Ca, which provides a plausible explanation for the changes in Oe chemistry measured in the North and South Buck and Big Moose watersheds. The low levels and small change in atmospheric deposition of Ca over the study period are not likely to have affected exchangeable Ca concentrations to a large degree in this horizon, particularly since the pool size of Ca in the Oe horizon in 2014 exceeded 100 Mg ha−1 [31], whereas deposition was less than 1 kg ha−1 y−1. At this rate of deposition, it would take 100,000 years of deposition to equal the exchangeable Ca stored in these Oe horizons. The increase in Ca concentrations is more likely the result ofincreased root uptake, higher Ca in litterfall [58] and, possibly higher rates of upward transport of Ca from deeper in the mineral soil by increased activity of wood decay fungi [59]. The changes in Ca and Al concentrations were primarily responsible for higher Oe base saturation. However, changes in pH were minimal, as were changes in CEC, which is not unexpected due to the naturally high cation exchange capacity and acid buffering by organic anions in the Oe horizon, which is typical in forest soils [41].
Changes consistent with recovery were less apparent in the Oa horizon than in the Oe horizon for North and South Buck and Big Moose soils. The only changes that would suggest recovery were decreases in Al in North and South Buck, which were considerably less pronounced than in the Oe horizon of these watersheds, and also an increase in pH in Big Moose. Results from the WASS watersheds also suggested minimal recovery, with pH increases seen only in watersheds 27020 and 28030 and no significant changes (p > 0.10) in the other two watersheds. It should be noted, however, that non-significant decreases in Al in six of the seven watersheds did occur, and when taken as a group, the decrease in Al was significant (p = 0.03). The power of the significance testing for the individual WASS watersheds was limited by the unbalanced sample numbers of n = 5 in the initial sampling and n = 18 in the final sampling. Regardless, the less pronounced decrease in Al in the Oa horizon than the Oe horizon is likely a reflection of differences in how these horizons are formed, with the Oe being the product of litterfall and the Oa being the product of the decomposing Oe. The contact between the Oa horizon and upper mineral soil also provides greater opportunity for upward transport of Al from the mineral soil into the Oa horizon through hydrologic transport and fungal translocation [58]. Based on data from the same sites used in Lawrence et al. [28], Hazlett et al. [60] recently found that O horizon Al was most likely to have decreased in soils with an O horizon pH less than 3.5 in the initial sampling, a criteria met by all but one of the watersheds in this study. The lack of increase in Ca concentrations in the Oa suggests that Ca availability in the overall rooting zone has not increased appreciably. Because the Oa horizon is largely dependent on the Oe horizon as its direct source of Ca, efficient recycling in the Oe horizon under conditions of limited Ca reduces translocation of mineralized Ca from the Oe into the Oa horizon. Furthermore, root uptake of Ca within the Oa horizon is high, although fine root density in the Oa horizon is lower than in the Oe horizon, and higher concentrations of Al in the Oa than the Oe horizon limit Ca availability in the Oa horizon. These factors all provide potential reasons for why increases in Ca were seen in the Oe but not the Oa horizon.
Efficient recycling of Ca in the Oe and Oa horizons reduced the degree to which available Ca was depleted in these horizons from the prior elevated leaching by acidic deposition and helped to speed up recovery by transferring Ca into this horizon through root uptake deeper in the profile. However, the upper mineral soil horizon was more susceptible than organic horizons to exchangeable cation loss from mobile anion leaching due to much lower CEC and a large supply of potentially mobile secondary mineral Al. For these reasons, Ca depletion has been most acute in the upper mineral soil, which has been especially problematic because Ca released through mineral weathering in this part of the soil profile is important for maintaining Ca availability throughout the rooting zone. Availability of Ca in the upper B horizon can therefore serve as an important index of soil recovery from acidic deposition.
The most recent study across eastern North America suggested that Ca depletion in the upper B horizon has slowed or perhaps stopped, but these data do not extend past 2010, with the exception of one watershed in 2014 [28]. Although none of the individual watersheds in the study presented here showed statistically significant increases in Ca, the significant increase in Ca for the watersheds as a whole (Figure 4) suggests the possibility that Ca availability may have begun to improve in the upper B horizon in the western Adirondack region, which also tends to have the lowest Ca2+ concentrations in stream water. The base saturation results in the B horizon may also suggest signs of recovery, despite movement of Al out of the forest floor as organic horizons recover. However, base saturation responses are less consistent among watersheds. North and South Buck showed decreases in base saturation, but these watersheds had the earliest sampling window. Of the remaining five watersheds, one showed a significant increase and none showed significant decreases in base saturation. Interpretation of base saturation responses is complicated by significant increases in Al that were quite large in North and South Buck and Big Moose. These increases could reflect the movement of Al out of the Oa horizon that is being deposited in the upper B horizon under conditions of reduced Al mobility. However, ongoing mobilization of inorganic Al by strong inorganic acids could have a similar effect on B horizon base saturation. Continued inorganic anion leaching from mineralization of S and N was identified through 2015 as an ongoing problem in North and South Buck, although the effect has been decreasing [31].
