Abstract
Soil CO2 efflux (FCO2) is a major component of the terrestrial carbon (C) cycle but challenges in explaining local variability hamper efforts to link broad-scale fluxes to their biotic drivers. Trees are the dominant C source for forest soils, so linking tree properties to FCO2 could open new avenues to study plant-soil feedbacks and facilitate scaling; furthermore, FCO2 responds dynamically to meteorological conditions, complicating predictions of total FCO2 and forest C balance. We tested for proximity effects of individual Acer saccharum Marsh. trees on FCO2, comparing FCO2 within 1 m of mature stems to background fluxes before and after an intense rainfall event. Wetting significantly increased background FCO2 (6.4 ± 0.3 vs. 8.6 ± 0.6 s.e. μmol CO2 m−2s−1), with a much larger enhancement near tree stems (6.3 ± 0.3 vs. 10.8 ± 0.4 μmol CO2 m−2s−1). FCO2 varied significantly among individual trees and post-rain values increased with tree diameter (with a slope of 0.058 μmol CO2 m−2s−1cm−1). Post-wetting amplification of FCO2 (the ‘Birch effect’) in root zones often results from the improved mobility of labile carbohydrates and further metabolization of recalcitrant organic matter, which may both occur at higher densities near larger trees. Our results indicate that plant-soil feedbacks change through tree ontogeny and provide evidence for a novel link between whole-system carbon fluxes and forest structure.
1. Introduction
Soil carbon dioxide efflux (FCO2) is the predominant contributor of CO2 to the atmosphere from terrestrial ecosystems, with the balance between net photosynthesis and FCO2 largely determining whether a given ecosystem constitutes a net carbon (C) source or sink [1]. Recent decades have seen a shift in how FCO2 is conceptualized—from a flux largely reflecting ecosystem-specific decomposition and its response to soil temperature and moisture, to a process highly influenced by active and mutual exchanges between plants and soil biota [2]. Such feedbacks between plants and soil biota play a stabilizing role in numerous ecosystem functions and allow for plant regulation of biogeochemical cycles [3,4]; however, the specific role of biota is often neglected in studies of FCO2 conducted at higher levels of aggregation, because belowground sources are difficult to disentangle, and because abiotic signals, especially temperature, may be more clearly expressed on spatially integrated data [2]. As a result, the representation of plants and associated biota in ecosystem process models remains rather simplistic and temperature generally stands as the most common driving variable considered in ecosystem models of soil C processes [5,6,7]. However, without deeper insight into the biotic drivers of soil FCO2, it will remain extremely challenging to link localized observations to broader scale, ecosystem-level fluxes and to develop process-driven predictions of future FCO2 dynamics [2,4].
Studies quantifying FCO2 in forest ecosystems commonly find high spatial variability that is not readily explained by edaphic or other environmental variables [8,9,10]. Spatial patchiness in FCO2 partially derives from the poor mobility of belowground C inputs through the soil matrix, with further temporal variability contributed by the responses of autotrophs and heterotrophs to short timescale (hourly to daily) meteorological variability [11,12]. A prominent phenomenon exemplifying the spatiotemporal complexity of localized FCO2 involves the dramatic spikes in FCO2 that follow rainfall events, also known as the ‘Birch effect’ [13,14]. The Birch effect is commonly attributed to the sudden mobilization of labile carbon inputs (e.g., energy-rich carbohydrates from litter and root exudates) by rainwater, which primes microbial assemblages, allowing further decomposition of lower-quality organic matter (e.g., from macroscopic necrotic tissues and humified organic matter) [14]. Increased osmoregulatory activity and rapid turnover of lysed microbes may also contribute to enhanced C fluxes [14]. Linking biotic sources of FCO2 to the broad-scale outputs of ecosystem models may therefore require attention to high temporal variability in meteorology and could further benefit from spatially explicit consideration of C inputs [15,16].
FCO2 depends directly on primary productivity, and, surprisingly, productivity effects can be even larger than temperature effects [17,18]. Tree girdling experiments and isotopic tracing measurements suggest that as much as 50% of CO2 emitted from forest soils is sustained by recently produced photosynthates [19,20], implying that physiological changes occurring in tree canopies likely impact FCO2. The ability to isolate the contribution of individual trees to FCO2 has further been demonstrated in a sparsely-treed savanna, where the diurnal pattern of FCO2 in individual tree root-zones was larger in amplitude than could be explained by temperature, and lagged the diurnal up-regulation of photosynthesis, corresponding to the transport time of sugars from foliage to roots [21]. Recognizing the potential to isolate tree-level FCO2 motivates further efforts to link such observations to traits governing the quantities of C exported from trees to external C sinks [4].
