Soil N2O, CH4, and CO2 Fluxes in Forest, Grassland, and Tillage/No-Tillage Croplands in French Guiana (Amazonia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
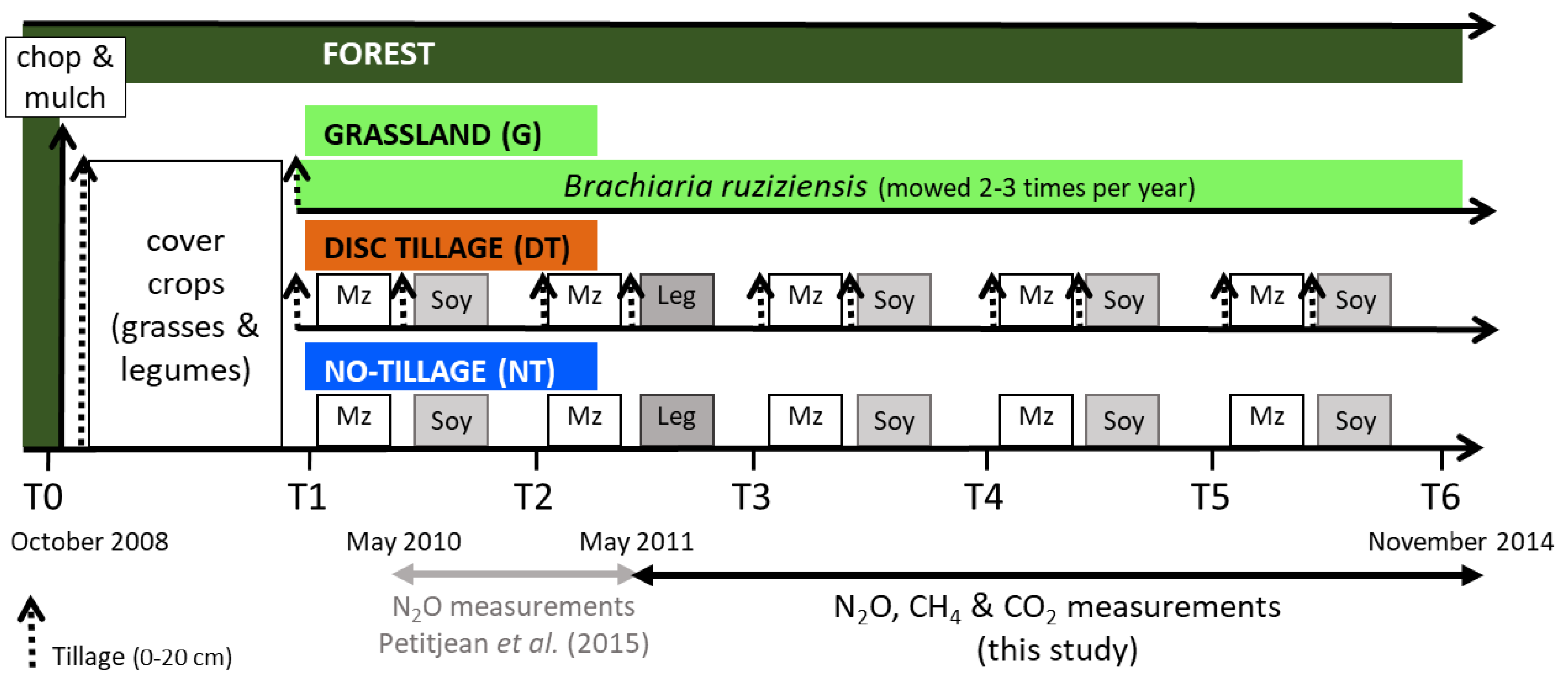
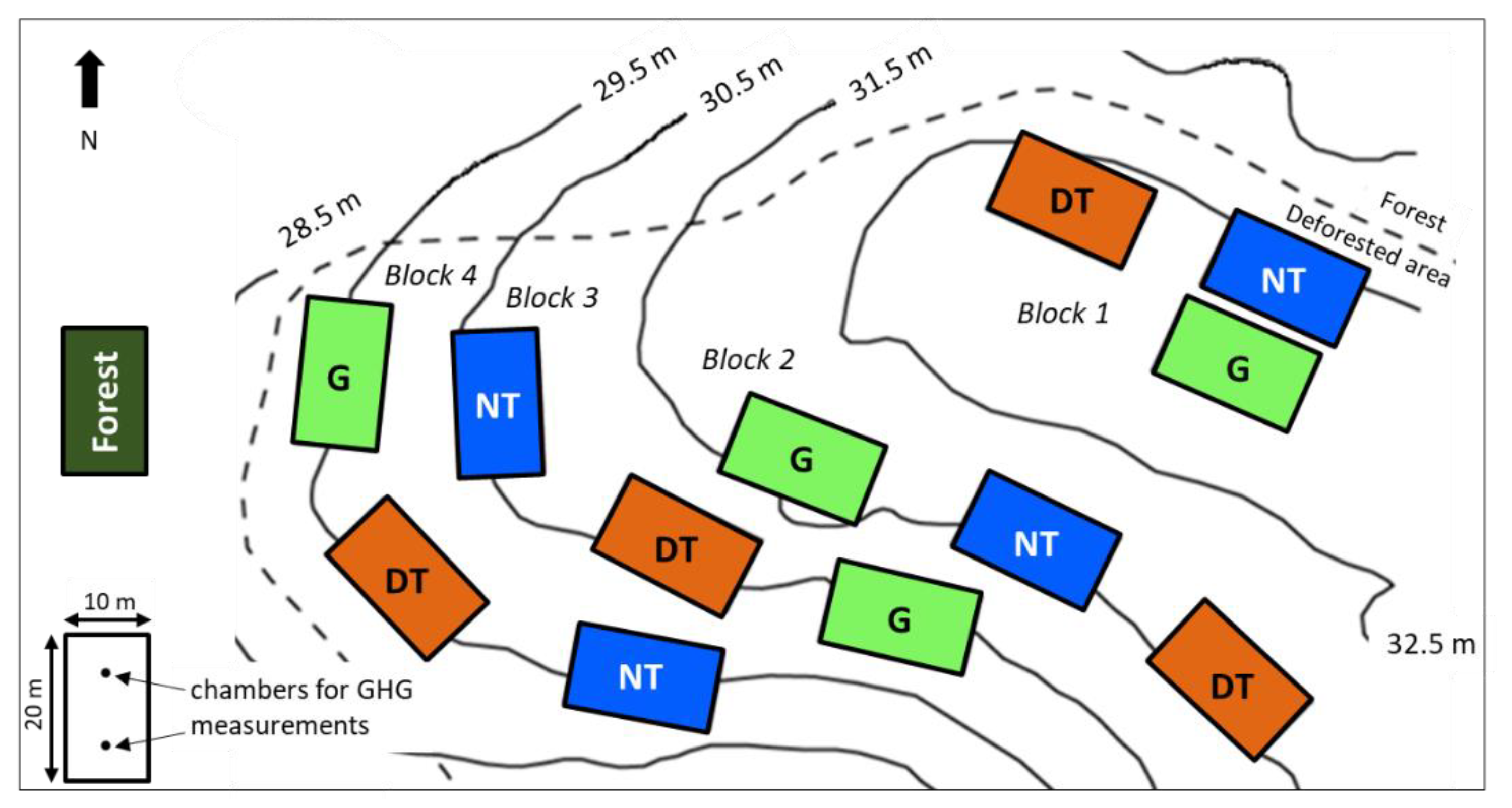
2.2. Experimental Design and Agricultural Systems
- Grassland (G): Grassland of Brachiaria ruziziensis cv. ruzi mowed 2–3 times per year;
- Disc tillage (DT): Maize (Zea mays L.)/soybean (Glycine max L. Merr) crop rotation with disc tillage using two passes of a heavy disc harrow (0–20 cm);
- No-tillage (NT): Maize (Zea mays L.)/soybean (Glycine max L. Merr) rotation under no-tillage with direct seeding.