4.2. Accelerating Recovery
The ranges in Ca2+ availability presented in Figure 1 relate to general geochemical and ecological relationships and are roughly based on soil conditions and acidic deposition levels for 2018, the year stream samples were collected. The range of extremely low Ca availability corresponds to minimal acid buffering; therefore, Adirondack streams with Ca2+ concentrations lower than 50 µeq L−1 are likely to experience periods of acidification that produce harmful Al concentrations [31]. Concentrations of Ca2+ lower than 75 µeq L−1 provide insufficient nutrition for the survival of key species in aquatic food webs [30], as well as low acid-buffering capacity. Concentrations of Ca2+ between 75 and 95 µeq L−1 provide marginal acid buffering. Concentrations of Ca2+ above 95 µeq L−1 are likely to provide sufficient acid buffering to avoid harmful acidification during most flow conditions. Streams with Ca2+ concentrations above 145 µeq L−1 can be considered well buffered, and streams with Ca2+ concentrations above 250 µeq L−1 can be considered extremely well buffered. These categories of Ca2+ concentrations in headwater streams indicate that even with the current low levels of acidic deposition, nearly half of the Adirondack region is experiencing impairment from low Ca availability. Figure 1 also indicates that a large portion of the western Adirondack region is still subject to severe acidification, with questionable ability for recovery.
The streams in the Honnedaga watersheds fall in this category of ongoing severe acidification (Figure 1). The soil sampling in the reference watershed T24 in 2013 and 2018 did not indicate any increases in exchangeable Ca concentrations in soil over this period. Furthermore, recent modeling of 25 Adirondack watersheds using PnET-BGC showed no change in average B horizon base saturation for these watersheds through the year 2200 [7]. Included in the group of modeled watersheds were North and South Buck, and a watershed 1.5 km from 27020 (watershed 27026), with highly similar soil Ca and Al concentrations. Model projections for those three watersheds indicated that past base cation depletion was not sufficiently reversible to increase the acid-neutralizing capacity (ANC) of stream water to within 20 µeq L−1 of the modeled pre-industrial value of ANC, even if acidic deposition was decreased to zero [7]. This information, coupled with soil monitoring data presented herein showing limited recovery of soil Ca availability to date, suggests that any further increases in soil Ca availability in these systems will occur slowly even with the current low levels of acidic deposition.
The lime addition to T16 provided a relatively rapid boost to the pool of available Ca that extended into the mineral soil within 5 years. The input of CaCO3 neutralized soil acidity in addition to increasing exchangeable Ca concentrations, which resulted in large changes in soil chemistry that were largely restricted to the Oe horizon. Nevertheless, even with the two-unit increase in Oe horizon pH, the soil pH did not increase above a mildly acidic value of 5.25 by 2018. The decrease in Al in the Oe horizon was also large but similar to the decreases measured in untreated North Buck and Big Moose. However, at T16, the decrease occurred in 5 years, whereas the decreases at North Buck and Big Moose occurred in 11 and 12 years, respectively. Chemical changes in the T16 Oa horizon were much less pronounced than in the Oe horizon. Increases in Ca and base saturation were relatively small, no change occurred in Al and the pH increase of 0.27 was minimal. In the B horizon, there was no change in pH or Al. The T16 results for Al and pH did not stand out from the reference watershed T16 or from the results of the other untreated watersheds for the Oa or B horizon, suggesting that the lime dose would not cause a severe neutralization response in low-Ca soils in this region. The T16 treatment was successful in increasing available Ca throughout the primary rooting zone without causing a large degree of acid neutralization in the profile of this naturally acidic soil. Increases in Ca down into the B horizon without a large reduction in soil acidity were helped by the low levels of mobile inorganic anion leaching in the five post-treatment years.
