Biochar Enhances Nitrous Oxide Reduction in Acidic but Not in Near-Neutral pH Soil
Abstract
1. Introduction
2. Materials and Methods
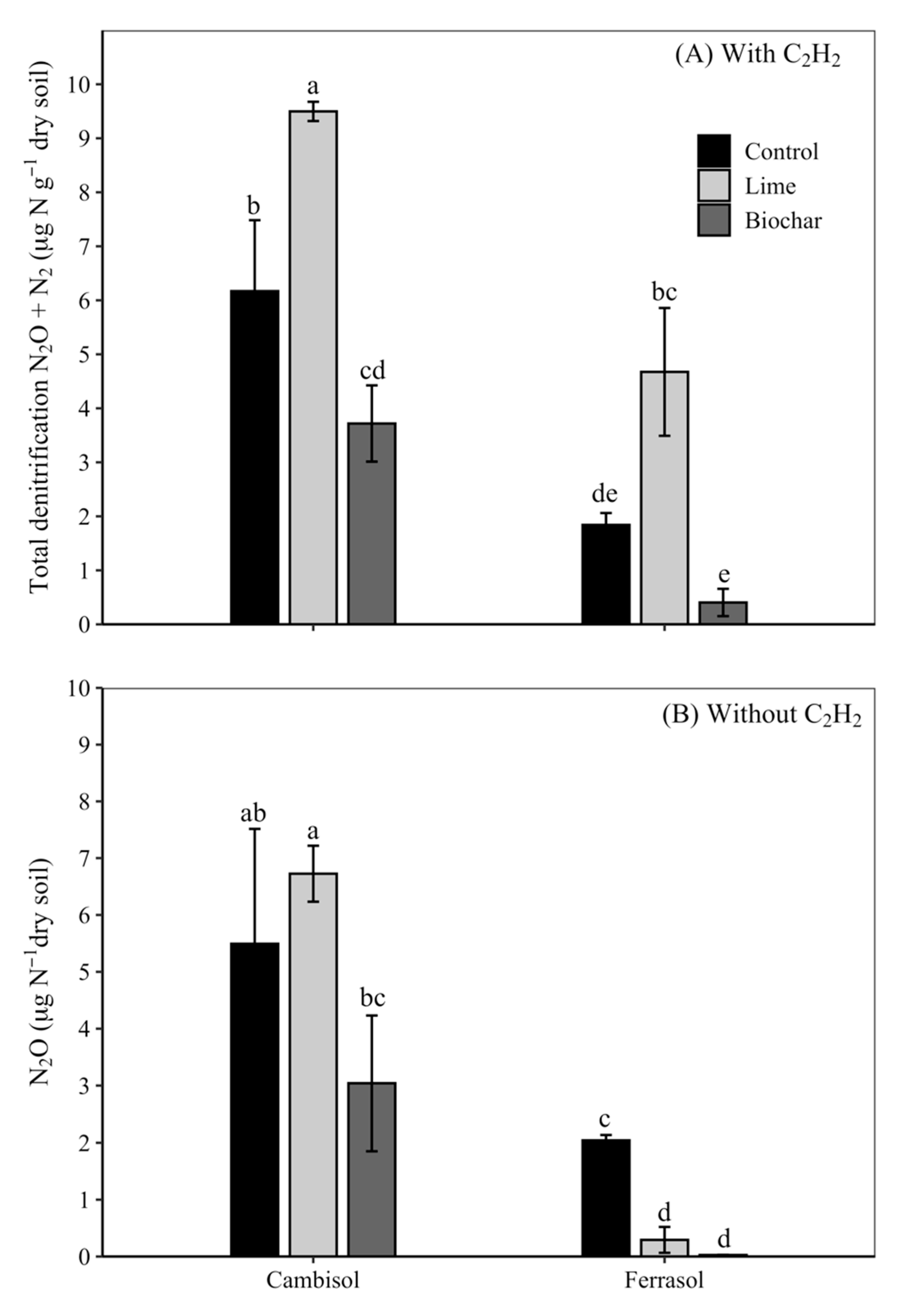
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s Role in Mitigating Soil Nitrous Oxide Emissions: A Review and Meta-Analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Simek, M.; Cooper, J.E. The Influence of Soil PH on Denitrification: Progress towards the Understanding of This Interaction over the Last 50 Years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Liu, B.; Frostegård, Å.; Bakken, L.R. Impaired Reduction of N2O to N2 in Acid Soils Is Due to a Posttranscriptional Interference with the Expression of NosZ. MBio 2014, 5, e01314–e01383. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.I.P.; Suddick, E.C.; Mansour, I.; Mukome, F.N.D.; Parikh, S.J.; Scow, K.; Six, J. Biochar Alters Nitrogen Transformations but Has Minimal Effects on Nitrous Oxide Emissions in an Organically Managed Lettuce Mesocosm. Biol. Fertil. Soils 2015, 51, 573–582. [Google Scholar] [CrossRef]
- Verhoeven, E.; Pereira, E.; Decock, C.; Suddick, E.; Angst, T.; Six, J. Toward a Better Assessment of Biochar–Nitrous Oxide Mitigation Potential at the Field Scale. J. Environ. Qual. 2017, 46, 237. [Google Scholar] [CrossRef] [PubMed]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Melbourne, Australia, 1992; p. 330. [Google Scholar]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of Chemical and Physical Characteristics to Investigate Trends in Biochar Feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Drury, C.F.; Myrold, D.D.; Beauchamp, E.G.; Reynolds, W.D. Denitrification Techniques for Soils. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; p. 1264. [Google Scholar] [CrossRef]
- Culman, S.W.; Bukowski, R.; Gauch, H.G.; Cadillo-Quiroz, H.; Buckley, D.H. T-REX: Software for the Processing and Analysis of T-RFLP Data. BMC Bioinform. 2009, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; p. 409. [Google Scholar] [CrossRef]
- Thomsen, J.K.; Geest, T.; Cox, R.P. Mass Spectrometric Studies of the Effect of PH on the Accumulation of Intermediates in Denitrification by Paracoccus Denitrificans. Appl. Environ. Microbiol. 1994, 60, 536–541. [Google Scholar] [PubMed]
- Felber, R.; Leifeld, J.; Horák, J.; Neftel, A. Nitrous Oxide Emission Reduction with Greenwaste Biochar: Comparison of Laboratory and Field Experiments. Eur. J. Soil Sci. 2014, 65, 128–138. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar Suppresses N2O Emissions While Maintaining N Availability in a Sandy Loam Soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of Soil PH Increase by Biochar on NO, N2O and N2 Production during Denitrification in Acid Soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Singh, B.; Singh, B.P. Interactive Priming of Biochar and Labile Organic Matter Mineralization in a Smectite-Rich Soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of Sugarcane Bagasse-Derived Biochar Application on Nitrate Leaching in Calcaric Dark Red Soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.N.; Jones, D.L.; Murphy, D.V. Clay and Biochar Amendments Decreased Inorganic but Not Dissolved Organic Nitrogen Leaching in Soil. Soil Res. 2012, 50, 216. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Arbestain, M.C.; Hedley, M.; Bishop, P. Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Peralta, A.L.; Matthews, J.W.; Kent, A.D. Microbial Community Structure and Denitrification in a Wetland Mitigation Bank. Appl. Environ. Microbiol. 2010, 76, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, G.; Wang, Y.; Wang, S.; Yin, C. Nitrous Oxide Reductase Gene (NosZ) and N2O Reduction along the Littoral Gradient of a Eutrophic Freshwater Lake. J. Environ. Sci. 2013, 25, 44–52. [Google Scholar] [CrossRef]
| Soil | Amendment | Soil pH | N2O/N2O + N2 | nirK | nirS | nosZ | Shannon H’ |
|---|---|---|---|---|---|---|---|
| Log Gene Copies g−1 soil | |||||||
| Cambisol | Control | 6.32 c | 0.88 a | 7.79 a | 6.89 a | 7.15 ab | 2.17 b |
| Lime | 7.99 b | 0.71 a | 7.99 a | 7.10 a | 6.93 abc | 1.84 b | |
| Biochar | 8.05 b | 0.84 a | 8.54 a | 7.36 a | 7.29 a | 1.92 b | |
| Ferralsol | Control | 5.37 d | 1.12 a | 7.72 a | 7.26 a | 6.38 cd | 2.58 a |
| Lime | 8.24 ab | 0.06 b | 8.11 a | 7.22 a | 6.27 cd | 2.43 a | |
| Biochar | 8.64 a | 0.08 b | 8.02 a | 7.06 a | 6.00 d | 2.43 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol Pereira, E.I.; Léchot, J.; Feola Conz, R.; da Silva Cardoso, A.; Six, J. Biochar Enhances Nitrous Oxide Reduction in Acidic but Not in Near-Neutral pH Soil. Soil Syst. 2019, 3, 69. https://doi.org/10.3390/soilsystems3040069
Pujol Pereira EI, Léchot J, Feola Conz R, da Silva Cardoso A, Six J. Biochar Enhances Nitrous Oxide Reduction in Acidic but Not in Near-Neutral pH Soil. Soil Systems. 2019; 3(4):69. https://doi.org/10.3390/soilsystems3040069
Chicago/Turabian StylePujol Pereira, Engil Isadora, Jérôme Léchot, Rafaela Feola Conz, Abmael da Silva Cardoso, and Johan Six. 2019. "Biochar Enhances Nitrous Oxide Reduction in Acidic but Not in Near-Neutral pH Soil" Soil Systems 3, no. 4: 69. https://doi.org/10.3390/soilsystems3040069
APA StylePujol Pereira, E. I., Léchot, J., Feola Conz, R., da Silva Cardoso, A., & Six, J. (2019). Biochar Enhances Nitrous Oxide Reduction in Acidic but Not in Near-Neutral pH Soil. Soil Systems, 3(4), 69. https://doi.org/10.3390/soilsystems3040069







