Plant Organic Matter Really Matters: Pedological Effects of Kūpaoa (Dubautia menziesii) Shrubs in a Volcanic Alpine Area, Maui, Hawai’i
Abstract
“The pedogenic impact of organisms is inferred from comparisons of bare and vegetated soil… or of soils covered by combinations of species.”Hans Jenny, 1980 [1], p. 359.
1. Introduction
1.1. Influence of Plants on Soil Development
1.2. Study Species: Kūpaoa
1.3. Study Objectives
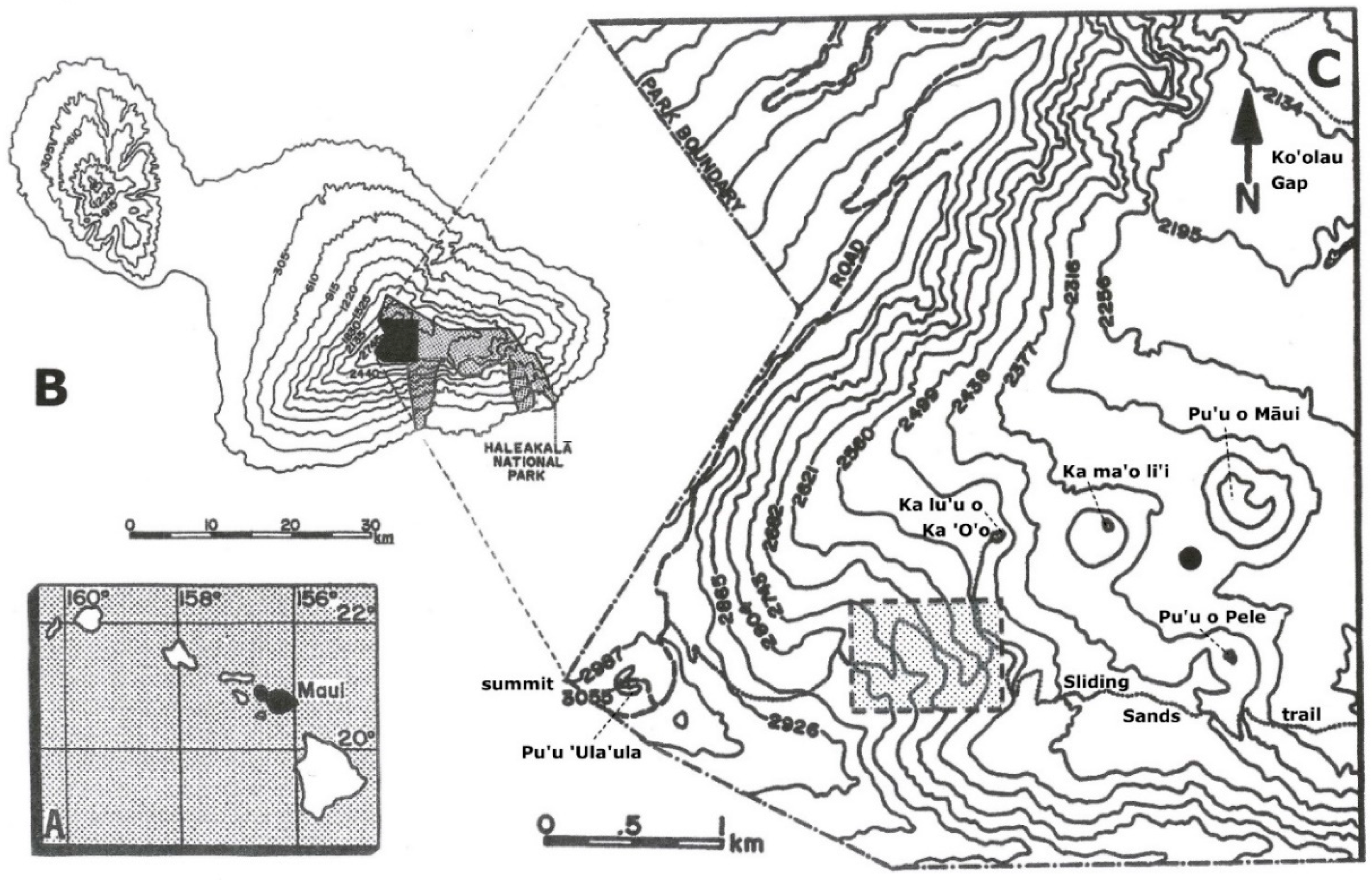
2. Study Area
2.1. Physical Setting and Climate
2.2. Soils at Haleakalā Crater and the Study Site
3. Materials and Methods
3.1. Field Sampling and Observations
3.2. Laboratory and Analytical Techniques
3.3. Statistical Procedures
4. Results
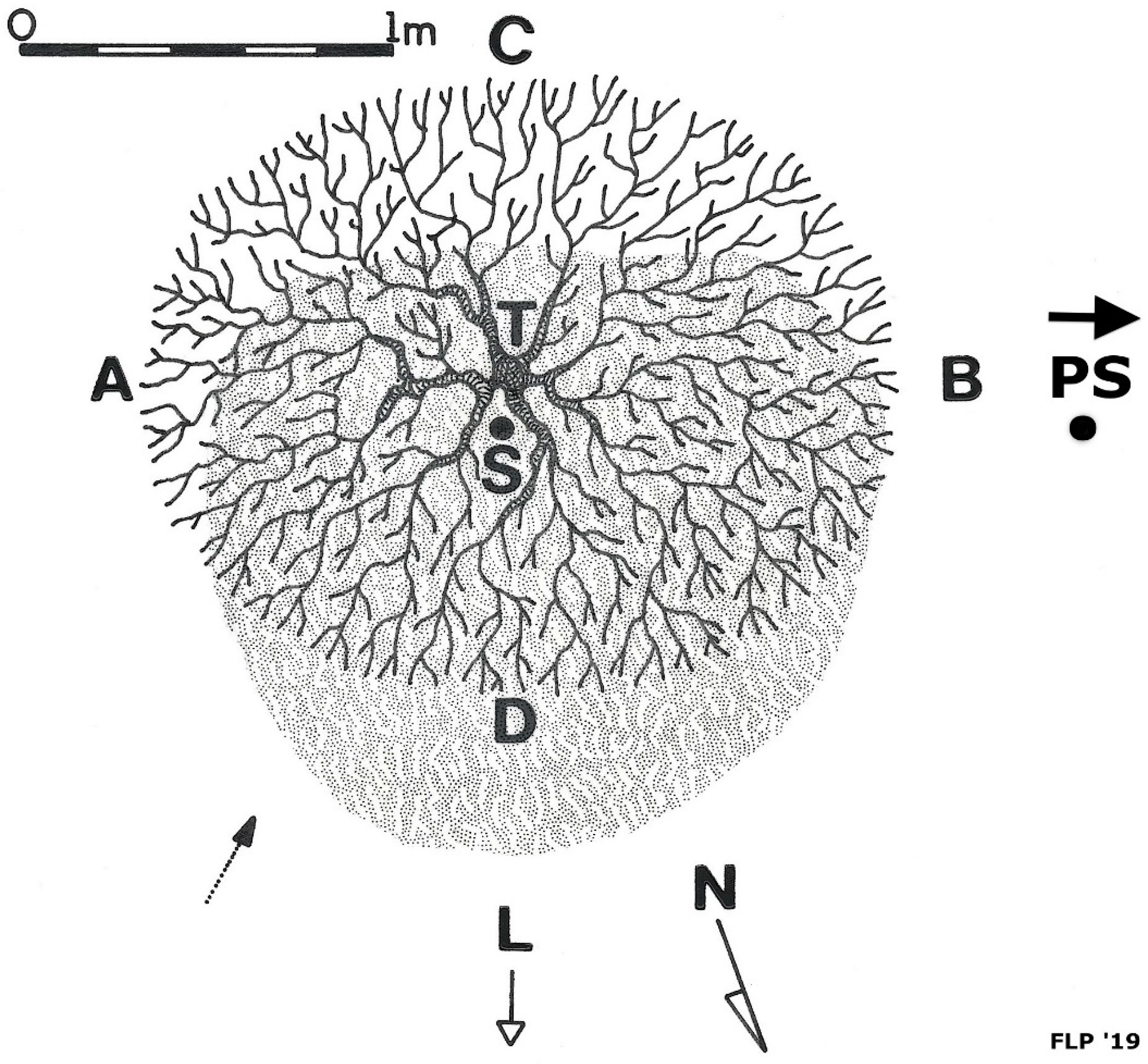
4.1. Litterfall Accumulation under Kūpaoa and Development of Litter Islands
4.2. Physical and Chemical Properties of Kūpaoa Litter
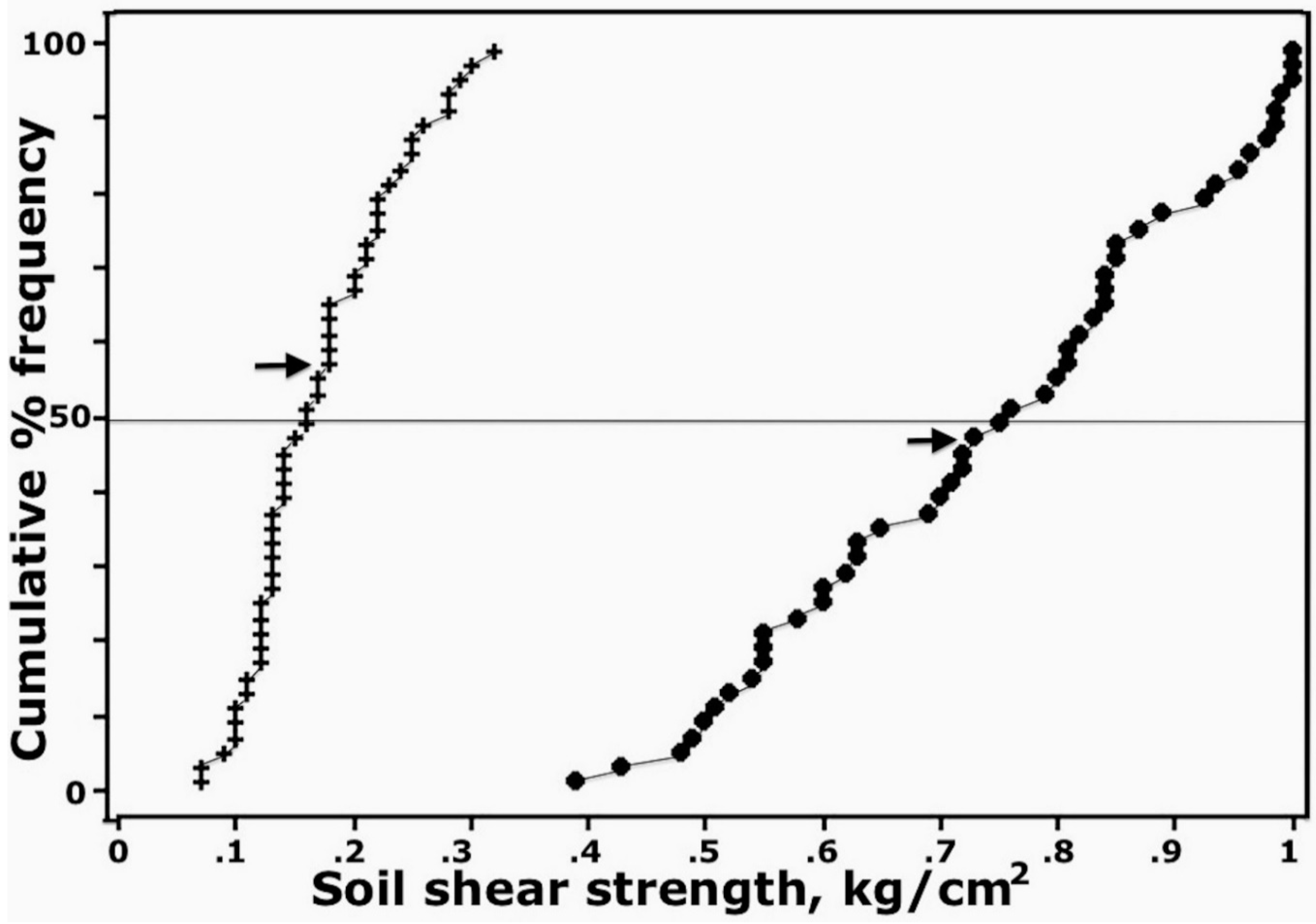
4.3. Physical Strength of Aggregated Surface Crusts
4.4. Modification of Surface-Soil Characteristics
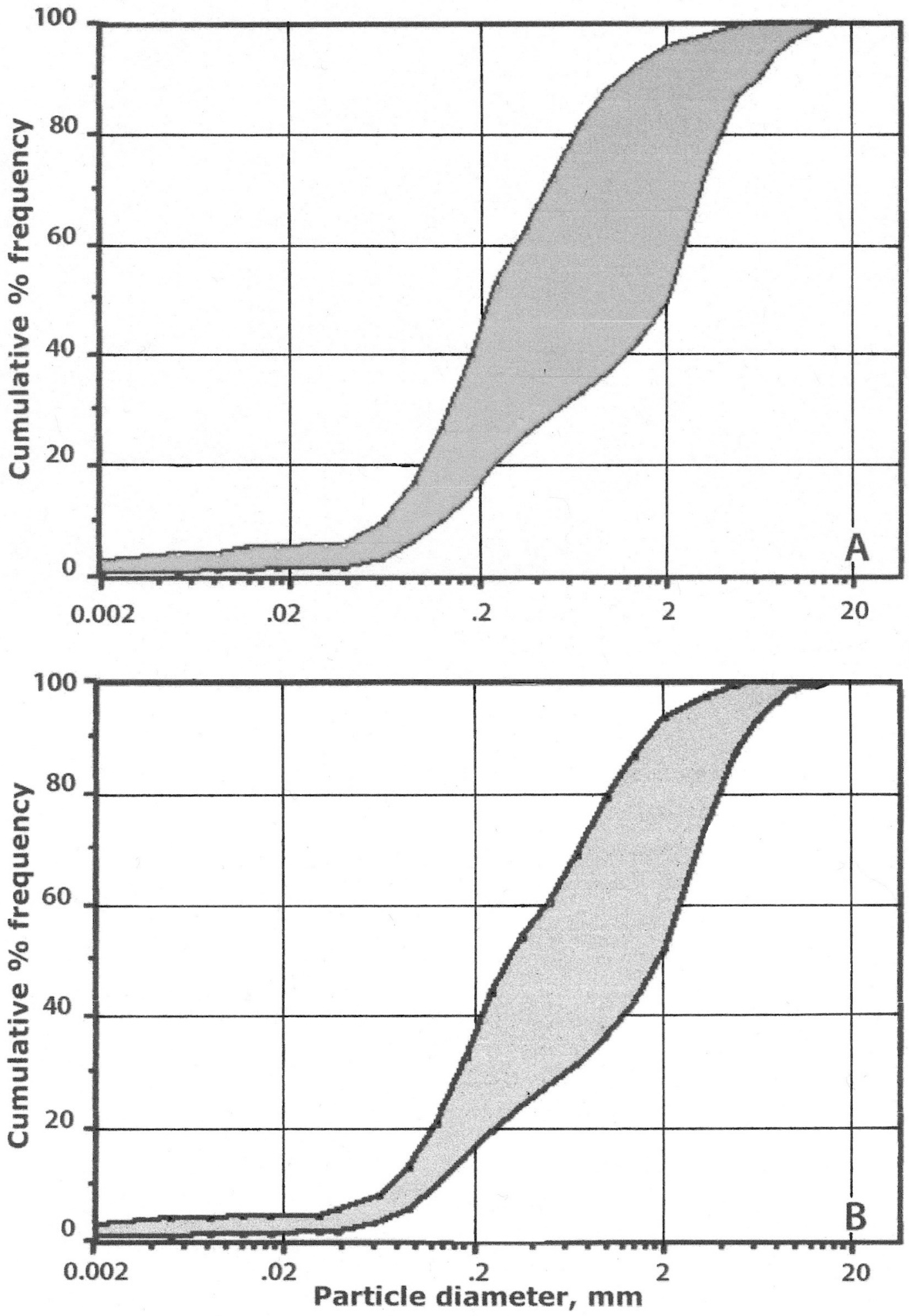
4.4.1. Mineral Particle-Size Distribution
4.4.2. Soil Organic-Matter Content, and Soil Color
4.4.3. Influence of Organic Matter on Chemical Soil Properties
4.5. Soil Changes with Depth: Characteristics of Soil Profiles
4.6. Influence of Shrubs on Water Relationships
4.7. Microclimatic modification under Shrubs
5. Discussion
5.1. Litter Accumulation, and Formation of Fertility Islands
5.1.1. Development of Litter Islands
5.1.2. Soil Enrichment under Fertility Islands
5.2. Possible Sources of Soil Aggregation in Surface Crusts
5.3. Differences between Pedological Effects of Kūpaoa and Silversword
5.3.1. Comparison with Silversword Litter
5.3.2. Comparison with Soils under Silversword
5.4. Additional Influences of Kūpaoa on Soil
5.4.1. Alteration of Soil-Water Relationships
5.4.2. Modifications of Soil Microclimate
5.4.3. Other Possible Plant Influences
6. Conclusions: Kūpaoa as a Control Factor of Alpine Pedogenesis
Funding
Acknowledgments
Conflicts of Interest
References
- Jenny, H. The Soil Resource. Origin and Behavior; Springer: New York, NY, USA, 1980. [Google Scholar]
- Jenny, H. Role of the plant factor in the pedogenic functions. Ecology 1958, 39, 5–16. [Google Scholar] [CrossRef]
- Fireman, M.; Hayward, H.E. Indicator significance of some shrubs in the Escalante desert, Utah. Bot. Gaz. 1952, 114, 143–155. [Google Scholar] [CrossRef]
- Rickard, W.H.; Keough, R.F. Soil-plant relationships of two steppe desert shrubs. Plant Soil 1968, 29, 205–212. [Google Scholar] [CrossRef]
- García-Moya, E.; McKell, C.M. Contribution of shrubs to the nitrogen economy of a desert-wash plant community. Ecology 1970, 51, 81–88. [Google Scholar] [CrossRef]
- Charley, J.L.; West, N.E. Plant-induced soil chemical patterns in some shrub-dominated semi-desert ecosystems of Utah. J. Ecol. 1975, 63, 945–963. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Raikes, J.A.; Hartley, A.E.; Cross, A.F. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 1996, 77, 364–374. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Pilmanis, A.M. Plant-soil interactions in deserts. Biogeochemistry 1998, 42, 169–187. [Google Scholar] [CrossRef]
- Wilson, T.B.; Thompson, T.L. Soil nutrient distributions of mesquite-dominated desert grasslands: Changes in time and space. Geoderma 2005, 126, 301–315. [Google Scholar] [CrossRef]
- Ruiz, T.G.; Zaragoza, S.R.; Cerrato, R.F. Fertility islands around Prosopis laevigata and Pachycereus hollianus in the drylands of Zapotitlán Salinas, Mexico. J. Arid Environ. 2008, 72, 1202–1212. [Google Scholar] [CrossRef]