The effects of the T16 liming on Ca availability showed both differences and similarities to the effects of adding wollastonite (CaSiO3) to a forested watershed (W1) in the White Mountains of New Hampshire, USA [61]. The W1 watershed was similar to T16 and T24 in terms of vegetation, climate, slow weathering geologic materials and Ca-depleted soils [62]. Prior to treatments, Ca concentrations in the Oe, Oa and upper B horizons were highly similar between T16 and W1. The largest difference between treatment effects in these experiments occurred in the Oe horizon, where the exchangeable Ca concentration after the first treatment year (and each subsequent year) at T16 was at least 35% higher than that measured at W1 during any year of that study, which lasted 11 years. The T16 dose of 1.4 Mg Ca ha−1 was similar to the W1 dose of 1.3 Mg Ca ha−1 [63], so the difference in Oe-exchangeable Ca concentrations can be attributed to the silicate structure of wollastonite, which results in a slower weathering rate than that of CaCO3. However, in the Oa horizon, the year three Ca concentration at T16 was nearly identical to the year three value at W1, and the T16 value for year five was close to that of year seven at W1 (T16: 15 cmolc kg−1; W1: 13.5 cmolc kg−1). Sampling was not performed at W1 in years five or six. Concentrations of Ca in the upper B horizon of T16 in year five were about one-third of those in W1 in year seven.
The faster weathering rate of lime than wollastonite suggests that with similarity of added Ca doses and response periods, Ca concentrations would be considerably greater in the Oa and upper B horizons at T16 than at W1. The similarity of the results seen in the Oa and upper B horizons is contrary to this expectation and may be related to different acidic deposition levels when the experiments were conducted. In the first 7 years of treatment in the W1 experiment, which occurred from 1999 to 2006, acidic deposition levels decreased from approximately 8 to 6.5 kg S ha−1 y−1 (http://nadp.slh.wisc.edu/data/NTN/; NADP site NH02, accessed on 9 September 2020), whereas deposition of sulfur in the central Adirondack region decreased from about 1.5 to 1.0 kg S ha−1 y−1 during the five post-treatment years in T16 (http://nadp.slh.wisc.edu/data/NTN/; NADP site NY20, accessed on 9 September 2020). The lower S deposition rate following the T16 treatment than that following the W1 treatment meant that weathering and leaching by mobile inorganic anions was likely to have been much higher in W1 than T16 than W1, which would increase the movement of Ca from the Oe horizon into the lower horizons in W1. With less leaching pressure, the added Ca at T16 was better retained within the rooting zone in an available form, which increased the opportunity for Ca biocycling, and aided in the retention of exchangeable Ca in the upper soil. This interpretation is supported by Hazlett et al. [60] who found that past S deposition levels were inversely related to the recovery potential of the B horizon.
By reducing the leaching of Ca added to T16, the lowered rates of acidic deposition enhanced the effectiveness of the treatment in accelerating increases in soil Ca availability. However, better Ca retention within the terrestrial ecosystem meant that less Ca reached the stream. This effect applies to recovering untreated watersheds as well, as seen in the ongoing decreasing trends of Ca in the streams. In some watersheds, dilution of surface waters is likely to progress beyond pre-acid rain conditions. To reverse this process, Ca leaching in these watersheds needs to shift from SO42− and NO3− control to HCO3− control, which will require s further reductions in soil acidity.
The effects of soil recovery reported here, whether from decreased acidic deposition or a combination of decreased acidic deposition and liming, have ecological significance for both aquatic and terrestrial ecosystems. The large decreases in Oe horizon Al in North Buck, South Buck and Big Moose as well as T16 imply a substantial decrease in Al mobility in the upper soil [28], which is the key to reducing surface water concentrations of harmful Al [55]. Josephson et al. [64] previously reported that prior to the T16 liming in 2013, inorganic Al concentrations in the T16 stream were above 4 µmol L−1 in nearly all samples, but from 2014 to 2016 concentrations were below 2.0 µmol L−1, the threshold needed for large reductions in brook trout mortality. Terrestrial effects of the wollastonite addition that caused increases in soil Ca which were similar to the T16 treatment were apparent in increases of sugar maple seedling density and growth within 2 to 4 years of treatment and in the improved crown condition of sugar maple in 6 years [65]. Soil Ca availability has also been strongly linked to stress resistance in trees [66]. The wollastonite addition provided experimental evidence of improved stress resistance in red spruce when the effects of a regional winter injury event were substantially reduced within the treated watershed relative to untreated adjacent areas [67].