A number of recent studies have documented detectable local effects of tree proximity and even tree species on FCO2 [3,22,23,24], but the hypothetical mechanisms behind these effects are numerous and remain poorly resolved. Studies have also described effects of tree size on FCO2 in forest ecosystems, generally finding higher FCO2 in the immediate neighborhood of larger trees [25,26,27,28,29,30]. However, counterexamples exist [10,31], and several chrono sequence studies have found reduced FCO2 with stand age in even-aged plantations [32,33], or no consistent relationship [34]. Rodríguez-Calcerrada et al., [35] tested for associations between visible crown health indicators and FCO2 in an open woodland in Spain but found that increased plant recruitment near declining trees offset potential reductions in FCO2 related to tree decline. The character of C supply could theoretically shift as trees age from active extrusion of labile root exudates by more productive trees to less bioavailable necrotic tissues, but the connection between local tree effects on FCO2 and tree senescence or health status has received little attention.
Age-related trends in tree physiology are particularly large and well-documented in Acer saccharum Marsh. (hereafter sugar maple), a dominant tree in northern hardwood forests in Eastern North America. Leaf-level photosynthetic capacity peaks at intermediate sizes and declines later in ontogeny [36], and there is evidence for declines in whole-tree leaf area through ontogeny [37]. These trends are expected to constrain the amount of C available for belowground allocation, consistent with age-related declines in concentrations of non-structural carbohydrates in other sinks, such as wood [38]. As the capacity to replace fine roots declines with overall function, roots are more likely to be constructed to last longer, have increased C:N ratios, and be more resistant to decomposition [39]. If accumulation of high-C:N organic matter in tree root zones is an important chemical change that occurs through ontogeny, then the moisture-induced priming of microbial communities (Birch effect) may likewise vary through tree ontogeny. Additional processes associated with large old trees that may affect FCO2 include the potential for warmer soils beneath sparser canopies and increased maintenance respiration of older root tissue, paralleling age-related trends in leaf respiration [40]. The magnitude of Birch-effect FCO2 pulses could be more pronounced near large trees, but studies to date have not examined potential local tree size influences on Birch effect peaks in FCO2.
In the present study we collected spatially explicit FCO2 data in a large mapped forest plot to address the following questions: (1) Does FCO2 in the immediate vicinity of adult sugar maple stems differ from background soil flux? (2) Is there detectable tree-to-tree variation in root zone FCO2, and if so, is such variation dependent on tree diameter, growth rate, or health status? (3) How does FCO2 in background vs. root-zone locations respond to soil conditions, in particular wetting following an intense rainfall event?
2. Materials and Methods
2.1. Study Site
The Haliburton Forest Dynamics Plot (HFDP), is located within Haliburton Forest and Wildlife Reserve Ltd., Ontario, Canada (44°55′ N, 78°45′ W), and belongs to the CTFS-ForestGEO global network of large-scale forest research plots [41]. HFDP encompasses several forest community types characteristic of the Great Lakes–St. Lawrence region. Total plot area is approximately 13.5 ha, of which approximately two thirds is occupied by a stand of shade-tolerant hardwoods. These interior, upland communities are dominated by sugar maple (Acer saccharum Marsh.), followed by American beech (Fagus grandifolia Ehrh.), and yellow birch (Betula alleghaniensis Britt.); the sub-canopy species striped maple (Acer pensylvanicum L.) is also abundant. Soils in the Haliburton region are shallow, predominantly sandy loams derived from till of the underlying granitic bedrock. Annual precipitation is approximately 1050 mm, with a mean annual temperature of 5 °C [42].
2.2. Data Collection
Flux collars consisted of 5-cm-long cross-sections of 10-cm diameter PVC plumbing, beveled with a power sander. The beveled edge of the collar was twisted into soil just enough to hold firmly (~1 cm); extra care was taken to ensure that collar insertion did not damage tree root tissues. If obstructions were encountered or a root was damaged, the collar was relocated within 20 cm of its initial location. Collars were always allowed to settle for at least 48 h before sampling.