- Gutiérrez, J.R.; Meserve, P.L.; Contreras, L.C.; Vásquez, H.; Jaksic, F.M. Spatial distribution of soil nutrients and ephemeral plants underneath and outside the canopy of Porlieria chilensis shrubs (Zygophyllaceae) in arid coastal Chile. Oecologia 1993, 95, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, L.F.; Gutiérrez, J.R.; Meserve, P.L. Variation in soil micro-organisms and nutrients underneath and outside the canopy of Adesmia bedwellii (Papilionaceae) shrubs in arid coastal Chile following drought and above average rainfall. J. Arid Environ. 1999, 42, 61–70. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Zhu, H.; Li, Y.; Wang, F. Effect of plant species on shrub fertile island at an oasis-desert ecotone in the South Junggar Basin, China. J. Arid Environ. 2007, 71, 350–361. [Google Scholar] [CrossRef]
- Li, P.-X.; Wang, N.; He, W.-M.; Krüsi, B.O.; Gao, S.-Q.; Zhang, S.-M.; Yu, F.-H.; Dong, M. Fertile islands under Artemisia ordosica in inland dunes of northern China: Effects of habitats and plant developmental stages. J. Arid Environ. 2008, 72, 953–963. [Google Scholar] [CrossRef]
- Jeddi, K.; Cortina, J.; Chaieb, M. Acacia salicina, Pinus halepensis and Eucalyptus occidentalis improve soil surface conditions in southern Tunisia. J. Arid Environ. 2009, 73, 1005–1013. [Google Scholar] [CrossRef]
- Ward, D.; Trinogga, J.; Wiegand, K.; du Toit, J.; Okubamichael, D.; Reinsch, S.; Schleicher, J. Large shrubs increase soil nutrients in a semi-arid savanna. Geoderma 2018, 310, 153–162. [Google Scholar] [CrossRef]
- Herrera-Arreola, G.; Herrera, Y.; Reyes-Reyes, B.G.; Dendooven, L. Mesquite (Prosopis juliflora (Sw.) DC.), huisache (Acacia farnesiana (L.) Willd.) and catclaw (Mimosa biuncifera Benth.) and their effect on dynamics of carbon and nitrogen in soils of the semi-arid highlands of Durango, Mexico. J. Arid Environ. 2007, 69, 583–598. [Google Scholar] [CrossRef]
- Jackson, R.B.; Caldwell, M.M. Geostatistical patterns of soil heterogeneity around individual perennial plants. J. Ecol. 1993, 81, 683–692. [Google Scholar] [CrossRef]
- Hirobe, M.; Ohte, N.; Karasawa, N.; Zhang, G.; Wang, L.; Yoshikawa, K. Plant species effect on the spatial patterns of soil properties in the Mu-us desert ecosystem, Inner Mongolia, China. Plant Soil 2001, 234, 195–205. [Google Scholar] [CrossRef]
- Zheng, J.; He, M.; Li, X.; Chen, Y.; Li, X.; Liu, L. Effects of Salsola passerina shrub patches on the microscale heterogeneity of soil in a montane grassland, China. J. Arid Environ. 2008, 72, 150–161. [Google Scholar] [CrossRef]
- Escudero, A.; Giménez-Benavides, L.; Iriondo, J.M.; Rubio, A. Patch dynamics and islands of fertility in a high mountain Mediterranean community. Arct. Antarct. Alp. Res. 2004, 36, 518–527. [Google Scholar] [CrossRef]
- Certini, G.; Fernández-Sanjurjo, M.J.; Corti, G.; Ugolini, F.C. The contrasting effect of broom and pine on pedogenic processes in volcanic soils (Mt. Etna, Italy). Geoderma 2001, 102, 239–254. [Google Scholar] [CrossRef]
- Fernández-Sanjurjo, M.J.; Corti, G.; Certini, G.; Ugolini, F.C. Pedogenesis induced by Genista aetnensis (Biv.) DC. on basaltic pyroclastic deposits at different altitudes, Mt. Etna, Italy. Geoderma 2003, 115, 223–243. [Google Scholar] [CrossRef]
- Rodríguez, A.; Durán, J.; Fernández-Palacios, J.M.; Gallardo, A. Spatial pattern and scale of soil N and P fractions under the influence of a leguminous shrub in a Pinus canariensis forest. Geoderma 2009, 151, 303–310. [Google Scholar] [CrossRef]
- Walker, L.R.; Powell, E.A. Regeneration of the Mauna Kea silversword Argyroxiphium sandwicense (Asteraceae) in Hawaii. Biol. Conserv. 1999, 89, 61–70. [Google Scholar] [CrossRef]