5. Conclusions
The most recent resampling data obtained from these seven Adirondack watersheds provide the first evidence suggesting some reversal of Ca depletion in the B horizon in regions of North America that have been impacted by acidic deposition. These results also suggest the possibility of soil Ca recovery across the southwestern portion of the Adirondack region, which has probably experienced a greater degree of soil Ca depletion than any other area in the Adirondack region. The possibility that these soils are beginning to recover is supported by recent eastern North American results that showed the highest recovery potential in soils that were the most affected by acidic deposition and that soil recovery potential was more strongly tied to the chemical conditions of the upper profile than soil parent material [60]. However, the finding of Hazlett et al. [60] of an inverse relationship between past S deposition levels and recovery potential of the B horizon suggests that the acidic deposition impacts on this horizon in the Adirondack region, and likely, elsewhere, will be reversed slowly. The role of parent material ultimately must play a role in recovery but may operate at a longer time scale than recovery processes in the upper soil, which are more directly linked with acidic deposition levels and vegetative processes.
Confirmation of a widespread recovery of soils in the Adirondack region is limited by the number of watersheds with resampling data, the duration of resampling windows and the length of time over which acidic deposition levels have been near pre-industrial levels. Determining if regional recovery of Ca availability has begun in the upper mineral soil, as well as the forest floor, and determining the rate at which it is occurring will require monitoring of a larger number of watersheds to better account for innate variations in soils among watersheds that cause variations in recovery responses.
The continued monitoring of soil changes will be critical for understanding how soils are affecting forests and aquatic ecosystems, and this study suggests that some changes are occurring at less than a decadal timescale. Results from the T16 experiment also showed that moderate application of CaCO3 can markedly increase soil Ca availability within 5 years and improve water quality without severe effects on the naturally acidic soil ecosystem. It is important to note, however, that the efficacy of the lime treatment was dependent on the extremely low levels of acidic deposition in the period following treatment. For selected high-value watersheds that have undergone a high degree of soil Ca depletion, lime treatment may be a useful approach for accelerating recovery, but whether through the use of lime additions or reliance on natural processes, recovery of soil Ca depletion will be most readily achieved through the lowest acidic deposition levels attainable.
Supplementary Materials
The following are available online at https://www.mdpi.com/2571-8789/5/1/6/s1, Table S1: National Water Information System (NWIS) codes, project codes and location coordinates for all streams sampled in the study.
Author Contributions
G.L. contributed to this paper in the areas of conceptualization by identifying the problem, developing the research program that produced this paper, participating in the field program, writing the paper and working with co-authors on editing. J.S. contributed to this paper in the areas of method development to improve sampling techniques for detection of soil changes, investigation to collect samples and field data, organization of the field program and review and editing of the manuscript. M.A. contributed to conceptualization through original research on which some of this paper is based, and to the field investigation work. D.B. contributed to the paper by working on the field and laboratory components of the investigation and the development of field methods. M.M. contributed to this paper by playing a large role in the conceptualization of the research, supervising field and laboratory work in the investigation, assisting in the funding acquisition and reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the New York State Energy Research and Development Authority under Agreements 34356 and 122529.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to develop this paper are publicly available at the links provided in the references cited in the text.