Background sites were selected to ensure that locations were displaced at least two meters from any trees ≥10 cm diameter at breast height (dbh, measured using a diameter tape at 1.3 m height). Each background site consisted of a set of three collars, arrayed in a triangle with an approximate distance of one meter between collars. Background sites were distributed among 21 fixed points on a 57-m grid, with nine more randomly selected locations (Figure 1); a combination of uniform and random points was used to achieve broad coverage of spatial variation yet ensure that background estimates were not biased by unobserved factors with spatially regular distributions. The locations of background sampling locations were adjusted slightly upon collar installation to exclude local low points with a tendency to accumulate water and locations with shallow soils or exposed bedrock that prevented insertion of flux collars. Patches of understory vegetation may also contribute to soil FCO2 [35], and so were also avoided.
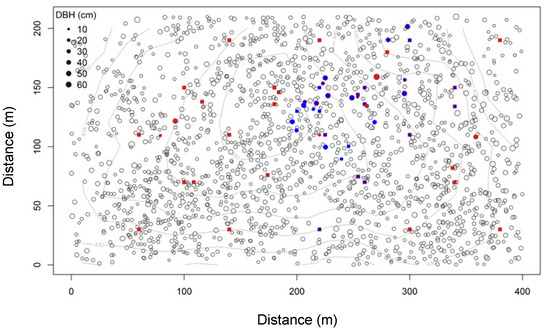
Figure 1.
Map of adult sugar maple trees and soil efflux observations: all sugar maple stems with a diameter larger than 15 cm are indicated by empty circles. Bolded points indicate locations of soil efflux measurements. Squares are background fluxes; circles are tree-associated fluxes. Red symbols indicate pre-rain and blue symbols post-rain observations; blue symbols with a red outline indicate the locations with both pre- and post-rain observations. Topographic contours indicate 5-m elevation increments, ranging from 425 to 450 m.
Root zone FCO2 observations were collected from trees that were well spaced from neighboring individuals (minimum distance 3 m) on level terrain. Sampled focal trees varied from 10 to 55 cm dbh (corresponding to a crown radius of ~2 to 5 m). The majority of sampled trees were located on the interior plateau at the center of the forest plot, where composition is dominated by Acer saccharum and individual trees are well spaced (Figure 1). Canopy cover in the area is dense and spatially uniform and leaf-litter collection traps indicate that organic inputs in this section are also spatially uniform (data not shown). Measuring trees in the same neighborhood had the added advantage of minimizing underlying variation in soil drainage, texture, depth, and chemistry.
After study trees were selected, six soil efflux collars were inserted at a distance of approximately one meter from the base of each tree. Collars were distributed uniformly around each tree, except where obvious visible obstructions (vegetation, rocks, decayed logs) interfered. Several tree-level measurements were made to assess whether tree vitality influenced root-zone FCO2. Tree dbh was recorded at the time of FCO2 sampling using a diameter tape and compared to a former census measurement collected in the year 2011 to estimate mean annual growth increment over the two-year period (radial increment in cm/y). Crown transparency measurements from a maple health survey conducted in July 2009 with moosehorn densiometers [43] were also used as a health status indicator for sampled trees.
2.3. Pre- and Post-Rain Sampling Intervals
The first round of sampling was done from 17–19 August 2013, with a subset of ten background and two root zone locations resampled on August 24th. A 42.2-mm rain event occurred on August 25th (Figure 2, Historical Climate Data, Environment Canada, Government of Canada) followed by a second round of sampling between August 27th and 29th. We were additionally able to validate the correspondence between temperature data with regional weather station and local temperatures at the soil surface with a network of 46 temperature loggers (Logtag, Auckland, New Zealand), which recorded soil temperatures just below the organic layer, spaced on a 28-m square grid throughout the study site. Mean daily soil surface temperatures and mean daily air temperatures from the regional station day were highly correlated (r = 0.94, data not shown).
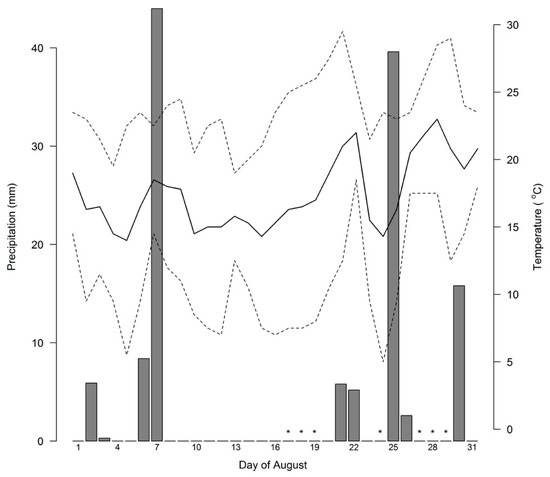
Figure 2.