- Halvorson, J.J.; Smith, J.L.; Franz, E.H. Lupine influence on soil carbon, nitrogen and microbial activity in developing ecosystems at Mount St. Helens. Oecologia 1991, 87, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Onipchenko, V.G.; Makarov, M.I.; van der Maarel, E. Influence of alpine plants on soil nutrient concentrations in a monoculture experiment. Folia Geobot. 2001, 36, 225–241. [Google Scholar] [CrossRef]
- Nuñez, C.; Aizen, M.A.; Ezcurra, C. Species associations and nurse plant effects in patches of high-Andean vegetation. J. Veg. Sci. 1999, 10, 357–364. [Google Scholar] [CrossRef]
- Lee, W.G.; Hewitt, A.E. Soil changes associated with development of vegetation on an ultramafic scree, northwest Otago, New Zealand. J. R. Soc. N. Z. 1982, 12, 229–241. [Google Scholar] [CrossRef][Green Version]
- Holtmeier, F.K.; Broll, G. The influence of tree islands and microtopography on pedoecological conditions in the forest-alpine tundra ecotone on Niwot Ridge, Colorado Front Range, U.S.A. Arct. Alp. Res. 1992, 24, 216–228. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Adams, G.A. Effects of mobile tree islands on alpine tundra soils. Ecology 2001, 82, 8–17. [Google Scholar] [CrossRef]
- Pérez, F.L. Conifer litter and organic matter accumulation at timberline, Lassen Peak. In Examples of Resource Inventory and Monitoring in National Parks of California; van Riper, C., Stohlgren, T.J., Veirs, S.D., Castillo, S., Eds.; U.S. Department of the Interior National Park Service. Transactions and Proceedings Series: Washington, DC, USA, 1990; Volume 8, pp. 207–224. [Google Scholar]
- Pérez, F.L. The influence of organic matter addition by caulescent Andean rosettes on surficial soil properties. Geoderma 1992, 54, 151–171. [Google Scholar] [CrossRef]
- Pérez, F.L. The effects of giant Andean rosettes on surface soils along a high paramo toposequence. Geojournal 1996, 40, 283–298. [Google Scholar]
- Pérez, F.L. Geoecological alteration of surface soils by the Hawaiian silversword (Argyroxiphium sandwicense DC.) in Haleakala’s crater, Maui. Plant Ecol. 2001, 157, 215–233. [Google Scholar] [CrossRef]
- Zinke, P.J. The pattern of influence of individual forest trees on soil properties. Ecology 1962, 43, 130–133. [Google Scholar] [CrossRef]
- Pérez, F.L. Plant-induced spatial patterns of surface soil properties near caulescent Andean rosettes. Geoderma 1995, 68, 101–121. [Google Scholar] [CrossRef]
- Tiedemann, A.R.; Klemmedson, O. Nutrient availability in desert grassland soils under mesquite (Prosopis juliflora) trees and adjacent open areas. Proc. Soil Sci. Soc. Am. 1973, 37, 107–111. [Google Scholar] [CrossRef]
- Gersper, P.L.; Holowaychuk, N. Effects of stemflow water on a Miami soil under a beech tree: I. Morphological and physical properties. Proc. Soil Sci. Soc. Am. 1970, 34, 779–786. [Google Scholar] [CrossRef]
- Zinke, P.J.; Crocker, R.L. The influence of giant sequoia on soil properties. For. Sci. 1962, 8, 2–11. [Google Scholar]
- Thomas, D.S.G.; Tsoar, H. The geomorphological role of vegetation in desert dune systems. In Vegetation and Erosion Processes and Environments; Thornes, J.B., Ed.; Wiley: London, UK, 1990; pp. 471–489. [Google Scholar]
- Scarff, F.R.; Westoby, M. Leaf litter flammability in some semi-arid Australian woodlands. Funct. Ecol. 2006, 20, 745–752. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Gongalsky, K.B.; Persson, T.; Bengtsson, J. Connectivity of litter islands remaining after a fire an unburnt forest determines the recovery of soil fauna. Appl. Soil Ecol. 2014, 83, 101–108. [Google Scholar] [CrossRef]
- van Breemen, N.; Finzi, A.C. Plant-soil interactions: Ecological aspects and evolutionary implications. Biogeochemistry 1998, 42, 1–19. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Tongway, D.J.; Marsden, S.G. Stripes, strands or stipples: Modelling the influence of three landscape banding patterns on resource capture and productivity in semi-arid woodlands, Australia. Catena 1999, 37, 257–273. [Google Scholar] [CrossRef]