Acknowledgments
Support for this work was provided by the New York State Energy Research and Development Authority (NYSERDA), the New York State Department of Environmental Conservation (DEC), the U.S. Geological Survey, the Adirondack Long-Term Monitoring Program conducted by the Adirondack Lake Survey Corporation (ALSC) and the Adirondack Effects Assessment Program (AEAP). Special thanks to all those who participated in the WASS and ECASS sampling, especially Barry Baldigo and Susan Capone, who provided valuable leadership in those arduous field programs. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McLaughlin, S.B.; Wimmer, R. Tansley Review No. 104. Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Beier, C.M.; Woods, A.M.; Hotopp, K.P.; Gibbs, J.P.; Mitchell, M.J.; Dovčiak, M.; Leopold, D.J.; Lawrence, G.B.; Page, B.D. Changes in faunal and vegetation communities along a soil calcium gradient in northern hardwood forests. Can. J. For. Res. 2012, 42, 1141–1152. [Google Scholar] [CrossRef]
- Jeziorski, A.; Smol, J.P. The ecological impacts of lakewater calcium decline on softwater boreal ecosystems. Environ. Rev. 2017, 25, 245–253. [Google Scholar] [CrossRef]
- Lawrence, G.B.; David, M.B.; Lovett, G.M.; Murdoch, P.S.; Burns, D.A.; Stoddard, J.L.; Baldigo, B.P.; Porter, J.H.; Thompson, A.H. Soil calcium status and the response of stream chemistry to changing acidic deposition rates. Ecol. Appl. 1999, 9, 1059–1072. [Google Scholar] [CrossRef]
- Warby, R.A.F.; Johnson, C.E.; Driscoll, C.T. Chemical recovery of surface waters across the northeastern United States from reduced inputs of acidic deposition: 1984−2001. Environ. Sci. Technol. 2005, 39, 6548–6554. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.; Larssen, T.; Camarero, L.; Cosby, B.J.; Ferrier, R.C.; Helliwell, R.; Forsius, M.; Jenkins, A.; Kopáček, J.; Majer, V.; et al. Recovery of acidified European surface waters. Environ. Sci. Technol. 2005, 39, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Driscoll, C.T.; Sullivan, T.J.; Burns, D.A.; Baldigo, B.; Lawrence, G.B.; McDonnell, T.C. The response of stream ecosystems in the Adirondack region of New York to historical and future changes in atmospheric deposition of sulfur and nitrogen. Sci. Total Environ. 2020, 716, 137113. [Google Scholar] [CrossRef] [PubMed]
- Markewitz, D.; Richter, D.D.; Allen, H.L.; Urrego, J.B. Three decades of observed soil acidification in the Calhoun experimental forest: Has acid rain made a difference? Soil Sci. Soc. Am. J. 1998, 62, 1428–1439. [Google Scholar] [CrossRef]
- Fernandez, I.J.; Rustad, L.; Norton, S.A.; Kahl, J.S.; Jackson Cosby, B., Jr. Experimental acidification causes soil base cation depletion in a New England forested watershed. Soil Sci. Soc. Am. J. 2003, 67, 1909–1919. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Adams, M.B.; Peterjohn, W.T. Response of soil fertility to 25 years of experimental acidification in a temperate hardwood forest. J. Environ. Qual. 2020, 49, 961–972. [Google Scholar] [CrossRef]
- Long, R.P.; Horsley, S.B.; Hallett, R.A.; Bailey, S.W. Sugar maple growth in relation to nutrition and stress in the northeastern United States. Ecol. Appl. 2009, 19, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Page, B.D.; Mitchell, M.J. The influence of basswood (Tilia americana) and soil chemistry on soil nitrate concentrations in a northern hardwood forest. Can. J. For. Res. 2008, 38, 667–676. [Google Scholar] [CrossRef]
- Zarfos, M.R.; Dovciak, M.; Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J. Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States. Plant Soil 2019, 438, 461–477. [Google Scholar] [CrossRef]
- Horsley, S.B.; Bailey, S.W.; Risteau, T.E.; Long, R.P.; Hallett, R.A. Linking environmental gradients, species composition, and vegetation indicators of sugar maple health in the northeastern United States. Can. J. For. Res. 2008, 38, 1761–1774. [Google Scholar] [CrossRef]
- Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J.; Dovciak, M.; Bailey, S.W.; Antidormi, M.R.; Zarfos, M.R. Soil base saturation combines with beech bark disease to influence composition and structure of sugar maple-beech forests in an acid-rain impacted region. Ecosystems 2018, 21, 795–810. [Google Scholar] [CrossRef]
- Pabian, S.E.; Brittingham, M.C. Terrestrial liming benefits birds in an acidified forest in the northeast. Ecol. Appl. 2007, 17, 2184–2194. [Google Scholar] [CrossRef]
- Leach, T.H.; Winslow, L.A.; Hayes, N.M.; Rose, K.C. Decoupled trophic responses to long-term recovery from acidification and associated browning in lakes. Glob. Chang. Biol. 2019, 25, 1779–1792. [Google Scholar] [CrossRef]