Haliburton regional weather data for August 2013. Lines indicate daily range (dashed) and mean (solid) temperatures. Bars indicate magnitude of rainfall. Points marked with asterisks along the x-axis indicate dates of flux observations.
2.4. Data Processing and Quality Control
In total, 30 background and 18 root zone fluxes were sampled before wetting and 14 background and 22 root zones after wetting. Soil CO2 efflux (FCO2) observations were made with a portable infrared gas analyzer (IRGA) with soil chamber attachment (Li6400 with Li-6400-09 attachment, LiCor, Lincoln, Nebraska). Mean temperature of the top 10 cm of soil was also recorded with a Li-6400-09TC thermocouple probe. The IRGA cuvette was sealed to the collar and approximately 30 to 60 s were allowed for CO2 readings to stabilize before measurements. At least three FCO2 readings were logged, spaced at 30-s intervals, which were averaged prior to analysis. If collars demonstrated extreme values (greater than two standard deviations from the mean, or >50 µmol m−2 s−1), the collar was removed and data from this location were not integrated into the analysis (this was done for 4 out of 278 collars).
2.5. Data Analysis
A two-way analysis of variance (ANOVA) was conducted to compare FCO2 of background to root zone locations, before and after rainfall. Where duplicate observations were available, the average of the two dates was used. For root zone FCO2 values associated with tree stems, each tree’s FCO2 was calculated by averaging over its associated collars prior to analysis.
Increases in soil temperature characteristically result in multiplicative increases in FCO2. To correct for temperature effects we modeled the temperature response of FCO2 using an exponential equation (FCO2 = a*exp[b * Tsoil]), where Tsoil = mean soil temperature to 10-cm depth, a and b are fitted parameters) to the full dataset of soil collars. Q10 provides a concise indicator of the temperature sensitivity of a FCO2 temperature responses curve and was estimated to allow for comparison among similar temperature responses analyses (Q10 = exp[b * 10]). A t-test was conducted to compare soil temperatures during pre-rain sampling and post-rain sampling.
The statistical residuals from the exponential model temperature response model provided temperature-corrected FCO2 indices, which were then compared to tree-level characteristics. To assess among-tree variation in root zone FCO2, an additional one-way ANOVA with tree as a factor was performed, using temperature-corrected FCO2 values for pre- and post-precipitation root zone fluxes. Simple linear regressions were conducted to determine whether tree size, recent growth, or crown transparency were statistical predictors of root zone respiration. All analyses were performed in the ‘stats’ package of the R statistical programming language [44].
3. Results
3.1. Pre- vs. Post-Precipitation FCO2
Soil CO2 efflux preceding the rainfall event averaged 6.43 (±0.31 s.e.) μmol CO2 m-2 s-1 and 6.25 (±0.29 s.e.) μmol CO2 m−2 s−1 for background and root zones, respectively, which were not statistically distinguishable. Following the August 25th rainfall event, FCO2 significantly increased to 8.61 (±0.64 s.e.) μmol CO2 m−2 s−1 and 10.79 (±0.41 s.e.) μmol CO2 m−2 s−1 for background and root zone fluxes (pwet < 0.001), respectively. A significant root zone by pre-/post-rainfall interaction term was also detected (pRZ*Wet = 0.005), indicating that root zones fluxes experienced a larger enhancement following the rainfall event relative to background conditions (Figure 3).
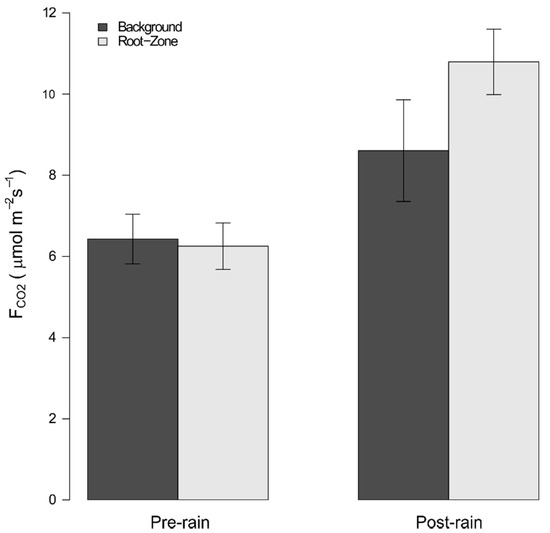
Figure 3.
Response of background and root zone soil CO2 flux to wetting. Error bars indicate 95% confidence intervals.