- Geesing, D.; Felker, P.; Bingham, R.L. Influence of mesquite (Prosopis glandulosa) on soil nitrogen and carbon development: Implications for global carbon sequestration. J. Arid Environ. 2000, 46, 157–180. [Google Scholar] [CrossRef]
- Whitford, W.G.; Anderson, J.; Rice, P.M. Stemflow contribution to the ‘fertile island’ effect in creosote bush, Larrea tridentata. J. Arid Environ. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Roberts, C.; Jones, J.A. Soil patchiness in juniper-sagebrush-grass communities of central Oregon. Plant Soil 2000, 223, 45–61. [Google Scholar] [CrossRef]
- Bochet, E.; Rubio, J.L.; Poesen, J. Modified topsoil islands within patchy Mediterranean vegetation in SE evolutionarySpain. Catena 1999, 38, 23–44. [Google Scholar] [CrossRef]
- Grieve, I.C. Some relationships between vegetation patterns and soil variability in the Forest of Dean, U.K. J. Biogeogr. 1977, 4, 191–199. [Google Scholar] [CrossRef]
- Crocker, R.L.; Dickson, B.A. Soil development on recessional moraines of the Herbert and Mendenhall glaciers, southeastern Alaska. J. Ecol. 1957, 45, 169–185. [Google Scholar] [CrossRef]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawai’i, Two Volumes; Bishop Museum Special Publication 83; University Hawai’i Press: Honolulu, HI, USA, 1990; 1854p. [Google Scholar]
- Friar, E.A.; Prince, L.M.; Roalson, E.H.; McGlaughlin, M.E.; Cruse-Sanders, J.M.; De Groot, S.J.; Porter, J.M. Ecological speciation in the east Maui-endemic Dubautia (Asteraceae) species. Evolution 2006, 60, 1777–1792. [Google Scholar] [PubMed]
- Pérez, F.L. Hot soils and cooler stones: Geoecological influence of volcanic tephra and boulders on soil temperature, and significance for plant distribution in Haleakalā Crater (Maui, Hawai’i). Catena 2017, 158, 9–19. [Google Scholar] [CrossRef]
- Kitayama, J.; Mueller-Dombois, D. Vegetation on the wet windward slope of Haleakala, Maui, Hawaii. Pac. Sci. 1992, 46, 197–220. [Google Scholar]
- Robichaux, R.H.; Canfield, J.E. Tissue elastic properties of eight Hawaiian Dubautia species that differ in habitat and diploid chromosome number. Oecologia 1985, 66, 77–80. [Google Scholar] [CrossRef]
- Robichaux, R.H.; Carr, G.D.; Liebmann, M.; Pearcy, R.W. Adaptive radiation of the Hawaiian silversword alliance (Compositae-Madiinae): Ecological, morphological, and physiological diversity. Ann. Mo. Bot. Gard. 1990, 77, 64–72. [Google Scholar] [CrossRef]
- Pérez, F.L. Steady as a rock: Biogeomorphic influence of nurse rocks and slope processes on kūpaoa (Dubautia menziesii) shrubs in Haleakalā Crater (Maui, Hawai’i). Geomorphology 2017, 295, 631–644. [Google Scholar] [CrossRef]
- Pérez, F.L. Influence of substrate on the distribution of the Hawaiian silversword (Argyroxiphium sandwicense DC.) in Haleakala (Maui, HI). Geomorphology 2003, 55, 173–202. [Google Scholar] [CrossRef]
- Macdonald, G.A.; Abbott, A.T.; Peterson, A.T. Volcanoes in the Sea. The Geology of Hawaii; University Hawai’i Press: Honolulu, HI, USA, 1983. [Google Scholar]
- Sherrod, D.R.; Nishimitsu, Y.; Tagami, T. New K-Ar ages and the geologic evidence against rejuvenated-stage volcanism at Haleakalā, East Maui, a postshield volcano of the Hawaiian island chain. GSA Bull. 2003, 115, 683–694. [Google Scholar] [CrossRef]
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.P.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.K.; Delparte, D.M. Online Rainfall Atlas of Hawaii. Bull. Am. Meteorol. Soc. 2013, 313–316. [Google Scholar] [CrossRef]
- Blumenstock, D.I.; Price, S. Climates of the States: Hawaii. Climatography of the United States 60-51; US Department Commerce Publ.: Washington, DC, USA, 1967; pp. 1–27.