2.3. Measurements of Trace Gas Fluxes
2.4. Rainfall and Soil Parameter Measurements
2.5. Statistical Analyses
3. Results
3.1. Rainfall
3.2. Soil Parameters and GHG Fluxes
4. Discussion
4.1. Soil N2O fluxes
4.2. Soil CH4 Fluxes
4.3. Soil CO2 Fluxes
4.4. Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- INSEE (Institut National de la Statistique et des Etudes Economiques). Recensement de la population en Guyane. La démographie guyanaise toujours aussi dynamique. INSEE Anal. Guyane 2018, 4. [Google Scholar]
- INSEE (Institut National de la Statistique et des Etudes Economiques). Projections de population à l’horizon 2040: Plus d’un demi-million de Guyanais. INSEE Guyane 2011, 71, 4. [Google Scholar]
- FAO and ITTO (Food and Agriculture Organization and International Tropical Timber Organization). The State of Forests in the Amazon Basin, Congo Basin and Southeast Asia a Report Prepared for the Summit of the Three Rainforest Basins; Food and Agriculture Organization: Rome, Italy, 2011; ISBN 978-92-5-106888-5. [Google Scholar]
- IGN (Institut National de l’Information Géographique et Forestière). Evolution de l’occupation des sols en Guyane française entre 1990 et 2012. L’if Feuille L’inventaire For. IGN 2015, 35, 9. [Google Scholar]
- Tsayem Demaze, M.; Manusset, S. L’agriculture itinérante sur brûlis en Guyane française: La fin des durabilités écologique et socioculturelle? Les Cah. D’outre-Mer 2008, 61, 31–48. [Google Scholar] [CrossRef]
- Tsayem Demaze, M. Croissance démographique, pression foncière et insertion territoriale par les abattis en Guyane française. Norois 2008, 206, 111–127. [Google Scholar] [CrossRef]
- Davidson, E.A.; de Abreu Sa, T.D.; Reis Carvalho, C.J.; de Oliveira Figueiredo, R.; Kato, M.D.; Kato, O.R.; Ishida, F.Y. An integrated greenhouse gas assessment of an alternative to slash-and-burn agriculture in eastern Amazonia. Glob. Chang. Biol. 2008, 14, 998–1007. [Google Scholar] [CrossRef]
- Mackensen, J.; Hölscher, D.; Klinge, R.; Fölster, H. Nutrient transfer to the atmosphere by burning of debris in eastern Amazonia. For. Ecol. Manag. 1996, 86, 121–128. [Google Scholar] [CrossRef]
- Sommer, R.; Vlek, P.L.G.; Deane de Abreu Sá, T.; Vielhauer, K.; de Fátima Rodrigues Coelho, R.; Fölster, H. Nutrient balance of shifting cultivation by burning or mulching in the Eastern Amazon—Evidence for subsoil nutrient accumulation. Nutr. Cycl. Agroecosyst. 2004, 68, 257–271. [Google Scholar] [CrossRef]
- Soares Neto, T.G.; Carvalho, J.A.; Cortez, E.V.; Azevedo, R.G.; Oliveira, R.A.; Fidalgo, W.R.R.; Santos, J.C. Laboratory evaluation of Amazon forest biomass burning emissions. Atmos. Environ. 2011, 45, 7455–7461. [Google Scholar] [CrossRef]
- Farella, N.; Lucotte, M.; Davidson, R.; Daigle, S. Mercury release from deforested soils triggered by base cation enrichment. Sci. Total Environ. 2006, 368, 19–29. [Google Scholar] [CrossRef]
- Feigl, B.J.; Steudler, P.A.; Cerri, C.C. Effects of pasture introduction on soil CO2 emissions during the dry season in the state of Rondônia, Brazil. Biogeochemistry 1995, 31, 1–14. [Google Scholar] [CrossRef]
- Steudler, P.A.; Melillo, J.M.; Feigl, B.J.; Neill, C.; Piccolo, M.C.; Cerri, C.C. Consequence of forest-to-pasture conversion on CH4 fluxes in the Brazilian Amazon Basin. J. Geophys. Res. Phys. 1996, 101, 18547–18554. [Google Scholar] [CrossRef]
- Garcia-Montiel, D.C.; Steudler, P.A.; Piccolo, M.C.; Melillo, J.M.; Neill, C.; Cerri, C.C. Controls on soil nitrogen oxide emissions from forest and pastures in the Brazilian Amazon. Glob. Biogeochem. Cycles 2001, 15, 1021–1030. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A.; Feigl, B.J.; Neill, C.; Garcia, D.; Piccolo, M.C.; Cerri, C.C.; Tian, H. Nitrous oxide emissions from forests and pastures of various ages in the Brazilian Amazon. J. Geophys. Res. Atmos. 2001, 106, 34179–34188. [Google Scholar] [CrossRef]