3.2. Temperature Effects on FCO2
In addition to increased soil moisture, soil temperatures were also higher during post-rain sampling (14.8 (±0.09 s.e.) °C vs. 16.8 (±0.09 s.e.) °C; Figure 4a). We detected a statistically significant influence of temperature on FCO2 and this relationship was accurately captured with an exponential model (FCO2 = 0.6189 × e 0.1651 . Tsoil; p < 0.001) (Figure 4b). From this relationship, we further estimated a Q10 value of 5.81 (±0.45 s.e.).
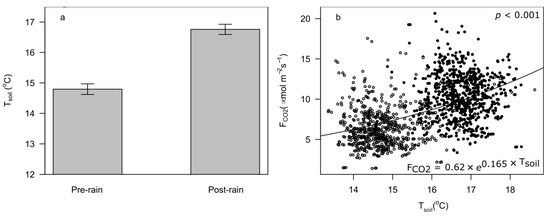
Figure 4.
Soil temperature patterns and effects on soil CO2 efflux. Soil temperatures (Tsoil) before and after wetting were significantly different ((a), p < 0.001, error bars indicate 95% confidence intervals). Observed fluxes were sensitive to soil temperatures (b). The exponential rate of increase was estimated to be Q10 = 5.81 (± 0.45 s.e.). Open dots indicate pre-rain soil fluxes observations and filled dots indicate post-rain fluxes.
3.3. Tree Effects on FCO2
Post-rain temperature-corrected root zone FCO2 was positively related to tree dbh (Figure 5b). Following rain, root-zone FCO2 increased at a rate of 0.058 µmol m−2 s−1 for every centimeter increase in tree dbh (p = 0.040, r2 = 0.154). We did not detect a significant effect of tree size on pre-rain FCO2 (Figure 5a). The other metrics examined, radial increment and crown transparency, were not able to explain any variation in temperature corrected effluxes among trees either pre- or post-rain. Although tree size appeared to be the only metric to influence FCO2, ANOVA results showed statistically significant variation among individuals in temperature-corrected efflux, both before and after the rainfall event (p < 0.001 for both ANOVAs) (Figure 5), indicating additional among-tree variation.
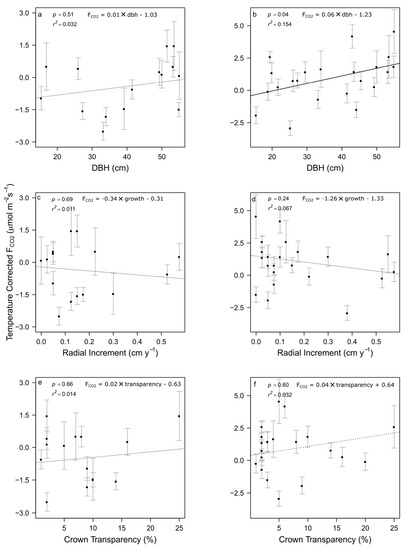
Figure 5.
Relationships between individual tree characteristics and temperature-corrected soil CO2 efflux. Error bars represent standard error (n = 6) in each individual tree’s root zone flux. Significant variation was detected among individual tree root zones both before (panels (a,c,e)) and after (panels (b,d,f)) wetting (pdry and pwet < 0.001). Post-rain variation in soil root zone fluxes was significantly related to tree dbh (solid regression line) but not to other variables (dotted regression lines).
4. Discussion
Our objective was to determine whether the contribution of individual trees to soil FCO2 could be detected by comparing root zone FCO2 to background variation, and to further link aboveground properties of A. saccharum trees to belowground C processes in a closed-canopy forest. Under typical conditions, root-zone FCO2 did not differ from background FCO2 but was significantly higher following an intense precipitation event. We also detected significant variation among individual root-zone FCO2 estimates, both before and after rainfall. Temperature-corrected FCO2 increased significantly with tree size, consistent with prior studies [25,26,27,28,29,30]; however, this pattern was only detected for post-precipitation FCO2. We did not find any trend with tree health indicators (either recent tree growth increment or crown transparency), suggesting that the size-dependent pattern in FCO2 is not related to tree senescence.
Transient ‘Birch effect’ increases in FCO2 in response to wetting is a well-documented phenomenon [13,14]. Elevated FCO2 typically persists for several days after moisture increases and is mainly attributed to the improved mobilization of labile carbon compounds, which primes microbial communities for further degradation of less bioavailable substrates [14]. Root exclusion studies have also found that the Birch effect depends on root presence [45]. Our results indicate that the Birch effect is both more pronounced in the vicinity of tree stems and is greater in magnitude near larger trees. Prior studies examining tree-size effects on FCO2 have not explicitly tested for Birch-effect patterns, but Søe and Buchmann [26] found that a best-fitting model describing variation in FCO2 included both tree size (mean dbh of trees within 4 m radius), and volumetric soil moisture content; Schwendenmann and Macinnis-Ng [30] noted relatively dry soil conditions near the base of large trees that could enhance the gas diffusion, and so increase FCO2.