- Johnson, A.H.; Moyer, A.; Bedison, J.E.; Richter, S.L.; Willig, S.A. Seven decades of calcium depletion in organic horizons of adirondack forest soils. Soil Sci. Soc. Am. J. 2008, 72, 1824–1830. [Google Scholar] [CrossRef]
- Watmough, S.A.; Dillon, P.J. Major element fluxes from a coniferous catchment in central Ontario, 1983–1999. Biogeochemistry 2004, 67, 369–399. [Google Scholar] [CrossRef]
- Warby, R.A.F.; Johnson, C.E.; Driscoll, C.T. Continuing acidification of organic soils across the northeastern USA: 1984–2001. Soil Sci. Soc. Am. J. 2009, 73, 274–284. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Lapenis, A.G.; Berggren, D.; Aparin, B.F.; Smith, K.T.; Shortle, W.C.; Bailey, S.W.; Varlyguin, D.L.; Babikov, B. Climate dependency of tree growth suppressed by acid deposition effects on soils in Northwest Russia. Environ. Sci. Technol. 2005, 39, 2004–2010. [Google Scholar] [CrossRef]
- Billett, M.F.; Parker-Jervis, F.; Fitzpatrick, E.A.; Cresser, M.S. Forest soil chemical changes between 1949/50 and 1987. Eur. J. Soil Sci. 1990, 41, 133–145. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Linnermark, N.; Tyler, G. Changes in acidity and cation pools of south Swedish soils between 1949 and 1985. Chemosphere 1987, 16, 2239–2248. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, H.Y.H.; Searle, E.B.; Sardans, J.; Ciais, P.; Peñuelas, J.; Huang, Z. Whole soil acidification and base cation reduction across subtropical China. Geoderma 2020, 361, 114107. [Google Scholar] [CrossRef]
- Cools, N.; De Vos, B. Availability and evaluation of European forest soil monitoring data in the study on the effects of air pollution on forests. iForest Biogeosciences For. 2011, 4, 205–211. [Google Scholar] [CrossRef]
- Berger, T.W.; Türtscher, S.; Berger, P.; Lindebner, L. A slight recovery of soils from Acid Rain over the last three decades is not reflected in the macro nutrition of beech (Fagus sylvatica) at 97 forest stands of the Vienna Woods. Environ. Pollut. 2016, 216, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Prietzel, J.; Falk, W.; Reger, B.; Uhl, E.; Pretzsch, H.; Zimmermann, L. Half a century of Scots pine forest ecosystem monitoring reveals long-term effects of atmospheric deposition and climate change. Glob. Chang. Biol. 2020. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Hazlett, P.W.; Fernandez, I.J.; Ouimet, R.; Bailey, S.W.; Shortle, W.C.; Smith, K.T.; Antidormi, M.R. Declining acidic deposition begins reversal of forest-soil acidification in the northeastern U.S. and eastern Canada. Environ. Sci. Technol. 2015, 49, 13103–13111. [Google Scholar] [CrossRef]
- Fraser, O.L.; Bailey, S.W.; Ducey, M.J. Decadal change in soil chemistry of northern hardwood forests on the White Mountain National Forest, New Hampshire, USA. Soil Sci. Soc. Am. J. 2019, 83. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Hartmann, J.; Hessen, D.O.; Kopáček, J.; Hejzlar, J.; Jacquet, S.; Hamilton, S.K.; Verburg, P.; Leach, T.H.; Schmid, M.; et al. Widespread diminishing anthropogenic effects on calcium in freshwaters. Sci. Rep. 2019, 9, 10450. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Scanga, S.E.; Sabo, R.D. Recovery of soils from acidic deposition may exacerbate nitrogen export from forested watersheds. JGR Biogeosciences 2020, 125, e2019JG005036. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Driscoll, K.M.; Fakhraei, H.; Civerolo, K. Long-term temporal trends and spatial patterns in the acid-base chemistry of lakes in the Adirondack region of New York in response to decreases in acidic deposition. Atmos. Environ. 2016, 146, 5–14. [Google Scholar] [CrossRef]
- Sullivan, T.; Cosby, B.J.; Driscoll, C.T.; McDonnell, T.C.; Herlihy, A.T.; Burns, D.A. Target loads of atmospheric sulfur and nitrogen deposition for protection of acid sensitive aquatic resources in the Adirondack Mountains, New York. Water Resour. Res. 2012, 48, 01547. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Burns, D.A.; Riva-Murray, K. A new look at liming as an approach to accelerate recovery from acidic deposition effects. Sci. Total Environ. 2016, 562, 35–46. [Google Scholar] [CrossRef]
- Baldigo, B.P.; George, S.D.; Lawrence, G.B.; Paul, E.A. Declining aluminum toxicity and the role of exposure duration on brook trout mortality in acidified streams of the Adirondack Mountains, New York, USA. Environ. Toxicol. Chem. 2020, 39, 623–636. [Google Scholar] [CrossRef] [PubMed]
- McNab, W.H.; Avers, P.E. Ecological subregions of the United States, Chapter 15—Adirondack Highlands, U.S. Department of Agriculture, Forest Service, W0-WSA-5. 1994. Available online: https://www.fs.fed.us/land/pubs/ecoregions/ (accessed on 1 December 2020).