- Giambelluca, T.W.; Nullet, D. Influence of the trade-wind inversion on the climate of a leeward mountain slope in Hawaii. Clim. Res. 1991, 1, 207–216. [Google Scholar] [CrossRef]
- Pérez, F.L. The role of tephra covers on soil moisture conservation at Haleakala’s crater (Maui, Hawai’i). Catena 2009, 76, 191–205. [Google Scholar] [CrossRef]
- Whiteaker, L.D. The vegetation and environment in the Crater District of Haleakala National Park. Pac. Sci. 1983, 37, 1–24. [Google Scholar]
- Noguchi, Y.; Tabuchi, H.; Hasegawa, H. Physical factors controlling the formation of patterned ground on Haleakala, Maui. Geogr. Annl. 1987, 69A, 329–342. [Google Scholar] [CrossRef]
- Siegel, S.M.; Carroll, P.; Corn, C.; Speitel, T. Experimental studies on Hawaiian silverswords (Argyroxiphium spp.): Some preliminary notes on germination. Bot. Gaz. 1970, 131, 277–280. [Google Scholar] [CrossRef]
- Goldstein, G.; Drake, D.R.; Melcher, P.; Giambelluca, T.W.; Heraux, J. Photosynthetic gas exchange and temperature-induced damage in seedlings of the tropical alpine species Argyroxiphium sandwicense. Oecologia 1996, 106, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.L. Biogeomorphic influence of soil depth to bedrock, volcanic ash soils, and surface tephra on silversword distribution, Haleakalā Crater (Maui, Hawai’i). Geomorphology 2015, 243, 75–86. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Sherrod, D.R.; Hagstrum, J.T.; McGeehin, J.P.; Champion, D.E.; Trusdell, F.A. Distribution, 14C chronology, and paleomagnetism of latest Pleistocene and Holocene lava flows at Haleakalā volcano, Island of Maui, Hawai’i: A revision of lava flow hazard zones. J. Geophys. Res. B 2006, 111, B05205. [Google Scholar] [CrossRef]
- USGS (US Geological Survey). The Age of Young Lava Flows on Haleakala’s Crater Floor, East Maui Volcano; U.S. Geological Survey, Hawaiian Volcano Observatory: Reston, VA, USA, 2003. Available online: www.accuracyingenesis.com/haleak.html (accessed on 8 March 2019).
- Kleier, C.; Rundel, P. Microsite requirements, population structure and growth of the cushion plant Azorella compacta in the tropical Chilean Andes. Aust. Ecol. 2004, 29, 461–470. [Google Scholar] [CrossRef]
- Kittredge, J. Some characteristics of forest floors from a variety of forest types in California. J. For. 1955, 53, 646–647. [Google Scholar]
- Brunori, F.; Penzo, M.C.; Torri, D. Soil shear strength: Its measurement and soil detachability. Catena 1989, 16, 59–71. [Google Scholar] [CrossRef]
- Davidson, D.T. Penetrometer measurements. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 472–484. [Google Scholar]
- Zimbone, S.M.; Vickers, A.; Morgan, R.P.C.; Vella, P. Field investigations of different techniques for measuring surface soil shear strength. Soil Technol. 1996, 9, 101–111. [Google Scholar] [CrossRef]
- Bouwer, H. Intake rate: Cylinder infiltrometer. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1986; Volume 9, pp. 825–844. [Google Scholar]
- Brotherson, J.D.; Rushforth, S.R. Influence of cryptogamic crusts on moisture relationships of soils in Navajo National Monument, Arizona. Great Basin Nat. 1983, 43, 73–78. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks, 3rd ed.; Hemphill: Austin, TX, USA, 1980. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy; Agriculture Handbook 436; Natural Resources Conservation Service: Washington, DC, USA, 1975.
- Munsell Soil Color Charts; Macbeth, Kollmorgen Instruments Corp.: Newburgh, NY, USA, 1992.
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Smith, R.T.; Atkinson, K. Techniques in Pedology. A Handbook for Environmental and Resource Studies; Elek Science: London, UK, 1975. [Google Scholar]
- Pitty, A.F. Geography and Soil Properties; Methuen: London, UK, 1979. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Nathan, M.V.; Stecker, J.A.; Sun, Y. Soil Testing in Missouri. A Guide for Conducting Soil Tests in Missouri; Division of Plant Sciences, College of Agriculture, Food and Natural Resources, University of Missouri: Columbia, MO, USA, 2012. [Google Scholar]
- Nathan, M.V.; Sun, Y. Methods for Plant Analysis. A Guide for Conducting Plant Analysis in Missouri; Division of Plant Sciences, University of Missouri Soil and Plant Testing Laboratory: Columbia, MO, USA, 2006. [Google Scholar]
- d’Agostino, R.; Pearson, E.S. Tests for departure from normality. Empirical results for the distributions of b2 and √b1. Biometrika 1973, 60, 613–622. [Google Scholar] [CrossRef]
- Jones, T.A. Skewness and kurtosis as criteria of normality in observed frequency distributions. J. Sediment. Petrol. 1969, 39, 1622–1627. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; Freeman: San Francisco, CA, USA, 1995. [Google Scholar]
- Miller, R.L.; Kahn, J.S. Statistical Analysis in the Geological Sciences; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables, 3rd ed.; Freeman: San Francisco, CA, USA, 1994. [Google Scholar]
- Bailey, N.T.J. Statistical Methods in Biology, 3rd ed.; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Tiedemann, A.R.; Klemmedson, O. Effect of mesquite trees on vegetation and soils in the desert grassland. J. Range Manag. 1977, 30, 361–367. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry. Genesis, Composition, Reactions; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Schaetzl, R.J.; Anderson, S. Soils. Genesis and Geomorphology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Mahaney, W.C.; Voros, J.; Krishnamani, R.; Hancock, R.G.V.; Aufreiter, S.; Milner, M.W.; Allen, C.C.R. Physico-geochemical and mineral composition of neem tree soils and relation to organic properties. Geogr. Annl. 2016, 98A, 143–154. [Google Scholar] [CrossRef]
- Kosmas, C.S.; Moustakas, N.; Danalatos, N.G.; Yassoglou, N. The Spata field site: I. The impacts of land use and management on soil properties and erosion. II. The effect of reduced moisture on soil properties and wheat production. In Mediterranean Desertification and Land Use; Brandt, C.J., Thornes, J.B., Eds.; Wiley: New York, NY, USA, 1996; pp. 207–228. [Google Scholar]
- Krull, E.S.; Skjemstad, J.O.; Baldock, J.A. Functions of Soil Organic Matter and the Effect on Soil Properties; CSIRO Land & Water, PMB 2: Glen Osmond, Australia, 2004; pp. 1–129. [Google Scholar]
- Nanzyo, M.; Shoji, S.; Dahlgren, R. Physical characteristics of volcanic ash soils. In Volcanic Ash Soils: Genesis, Properties and Utilization; Shoji, S., Nanzyo, M., Dahlgren, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 189–207. [Google Scholar]
- Gemma, J.N.; Koske, R.E.; Habte, M. Mycorrhizal dependency of some endemic and endangered Hawaiian plant species. Am. J. Bot. 2002, 89, 337–345. [Google Scholar] [CrossRef]
- Serna, M.D.; Borras, R.; Legaz, F.; Primo-Millo, E. The influence of nitrogen concentration and ammonium/nitrate ratio on N-uptake, mineral composition and yield of citrus. Plant Soil 1992, 147, 13–23. [Google Scholar] [CrossRef]
- Schmid, R. Descriptive nomenclature and classification of pyroclastic deposits and fragments: Recommendations of the IUGS Subcommission on the Systematics of Igneous Rocks. Geology 1981, 9, 41–43. [Google Scholar] [CrossRef]
- De Paepe, P.; Stoops, G. A classification of tephra in volcanic soils. A tool for soil scientists. In Soils of Volcanic Regions in Europe; Arnalds, O., Bartoli, F., Buurman, P., Oskarsson, H., Stoops, G., García-Rodeja, E., Eds.; Springer: Berlin, Germany, 2007; pp. 119–125. [Google Scholar]
- Soane, B.D. The role of organic matter in soil compactibility: A review of some practical aspects. Soil Tillage Res. 1990, 16, 179–201. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Water infiltration and soil structure related to organic matter and its stratification with depth. Soil Tillage Res. 2002, 66, 197–205. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Ann. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.P.D.; Voigt, P.J. Vegetation litter: An underestimated variable in hydrology and geomorphology. J. Biogeogr. 1977, 4, 253–274. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Foth, H.D. Fundamentals of Soil Science; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Shoji, S.; Nanzyo, M.; Dahlgren, R. Productivity and utilization of volcanic ash soils. In Volcanic Ash Soils: Genesis, Properties and Utilization; Shoji, S., Nanzyo, M., Dahlgren, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 209–251. [Google Scholar]
- Nanzyo, M.; Yamasaki, S.-I. Phosphorus-bearing mineral in fresh andesite and rhyolite tephras in northern part of Japan. Phosphorus Res. Bull. 1998, 8, 95–100. [Google Scholar] [CrossRef][Green Version]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Haydu-Houdeshell, C.-A.; Graham, R.G.; Hendrix, P.F.; Peterson, A.C. Soil aggregate stability under chaparral species in southern California. Geoderma 2018, 310, 201–208. [Google Scholar] [CrossRef]
- National Park Service. Subalpine Shrubland. Plants of Haleakala National Park. U.S. National Park Service. n.d. Available online: https://www.nps.gov/hale/learn/nature/upload/Subalpine-Shrubland.pdf (accessed on 25 September 2017).