Several mechanisms might contribute to the tree-size-dependent Birch effect detected in the present study. Mobility of labile C substrates, such as root exudates, is limited by diffusion, and these labile C compounds could thus accumulate in the immediate vicinity of their source [46]. Larger trees with larger canopies are generally more productive and may export larger quantities of carbohydrates to associated soil biota, which accumulate until rain-induced mobilization. It is furthermore likely that necrotic tissues such as dead fine roots, which are an important carbon source for heterotrophic respiration [47], occur at higher densities in the root zones of larger trees. These higher-density pools of low-quality organic matter are more accessible to microbial communities following wetting events. An additional possibility consists of a more biophysical mechanism, where larger canopy areas may intercept larger quantities of moisture, leading to higher stemflow and higher moisture near the bases of large trees [48].
Among-tree variation in root zone FCO2 was not significantly correlated with tree size prior to rainfall, but we did detect significant variation among individuals. This additional variation among locations could be explained by topography and unobserved edaphic variables, but also invites deeper explorations of how tree health status could also regulate soil FCO2. For example, Hancock et al. [49] found that the early stages of infection of Fagus grandifolia by the invasive pathogen Neonectria (beech-bark disease) corresponded with sharply increased local FCO2. Geddes et al. [50] reported anomalous ecosystem-level C losses from a stand co-dominated by Fagus grandifolia at HFWR and considered the onset of beech-bark disease a likely explanation. We speculate that pathogen infection may more generally contribute to high tree-to-tree variation in FCO2 and component processes.
Although biotic factors have been found to dominate soil FCO2 in studies conducted at comparable scales to the present analysis [17,18], we still found that correcting for temperature effects was essential for detecting tree size effects on soil FCO2. Soil temperatures were clearly sensitive to changes in ambient air temperature over the duration of the study, with impacts on FCO2 (Figure 3). However, the estimated sensitivity of FCO2 to temperature in the present study was substantially higher than estimates from comparable forest soils (compare our estimated Q10 of 5.8, to a prior estimate of 3.0 at HFWR [8], and 3.5 at a comparable site in New England [51]). This high sensitivity is almost certainly due to coupled increases in soil moisture and temperature, which likely had a compound effect on soil FCO2. This observation further highlights the need to sample over wider ranges of variation in meteorological conditions, whereas studies of plant-soil systems often aim to narrow the range of environmental variation in an effort to improve their ability to detect specific biotic interactions.
The ability to anticipate ecosystem-level responses of soil respiration to the changing climate is still limited by our understanding of relationships between soil moisture and microbial respiration [16]. The Birch effect is frequently left out of ecosystem-level models of C exchange entirely [16], even though this has been shown to result in a ~25% underestimation of soil C emissions, at least in northern deciduous forests [52]. Changes in the isotopic composition of C emissions over very short periods following moisture pulses indicate that inorganic C can also contribute to the Birch effect in some systems [53]. Uncertainty in these sources of C emissions limits the applicability of otherwise analytically tractable process-based models that incorporate the kinetics of C supply and microbe consumption [54]. Our results suggest that Birch effect fluxes may be specifically enhanced in forests with large, old trees, potentially indicating an underestimation of carbon losses from old-growth forests without explicit consideration.
5. Conclusions
The present study compliments prior work suggesting strong autotrophic control over FCO2. Our work replicates prior studies indicating a size-dependent increase in FCO2, while also demonstrating a strong ontogenetic trend in carbon flux associated with the Birch effect. Detecting tree size effects on soil CO2 efflux invites the investigation of additional biophysical and biogeochemical relations that may be impacted by the ontogeny and/or allometry of tree root functioning and organic matter production. Unifying physiological mechanisms with ecosystem modeling efforts requires replication of comparable measurements across tree species and over a wider range of environmental variation, including rainfall intensity, rainfall frequency, and seasonal variation in belowground photosynthate allocation.