- Influences of Wetlands and Lakes in the Adirondack Park of New York State: A Catalogue of Existing and New GIS Layers for the 400,000 Hectare Oswegatchie/Black River Watershed, Adirondack Park Agency; Final Report. Available online: https://apa.ny.gov/gis/gis_cd.html (accessed on 1 December 2020).
- Murdoch, P.S. Water budget comparison of two headwater lake basins subjected to low pH precipitation in the western Adirondack Mountains, New York. In Geology; SUNY: Binghamton, NY, USA, 1982; p. 98. [Google Scholar]
- Ito, M.; Mitchell, M.J.; Driscoll, C.T. Spatial patterns of precipitation quantity and chemistry and air temperature in the Adirondack region of New York. Atmos. Environ. 2002, 36, 1051–1062. [Google Scholar] [CrossRef]
- Lawrence, G.; Momen, B.; Roy, K.M. Use of stream chemistry for monitoring acidic deposition effects in the Adirondack region of New York. J. Environ. Qual. 2004, 33, 1002–1009. [Google Scholar] [CrossRef]
- Sullivan, T.J.; Fernandez, I.J.; Herlihy, A.T.; Driscoll, C.T.; McDonnell, T.C.; Nowicki, N.A.; Snyder, K.U.; Sutherland, J.W. Acid-base characteristics of soils in the Adirondack Mountains, New York. Soil Sci. Soc. Am. J. 2006, 70, 141–152. [Google Scholar] [CrossRef]
- Lawrence, G.; Shortle, W.C.; David, M.B.; Smith, K.T.; Warby, R.A.F.; Lapenis, A.G. Early indications of soil recovery from acidic deposition in U.S. red spruce forests. Soil Sci. Soc. Am. J. 2012, 76, 1407–1417. [Google Scholar] [CrossRef]
- Minocha, R.; Shortle, W.C.; Lawrence, G.B.; David, M.B.; Minocha, S.C. Relationships among foliar chemistry, foliar polyamines, and soil chemistry in red spruce trees growing across the northeastern United States. Plant Soil 1997, 191, 109–122. [Google Scholar] [CrossRef]
- Results of the 2003–2005 Western Adirondack Stream Survey (WASS); NYSERDA Report 08-22; New York State Energy Research and Technology Authority: Albany, NY, USA, 2008. Available online: https://www.nyserda.ny.gov/-/media/Files/Publications/Research/Environmental/EMEP/Western-Adirondack-Stream-Survey.pdf (accessed on 1 December 2020).
- Driscoll, C.T.; Cirmo, C.P.; Fahey, T.J.; Blette, V.L.; Bukaveckas, P.A.; Burns, D.A.; Gubala, C.P.; Leopold, D.J.; Newton, R.M.; Raynal, D.J.; et al. The experimental watershed liming study: Comparison of lake and watershed neutralization strategies. Biogeochemistry 1996, 32, 143–174. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Antidormi, M.R.; McDonnel, T.C.; Sullivan, T.J.; Bailey, S.W. Adirondack New York Soil Chemistry Data, 1992–2017 (ver. 1.1, December 2020); U.S. Geological Survey: Reston, VA, USA, 2020.
- Lawrence, G.B. Honnedaga Liming Project Soil and Vegetation Data, 2012–2018, Adirondack Region, New York, USA; U.S. Geological Survey: Reston, VA, USA, 2020.
- Lawrence, G.B.; Fernandez, I.J.; Hazlett, P.W.; Bailey, S.W.; Ross, D.S.; Villars, T.R.; Quintana, A.; Ouimet, R.; McHale, M.R.; Johnson, C.E.; et al. Methods of soil resampling to monitor changes in the chemical concentrations of forest soils. J. Vis. Exp. 2016, e54815. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.B.; George, S.D.; Burns, D.A.; Baldigo, B.P.; Roy, K.M.; Passy, S.I.; Pound, K.L. Results of the 2010–2011 East-Central Adirondack Stream Survey (ECASS); NYSERDA Report 18–26; New York State Energy Research and Development Authority: New York, NY, USA, 2018. Available online: https://www.nyserda.ny.gov/About/Publications/Research-and-Development-Technical-Reports/Environmental-Research-and-Development-Technical-Reports#eco (accessed on 1 December 2020).