- Krushelnycky, P.D.; Starr, F.; Starr, K.; Longman, R.J.; Frazier, A.G.; Loope, L.L.; Giambelluca, T.W. Change in trade wind inversion frequency implicated in the decline of an alpine plant. Clim. Chang. Response 2016, 3, 1. [Google Scholar] [CrossRef]
- Ekern, P.C. Direct interception of cloud water on Lanaihale, Hawaii. Proc. Soil Sci. Soc. Am. 1964, 28, 419–421. [Google Scholar] [CrossRef]
- Hartt, C.E.; Neal, M.C. The plant ecology of Mauna Kea, Hawaii. Ecology 1940, 21, 237–266. [Google Scholar] [CrossRef]
- Mueller-Dombois, D. Ecological relations in the alpine and subalpine vegetation on Mauna Loa, Hawaii. J. Indian Bot. Soc. 1967, 96, 403–411. [Google Scholar]
- Kobayashi, H.K. Ecology of the Silversword, Argyroxiphium sandwicense DC. (Compositae), Haleakala Crater, Hawaii. Ph.D. Dissertation, University of Hawai’i, Honolulu, HI, USA, 1973. [Google Scholar]
- Ritter, A.; Regalado, C.M.; Guerra, J.C. Quantification of fog water collection in three locations of Tenerife (Canary Islands). Water 2015, 7, 3306–3319. [Google Scholar] [CrossRef]
- Prada, S.; Menezes de Sequeira, M.; Figueira, C.; Vasconcelos, R. Cloudwater interception in the high altitude tree heath forest (Erica arborea L.) of Paul da Serra massif (Madeira, Portugal). Hydrol. Process. 2012, 26, 202–212. [Google Scholar] [CrossRef]
- Hendrix, L.B. Post-eruption succession on Isla Fernandina, Galápagos. Madroño 1981, 28, 242–254. [Google Scholar]
- Jäger, H.; Tye, A.; Kowarik, I. Destruction without extinction: Long-term impacts of an invasive tree species on Galápagos highland vegetation. J. Ecol. 2009, 97, 1252–1263. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T.A. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Pérez, F.L. Some effects of giant Andean stem-rosettes on ground microclimate, and their ecological significance. Int. J. Biometeorol. 1989, 33, 131–135. [Google Scholar] [CrossRef]
- van Breemen, N. Soils as biotic constructs favouring net primary productivity. Geoderma 1993, 57, 183–212. [Google Scholar] [CrossRef]



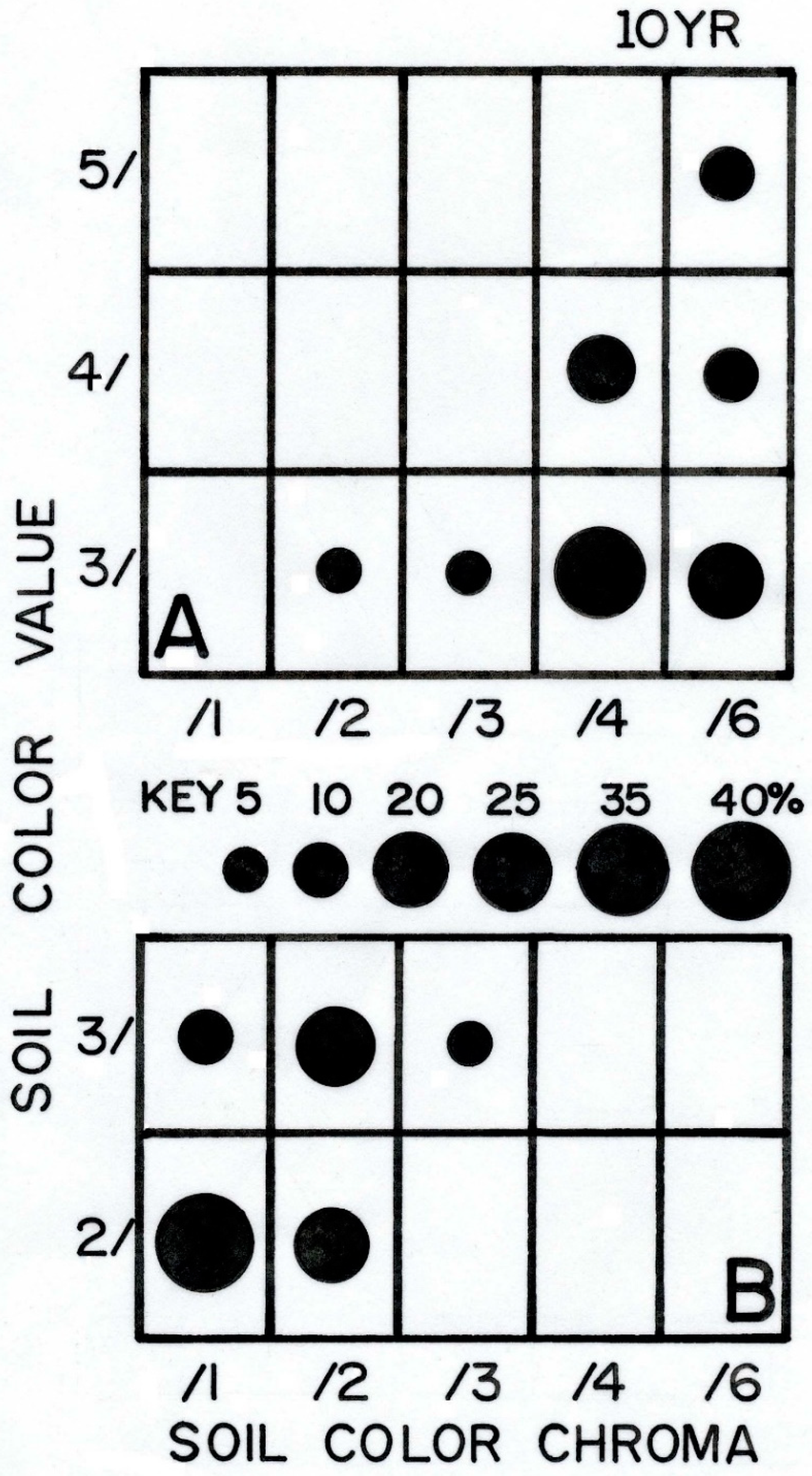


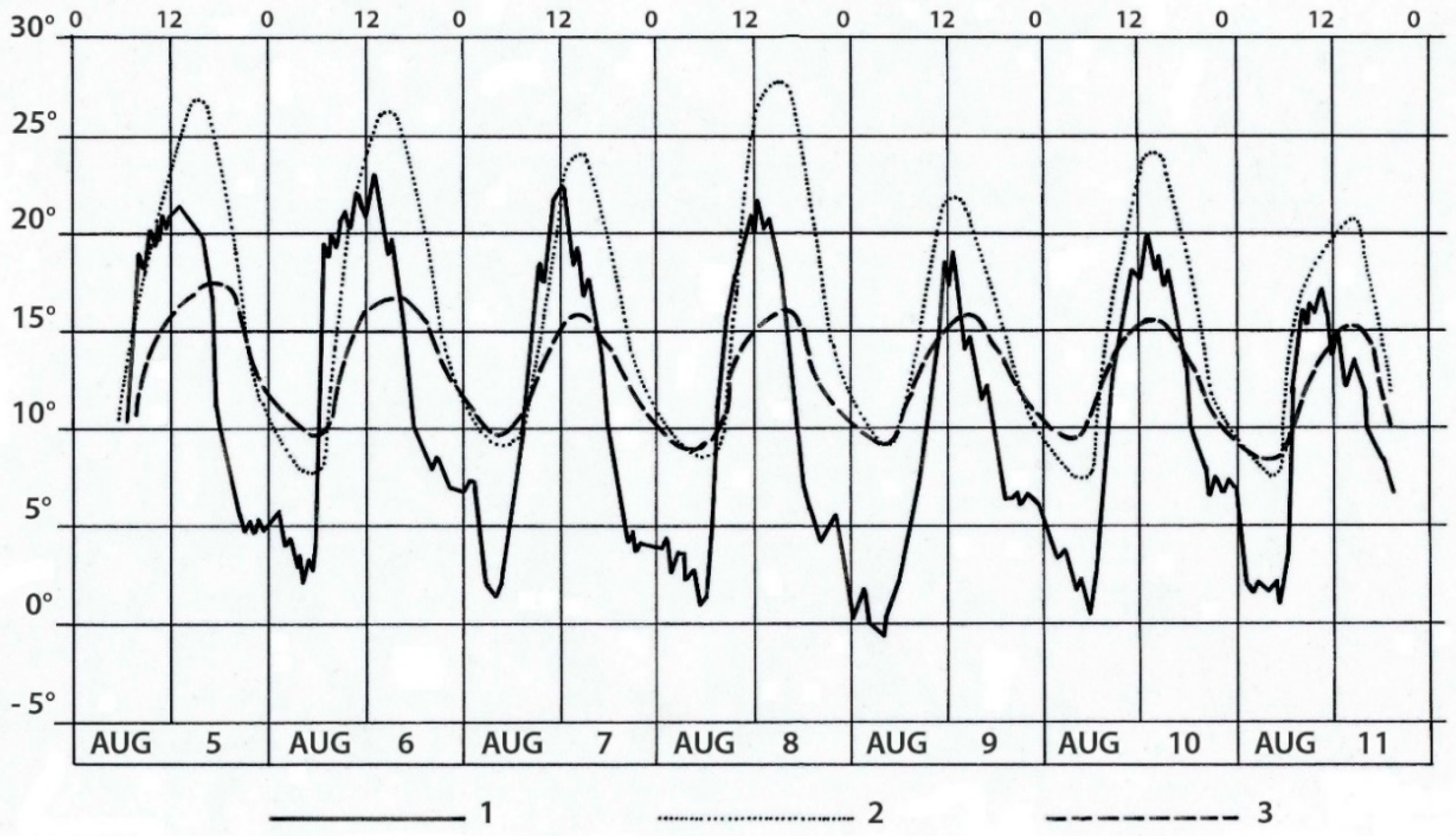
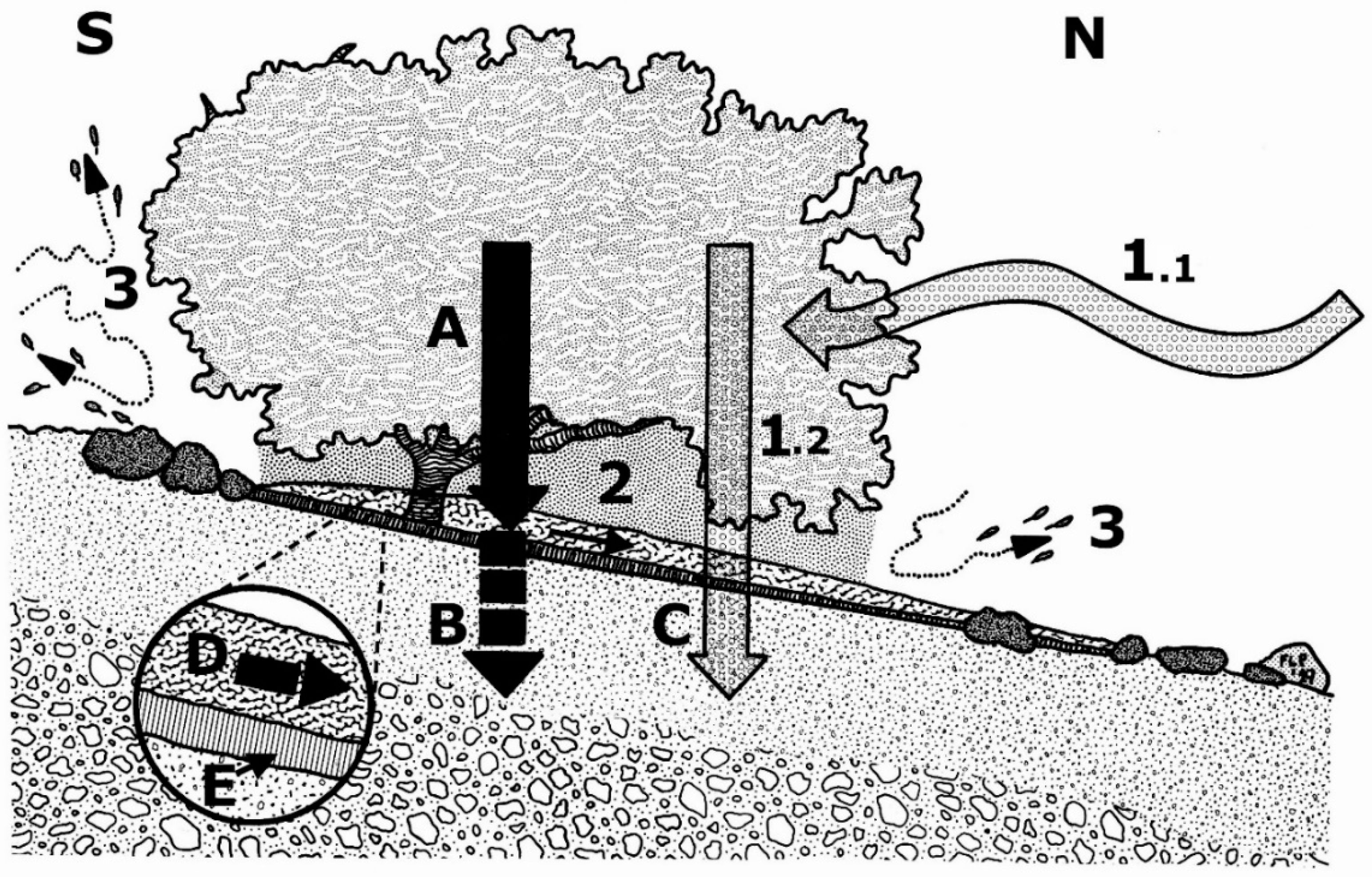
| Property | Average ± S.D. | Lowest Value | Highest Value |
|---|---|---|---|
| Litter thickness, mm | 41.9 ± 17.6 | 22 | 73 |
| Litter island width, cm | 177.0 ± 56.5 | 89 | 253 |
| Litter island length, cm | 130.4 ± 41.5 | 73 | 206 |
| Litter island area, m2 | 1.917 ± 1.0 | 0.566 | 3.608 |
| Slope angle, ° | 16.2 ± 6.8 | 4 | 24 |
| Shrub height, cm | 110.6 ± 25.7 | 68 | 152 |
| Shrub width, cm | 202.5 ± 37.0 | 131 | 253 |
| Shrub length, cm | 175.7 ± 47.8 | 111 | 268 |
| Litter Properties | Kūpaoa Litter | Silversword Litter |
|---|---|---|
| Color, mode | 10YR 5/1 to 6/1, gray | 10YR 4/1, dark gray |
| Water content, % | 309.7 ± 50.4 | 450.9 ± 121.3 |
| Total Nitrogen, % | 0.504 ± 0.08 | 0.281 ± 0.22 |
| Calcium, % | 2.81 ± 0.23 | 0.78 ± 0.43 |
| Magnesium, % | 0.42 ± 0.09 | 0.04 ± 0.03 |
| Potassium, % | 0.21 ± 0.04 | 0.02 ± 0.01 |
| Phosphorus, % | 0.048 ± 0.01 | 0.004 ± 0.001 |
| CEC, cmol+ kg−1 | 182.6 ± 10.1 | 43.4 ± 24.0 |
| pH | 5.92 ± 0.17 | 6.31 ± 0.2 |
| Soil Properties | Bare Soils (Control) near Kūpaoa | Soils under Kūpaoa | Soils under Silverswords |