Author Contributions
Conceptualization, J.S.S. and S.C.T.; methodology, J.S.S. and S.C.T.; formal analysis, J.S.S.; investigation, J.S.S.; data curation, J.S.S.; writing—original draft preparation, J.S.S. and S.C.T.; writing—review and editing, J.S.S. and S.C.T.; supervision, S.C.T.; funding acquisition, S.C.T. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was supported through grants from the Natural Sciences and Engineering Research Council of Canada.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Haliburton Forest and Wild Life Reserve and their staff for research support, including property use.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 481, 7–20. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- van Haren, J.; de Oliveira, R.C., Jr.; Beldini, P.T.; de Camargo, P.B.; Keller, M.; Saleska, S. Tree species effects on soil properties and greenhouse gas fluxes in East-Central Amazonia: Comparison between monoculture and diverse forest. Biotropica 2013, 456, 709–718. [Google Scholar] [CrossRef]
- Chapin, F.S., III; McFarland, J.; McGuire, A.D.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global carbon cycle: Linking plant-soil carbon dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; Von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I.; et al. Climate–carbon cycle feedback analysis: Results from the C 4 MIP model intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Subke, J.A.; Bahn, M. On the “temperature sensitivity” of soil respiration: Can we use the immeasurable to predict the unknown? Soil Biol. Biochem. 2010, 42, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Thomas, S.C. Soil CO2 efflux in uneven-aged managed forests: Temporal patterns following harvest and effects of edaphic heterogeneity. Plant Soil 2006, 289, 253–264. [Google Scholar] [CrossRef]
- Knohl, A.; Søe, A.R.; Kutsch, W.L.; Göckede, M.; Buchmann, N. Representative estimates of soil and ecosystem respiration in an old beech forest. Plant Soil 2008, 302, 189–202. [Google Scholar] [CrossRef]
- Ngao, J.; Epron, D.; Delpierre, N.; Bréda, N.; Granier, A.; Longdoz, B. Spatial variability of soil CO2 efflux linked to soil parameters and ecosystem characteristics in a temperate beech forest. Agric. For. Meteorol. 2012, 154, 136–146. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Högberg, P.; Read, D.J. Towards a more plant physiological perspective on soil ecology. Trends Ecol. Evol. 2006, 21, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Vargas, R.; Carbone, M.S.; Reichstein, M.; Baldocchi, D.D. Frontiers and challenges in soil respiration research: From measurements to model-data integration. Biogeochemistry 2011, 102, 1–13. [Google Scholar] [CrossRef]
- Vicca, S.; Bahn, M.; Estiarte, M.; van Loon, E.E.; Vargas, R.; Alberti, G.; Ambus, P.; Arain, M.A.; Beier, C.; Bentley, L.P.; et al. Can current moisture responses predict soil CO2 efflux under altered precipitation regimes? A synthesis of manipulation experiments. Biogeosciences 2014, 11, 853–899. [Google Scholar]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T.; et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Bahn, M.; Rodeghiero, M.; Anderson-Dunn, M.; Dore, S.; Gimeno, C.; Drösler, M.; Williams, M.; Ammann, C.; Berninger, F.; Flechard, C.; et al. Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems 2008, 11, 1352–1367. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar]
- Högberg, P.; Högberg, M.N.; Göttlicher, S.G.; Betson, N.R.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Schindlbacher, A.; Hurry, V.; Lundmark, T.; et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 2008, 177, 220–228. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D. Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Roy, J.; Freycon, V.; Coûteaux, M.M.; Bonal, D.; Epron, D. Do tree species characteristics influence soil respiration in tropical forests? A test based on 16 tree species planted in monospecific plots. Plant Soil 2009, 319, 235–246. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2009, 264, 185–196. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Jin, C.; Zhang, X.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The influence of tree species on small scale spatial heterogeneity of soil respiration in a temperate mixed forest. Sci. Total Environ. 2017, 590, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bréchet, L.; Ponton, S.; Alméras, T.; Bonal, D.; Epron, D. Does spatial distribution of tree size account for spatial variation in soil respiration in a tropical forest? Plant Soil 2011, 347, 293. [Google Scholar] [CrossRef]