- Lawrence, G.B.; Roy, K.M. Ongoing increases in dissolved organic carbon are sustained by decreases in ionic strength rather than decreased acidity in waters recovering from acidic deposition. Sci. Total Environ. 2020, 2020, 142529. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. USGS Water Data for the Nation: U.S.; Geological Survey National Water Information System Database. 2020. Available online: http://dx.doi.org/10.5066/F7P55KJN. (accessed on 1 March 2020).
- Sibson, R. A brief description of natural neighbor interpolation. In Interpolating Multivariate Data; John Wiley & Sons: New York, NY, USA, 1981; pp. 21–36. [Google Scholar]
- Cincotta, M.M.; Perdrial, J.N.; Shavitz, A.; Libenson, A.; Landsman-Gerjoi, M.; Perdrial, N.; Armfield, J.; Adler, T.; Shanley, J.B. Soil aggregates as a source of dissolved organic carbon to streams: An experimental study on the effect of solution chemistry on water extractable carbon. Front. Environ. Sci. 2019, 7, 172. [Google Scholar] [CrossRef]
- Johnson, J.; Pannatier, E.G.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Palmer, S.M.; Driscoll, C.T. Decline in mobilization of toxic aluminum. Nature 2002, 417, 242–243. [Google Scholar] [CrossRef]
- Dijkstra, F.A. Calcium mineralization in the forest floor and surface soil beneath different tree species in the northeastern US. For. Ecol. Manag. 2003, 175, 185–194. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Fitzhugh, R.D. Aluminum solubility and mobility in relation to organic carbon in surface soils affected by six tree species of the northeastern United States. Geoderma 2003, 114, 33–47. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Shortle, W.C.; Smith, K.T.; Jellison, J.; Schilling, J.S. Potential of decaying wood to restore root-available base cations in depleted forest soils. Can. J. For. Res. 2012, 42, 1015–1024. [Google Scholar] [CrossRef]
- Hazlett, P.; Emilson, C.E.; Lawrence, G.; Fernandez, I.J.; Ouimet, R.; Bailey, S.W. Reversal of forest soil acidification in the northeastern United States and eastern Canada: Site and soil factors contributing to recovery. Soil Syst. 2020, 4, 54. [Google Scholar] [CrossRef]
- Johnson, C.E.; Driscoll, C.T.; Blum, J.D.; Fahey, T.J.; Battles, J.J. Soil chemical dynamics after calcium silicate addition to a northern hardwood forest. Soil Sci. Soc. Am. J. 2014, 78, 1458–1468. [Google Scholar] [CrossRef]
- Likens, G.; Driscoll, C.; Buso, D.; Siccama, T.; Johnson, C.; Lovett, G.; Fahey, T.; Reiners, W.; Ryan, D.; Martin, C.; et al. The biogeochemistry of calcium at Hubbard Brook. Biogeochemistry 1998, 41, 89–173. [Google Scholar] [CrossRef]
- Cho, Y.; Driscoll, C.T.; Johnson, C.E.; Siccama, T.G. Chemical changes in soil and soil solution after calcium silicate addition to a northern hardwood forest. Biogeochemistry 2009, 100, 3–20. [Google Scholar] [CrossRef]
- Josephson, D.C.; Lawrence, G.B.; George, S.D.; Siemion, J.; Baldigo, B.P.; Kraft, C. Response of water chemistry and young-of-year brook trout to channel and watershed liming in streams showing lagging recovery from acidic deposition. Water Air Soil Pollut. 2019, 230, 144. [Google Scholar] [CrossRef]
- Juice, S.M.; Fahey, T.J.; Siccama, T.G.; Driscoll, C.T.; Denny, E.G.; Eagar, C.; Cleavitt, N.L.; Minocha, R.; Richardson, A.D. Response of sugar maple to calcium addition to northern hardwood forest. Ecology 2006, 87, 1267–1280. [Google Scholar] [CrossRef]
- Hallett, R.A.; Bailey, S.W.; Horsley, S.B.; Long, R.P. Influence of nutrition and stress on sugar maple at a regional scale. Can. J. For. Res. 2006, 36, 2235–2246. [Google Scholar] [CrossRef]
- Hawley, G.J.; Schaberg, P.G.; Eagar, C.; Borer, C.H. Calcium addition at the Hubbard Brook Experimental Forest reduced winter injury to red spruce in a high-injury year. Can. J. For. Res. 2006, 36, 2544–2549. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).