|---|---|---|---|
| Gravel, % c | 15.7 ± 12.1 | 19.0 ± 13.6 | 14.6 ± 10.5 |
| Sand, % c | 92.8 ± 2.3 | 92.2 ± 2.2 | 94.9 ± 2.0 |
| Fines, % c | 7.2 ± 2.3 | 7.8 ± 2.2 | 5.1 ± 2.0 |
| D50, mm c | 0.56 ± 0.50 | 0.57 ± 0.53 | - |
| Color, mode a | 10YR 3/4, dark yellowish brown | 10YR 2/1, black | 10YR 3/2, very dark grayish brown |
| Organic matter, % a | 1.25 ± 0.36 | 3.38 ± 1.73 | 1.90 ± 0.3 |
| Infiltration, mm/min a | 20.10 ± 9.38 | 103.24 ± 51.6 | - |
| Water content, % a | 30.4 ± 3.0 | 44.9 ± 6.5 | 30.6 ± 3.7 |
| Calcium, ppm a | 326.1 ± 139 | 1321.1 ± 562 | 271.3 ± 90 |
| Magnesium, ppm a | 37.7 ± 16 | 119.6 ± 66 | 22.5 ± 8 |
| Potassium, ppm a | 47.7 ± 20 | 362.5 ± 78 | 134.6 ± 41 |
| CEC (cmol+ kg−1) a | 3.44 ± 0.8 | 9.61 ± 3.6 | 1.89 ± 0.5 |
| Phosphorus, ppm b | 1.1 ± 0.36 | 6.8 ± 4.5 | 9.6 ± 2.7 |
| NO3− N, ppm b | 1.18 ± 0.08 | 41.93 ± 43.3 | - |
| NH4+ N, ppm b | 9.17 ± 8.8 | 16.0 ± 11.49 | - |
| (NO3/NH4) ratio b | 0.22 ± 0.14 | 4.64 ± 5.04 | - |
| Nitrogen, ppm a | 10.35 ± 8.9 | 57.92 ± 46.4 | - |
| pH c | 6.42 ± 0.15 | 6.65 ± 0.26 | 6.88 ± 0.18 |
| Profile 1.Vitrandic Haplustept, on a 7° slope, aspect: N41°E. All samples taken ~15 cm downslope from plant stem, and under the canopy of a kūpaoa shrub, 110 cm-h, 160 cm-w, 111 cm-l. Abundant volcanic tephra cobbles (~5–10 cm) with small rounded vesicles ≤2–5 mm diameter present on ground surface ≤15 cm upslope of the stem base. | |
| Horizon, Depth, cm | Description |
| Oi1 +5.8–0 to Oe1 | Litter horizon 58 mm thick near stem base. Foliage, with a few short twig (≤43 mm long, ≤2.5 mm thick) fragments; lanceolate leaves mostly whole (30-–5 mm long) on top to partially fragmented at the horizon bottom. Fibric organic material, only slightly decomposed. Dry modal color: 10YR 5/1 (gray); pH: 6.04; total N: 0.507%, water-retention capacity (WRC, −33 kPa): 319.1%; no discernible water repellency. Mor litter type with abrupt, smooth boundary to |
| A 0–2.6 | Horizon 8–26 mm thick is a firm, cohesive to slightly fragile, frangible, aggregated crust horizon; well-developed platy structure, can be detached but easily splits into fragments ≤44 × 40 mm. Dry color: 10YR 2/1(black). Shear strength (mean 10 readings): 0.459 kg/cm2. Shear strength and horizon thickness were highest near shrub stem; both gradually drop toward the periphery of litter island. Compressibility (mean 10 readings): 1.320 kg/cm2. Abrupt, smooth boundary to |
| A1 2.6–5 | Sand, with 29.1% gravel; D50: 0.82 mm; soil fraction: 91.7% sand, 8.3% fines. Friable, single-grain structureless dry consistence; slightly sticky, non-plastic wet consistence. Mild water repellency. Dry color: 10YR 3/2, very dark grayish brown, organic matter: 2.8%, pH: 6.70. CEC: 6.5 cmol+ kg−1; WRC: 39.3%. Clear, smootYes, it is correct. ‘kPa’ is the symbol for kilopascals, and is used in several other places in the text. h boundary to |
| A2 5–29 | Sand, with 45.6% gravel, mostly coarse volcanic cinder with small vesicles; D50: 1.83 mm; soil fraction: 91.3% sand, 8.7% fines. Friable, single-grain structureless dry consistence; slightly sticky, non-plastic wet consistence. No water repellency. Dry color: 10YR 4/4 (dark yellowish brown), organic matter: 2.4%, pH: 6.72. CEC: 6.0 cmol+ kg−1; WRC: 36.5%. Clear, smooth boundary to |