- Søe, A.R.; Buchmann, N. Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol. 2005, 2511, 1427–1436. [Google Scholar] [CrossRef]
- Katayama, A.; Kume, T.; Komatsu, H.; Ohashi, M.; Nakagawa, M.; Yamashita, M.; Otsuki, K.; Suzuki, M.; Kumagai, T. Effect of forest structure on the spatial variation in soil respiration in a Bornean tropical rainforest. Agric. For. Meteorol. 2009, 149, 1666–1673. [Google Scholar] [CrossRef]
- Luan, J.; Liu, S.; Zhu, X.; Wang, J.; Liu, K. Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biol. Biochem. 2012, 441, 143–150. [Google Scholar] [CrossRef]
- ArchMiller, A.A.; Samuelson, L.J.; Li, Y. Spatial variability of soil respiration in a 64-year-old longleaf pine forest. Plant Soil 2016, 403, 419–435. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Macinnis-Ng, C. Soil CO2 efflux in an old-growth southern conifer forest Agathis australis—magnitude, components and controls. Soil 2016, 23, 403–419. [Google Scholar] [CrossRef]
- Song, Q.H.; Tan, Z.H.; Zhang, Y.P.; Cao, M.; Sha, L.Q.; Tang, Y.; Liang, N.S.; Schaefer, D.; Zhao, J.F.; Zhao, J.B.; et al. Spatial heterogeneity of soil respiration in a seasonal rainforest with complex terrain. iForest-Biogeosci. For. 2013, 62, 65–72. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Saiz, G.; Green, C.; Butterbach-Bahl, K.; Kiese, R.; Avitabile, V.; Farrell, E.P. Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 2006, 287, 161–176. [Google Scholar] [CrossRef]
- Powers, M.; Kolka, R.; Bradford, J.; Palik, B.; Jurgensen, M. Forest floor and mineral soil respiration rates in a northern Minnesota red pine chrono sequence. Forests 2018, 9, 16. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Salomón, R.; Barba, J.; Gordaliza, G.G.; Curiel Yuste, J.; Magro, C.; Gil, L. Regeneration in the understory of declining overstory trees contributes to soil respiration homeostasis along succession in a sub-Mediterranean beech forest. Forests 2019, 10, 727. [Google Scholar] [CrossRef]
- Thomas, S.C. Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 2010, 30, 555–573. [Google Scholar] [CrossRef]
- Nock, C.A.; Caspersen, J.P.; Thomas, S.C. Large ontogenetic declines in intra-crown leaf area index in two temperate deciduous tree species. Ecology 2008, 89, 744–753. [Google Scholar] [CrossRef]
- Martin, A.R.; Thomas, S.C. Size-dependent changes in leaf and wood chemical traits in two Caribbean rainforest trees. Tree Physiol. 2013, 33, 1338–1353. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Salim, K.A.; Zambrano, A.M.A.; Alonso, A.; Baltzer, J.L.; et al. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Global Change Biol. 2014, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Environment Canada, Government of Canada, Historical Climate Data, Haliburton Ontraio. Available online: http://climate.weather.gc.ca/ (accessed on 9 November 2014).
- Garrison, G.A. Uses and modifications for the “moosehorn” crown closure estimator. J. For. 1949, 47, 733–735. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Tang, J.; Misson, L.; Gershenson, A.; Cheng, W.; Goldstein, A.H. Continuous measurements of soil respiration with and without roots in a ponderosa pine plantation in the Sierra Nevada Mountains. Agric. For. Meteorol. 2005, 132, 212–227. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Levia, D.F.; Keim, R.F.; Carlyle-Moses, D.E.; Frost, E.E. Throughfall and stemflow in wooded ecosystems. In Forest Hydrology and Biogeochemistry; Levia, D.F., Carlyle-Moses, D., Tanaka, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 425–443. [Google Scholar]
- Hancock, J.E.; Arthur, M.A.; Weathers, K.C.; Lovett, G.M. Carbon cycling along a gradient of beech bark disease impact in the Catskill Mountains, New York. Can. J. For. Res. 2008, 385, 1267–1274. [Google Scholar] [CrossRef]
- Geddes, J.A.; Murphy, J.G.; Schurman, J.S.; Petroff, A.; Thomas, S.C. Net ecosystem exchange of an uneven-aged managed forest in central Ontario, and the impact of a spring heat wave event. Agric. For. Meteorol. 2014, 198, 105–115. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Lee, X.; Wu, H.J.; Sigler, J.; Oishi, C.; Siccama, T. Rapid and transient response of soil respiration to rain. Glob. Chang. Biol. 2004, 106, 1017–1026. [Google Scholar] [CrossRef]
- Inglima, I.; Alberti, G.; Bertolini, T.; Vaccari, F.P.; Giolo, B.; Miglietta, F.; Cotrufo, M.F.; Peressotti, A. Precipitation pulses enhance respiration of Mediterranean ecosystems: The balance between organic and inorganic components of increased soil CO2 efflux. Glob. Chang. Biol. 2009, 155, 1289–1301. [Google Scholar] [CrossRef]
- Moyana, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).