- Garcia-Montiel, D.C.; Steudler, P.A.; Piccolo, M.; Neill, C.; Melillo, J.; Cerri, C.C. Nitrogen Oxide Emissions Following Wetting of Dry Soils in Forest and Pastures in Rondônia, Brazil. Biogeochemistry 2003, 64, 319–336. [Google Scholar] [CrossRef]
- Neill, C.; Steudler, P.A.; Garcia-Montiel, D.C.; Melillo, J.M.; Feigl, B.J.; Piccolo, M.C.; Cerri, C.C. Rates and controls of nitrous oxide and nitric oxide emissions following conversion of forest to pasture in Rondônia. Nutr. Cycl. Agroecosyst. 2005, 71, 1–15. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattânio, H.; Ackerman, I.L.; Erickson, H.E.; Keller, M. Land use change and biogeochemical controls of nitrogen oxide emissions from soils in eastern Amazonia. Glob. Biogeochem. Cycles 1999, 13, 31–46. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattânio, J.H.; Ackerman, I.L. Land-use change and biogeochemical controls of methane fluxes in soils of Eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Passianoto, C.C.; Ahrens, T.; Feigl, B.J.; Steudler, P.A.; do Carmo, J.B.; Melillo, J.M. Emissions of CO2, N2O, and NO in conventional and no-till management practices in Rondônia, Brazil. Biol. Fertil. Soils 2003, 38, 200–208. [Google Scholar] [CrossRef]
- Meurer, K.H.E.; Franko, U.; Stange, C.F.; Rosa, J.D.; Madari, B.E.; Jungkunst, H.F. Direct nitrous oxide (N2O) fluxes from soils under different land use in Brazil—A critical review. Environ. Res. Lett. 2016, 11, 023001. [Google Scholar] [CrossRef]
- Mosier, A.; Wassmann, R.; Verchot, L.; King, J.; Palm, C. Methane and Nitrogen Oxide Fluxes in Tropical Agricultural Soils: Sources, Sinks and Mechanisms. Environ. Dev. Sustain. 2004, 6, 11–49. [Google Scholar] [CrossRef]
- Mohanty, S.; Kollah, B.; Chaudhary, R.S.; Singh, A.B.; Singh, M. Methane uptake in tropical soybean–wheat agroecosystem under different fertilizer regimes. Environ. Earth Sci. 2015, 74, 5049–5061. [Google Scholar] [CrossRef]
- Palm, C.A.; Alegre, J.C.; Arevalo, L.; Mutuo, P.K.; Mosier, A.R.; Coe, R. Nitrous oxide and methane fluxes in six different land use systems in the Peruvian Amazon. Glob. Biogeochem. Cycles 2002, 16, 21–1–21–13. [Google Scholar] [CrossRef]
- Choudhary, M.; Akramkhanov, A.; Saggar, S. Nitrous oxide emissions from a New Zealand cropped soil: Tillage effects, spatial and seasonal variability. Agric. Ecosyst. Environ. 2002, 93, 33–43. [Google Scholar] [CrossRef]
- Mathieu, O.; Leveque, J.; Henault, C.; Milloux, M.; Bizouard, F.; Andreux, F. Emissions and spatial variability of N2O, N2, and nitrous oxide mole fraction at the field scale, revealed with 15N isotopic techniques. Soil Biol. Biochem. 2006, 38, 941–951. [Google Scholar] [CrossRef]
- McClain, M.E.; Boyer, E.W.; Dent, C.L.; Gergel, S.E.; Grimm, N.B.; Groffman, P.M.; Hart, S.C.; Harvey, J.W.; Johnston, C.A.; Mayorga, E.; et al. Biogeochemical Hot Spots and Hot Moments at the Interface of Terrestrial and Aquatic Ecosystems. Ecosystems 2003, 6, 301–312. [Google Scholar] [CrossRef]
- Potter, C.S.; Davidson, E.A.; Verchot, L.V. Estimation of global biogeochemical controls and seasonality in soil methane consumption. Chemosphere 1996, 32, 2219–2246. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Werner, C.; Kiese, R.; Butterbach-Bahl, K. Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Kort, E.A.; Patra, P.K.; Ishijima, K.; Daube, B.C.; Jiménez, R.; Elkins, J.; Hurst, D.; Moore, F.L.; Sweeney, C.; Wofsy, S.C. Tropospheric distribution and variability of N2O: Evidence for strong tropical emissions. Geophys. Res. Lett. 2011, 38, L15806. [Google Scholar] [CrossRef]