| A3 29–36 | Sand, with 45.2% gravel; D50: 1.70 mm; soil fraction: 89.6% sand, 10.4% fines. Friable, single-grain structureless; very slightly sticky, non-plastic wet consistence. No water repellency. Color: 10YR 3/3, (dark brown), organic matter: 2.5%, pH: 6.68; CEC: 6.3 cmol+ kg−1; WRC: 37.1%. Gradual, smooth boundary to |
| C 36–45+ | Coarse sand, with abundant (≥60%) gravel composed of pebble- and cobble-sized volcanic cinder and tephra prevented further auger penetration, D50: ~32.0 mm. |
| Profile 2Vitrandic Haplustept, ~200 cm away and on one side, of the canopy edge of shrub where profile 1 was examined, on bare soil, with same slope angle and orientation. Numerous volcanic-tephra cobbles (~5–10 cm) and pebbles (2.5–5 cm) present on the site. | |
| Horizon, Depth, cm | Description |
| A 0–2 | Sand, with 48.4% gravel, mainly coarse volcanic cinder with small vesicles; D50: 1.98 mm; soil fraction: 90.9% sand, 9.1% fines. Friable, single-grain structureless. Dry color: 10YR 4/6 (dark yellowish brown), organic matter: 1.7%, pH 6.63; WRC: 29.8%; no water repellency through whole profile. Clear, smooth boundary to |
| A1 2–11 | Dry, dusty sand horizon, with 30.4% gravel; D50: 0.81 mm; soil fraction: 88.7% sand, 11.3% fines. Friable, single-grain structureless. Dry color: 10YR 5/6 (yellowish brown), organic matter: 1.8%, pH: 6.6. CEC: 3.9 cmol+ kg−1; WRC: 27.4% water. Shear strength (mean 10 readings): 0.171 kg/cm2. Compressibility (mean 10 readings): 0.084 kg/cm2. Clear, smooth boundary to |
| A2 11–43 | Sand, with 40.9% gravel, volcanic cinder; D50: 1.54 mm; soil fraction: 93.6% sand, 6.4% fines. Friable, single-grain structureless. Dry color: 10YR 4/4 (dark yellowish brown), organic matter: 1.7%, pH: 6.7. Gradual smooth boundary to |
| C1 43–85 | Sand, with 45.3% gravel, volcanic cinder; D50: 1.77 mm; soil fraction: 92.2% sand, 7.8% fines. Friable, single-grain structureless. Dry color: 10YR 3/2 (very dark grayish brown), organic matter: 0.6%, pH: 6.73. Gradual, smooth boundary to |
| C2 85–95+ | Coarse sand, with abundant (≥75%) gravel composed of cobble- and pebble-sized volcanic tephra and cinder prevented further auger penetration, D50: ~45.0 mm. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, F.L. Plant Organic Matter Really Matters: Pedological Effects of Kūpaoa (Dubautia menziesii) Shrubs in a Volcanic Alpine Area, Maui, Hawai’i. Soil Syst. 2019, 3, 31. https://doi.org/10.3390/soilsystems3020031
Pérez FL. Plant Organic Matter Really Matters: Pedological Effects of Kūpaoa (Dubautia menziesii) Shrubs in a Volcanic Alpine Area, Maui, Hawai’i. Soil Systems. 2019; 3(2):31. https://doi.org/10.3390/soilsystems3020031
Chicago/Turabian StylePérez, Francisco L. 2019. "Plant Organic Matter Really Matters: Pedological Effects of Kūpaoa (Dubautia menziesii) Shrubs in a Volcanic Alpine Area, Maui, Hawai’i" Soil Systems 3, no. 2: 31. https://doi.org/10.3390/soilsystems3020031
APA StylePérez, F. L. (2019). Plant Organic Matter Really Matters: Pedological Effects of Kūpaoa (Dubautia menziesii) Shrubs in a Volcanic Alpine Area, Maui, Hawai’i. Soil Systems, 3(2), 31. https://doi.org/10.3390/soilsystems3020031