- Castaldi, S.; Bertolini, T.; Valente, A.; Chiti, T.; Valentini, R. Nitrous oxide emissions from soil of an African rain forest in Ghana. Biogeosciences 2013, 10, 4179–4187. [Google Scholar] [CrossRef]
- Buchmann, N.; Guehl, J.-M.; Barigah, T.S.; Ehleringer, J.R. Interseasonal comparison of CO2 concentrations, isotopic composition, and carbon dynamics in an Amazonian rainforest (French Guiana). Oecologia 1997, 110, 120–131. [Google Scholar] [CrossRef]
- Janssens, I.A.; Têtè Barigah, S.; Ceulemans, R. Soil CO2 efflux rates in different tropical vegetation types in French Guiana. Ann. Des. Sci. For. 1998, 55, 671–680. [Google Scholar] [CrossRef]
- Epron, D.; Bosc, A.; Bonal, D.; Freycon, V. Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana. J. Trop. Ecol. 2006, 22, 565–574. [Google Scholar] [CrossRef]
- Bonal, D.; Bosc, A.; Ponton, S.; Goret, J.-Y.; Burban, B.; Gross, P.; Bonnefond, J.-M.; Elbers, J.; Longdoz, B.; Epron, D.; et al. Impact of severe dry season on net ecosystem exchange in the Neotropical rainforest of French Guiana. Glob. Chang. Biol. 2008, 14, 1917–1933. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Alméras, T.; Bonal, D.; Epron, D. Does spatial distribution of tree size account for spatial variation in soil respiration in a tropical forest? Plant Soil 2011, 347, 293–303. [Google Scholar] [CrossRef]
- Courtois, E.A.; Stahl, C.; Van den Berge, J.; Bréchet, L.; Van Langenhove, L.; Richter, A.; Urbina, I.; Soong, J.L.; Peñuelas, J.; Janssens, I.A. Spatial Variation of Soil CO2, CH4 and N2O Fluxes Across Topographical Positions in Tropical Forests of the Guiana Shield. Ecosystems 2018, 21, 1445–1458. [Google Scholar] [CrossRef]
- Petitjean, C.; Hénault, C.; Perrin, A.-S.; Pontet, C.; Metay, A.; Bernoux, M.; Jehanno, T.; Viard, A.; Roggy, J.-C. Soil N2O emissions in French Guiana after the conversion of tropical forest to agriculture with the chop-and-mulch method. Agric. Ecosyst. Environ. 2015, 208, 64–74. [Google Scholar] [CrossRef]
- Perrin, A.-S.; Fujisaki, K.; Petitjean, C.; Sarrazin, M.; Godet, M.; Garric, B.; Horth, J.-C.; Balbino, L.C.; Filho, A.S.; de Almeida Machado, P.L.O.; et al. Conversion of forest to agriculture in Amazonia with the chop-and-mulch method: Does it improve the soil carbon stock? Agric. Ecosyst. Environ. 2014, 184, 101–114. [Google Scholar] [CrossRef]
- Fujisaki, K.; Perrin, A.-S.; Garric, B.; Balesdent, J.; Brossard, M. Soil organic carbon changes after deforestation and agrosystem establishment in Amazonia: An assessment by diachronic approach. Agric. Ecosyst. Environ. 2017, 245, 63–73. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; ISBN 978-92-5-108369-7. [Google Scholar]
- Parkin, T.B.; Venterea, R.T. Sampling Protocols. Chapter 3. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; Follett, R.F., Ed.; USDA-ARS: Washington, DC, USA, 2010; pp. 3-1–3-29. [Google Scholar]
- Aminot, A.; Kérouel, R. Dosage Automatique des Nutriments Dans les Eaux Marines: Méthodes en flux continu. In Méthodes D’analyse en Milieu Marin, 1st ed.; Quae, Ed.; MEDD: Paris, France, 2007; ISBN 978-2-7592-0023-8. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Lmertest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 82. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models: Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, Structure, and Size of N2O-Producing Microbial Communities in Soils—What Matters for Their Functioning? In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 75, pp. 33–70. ISBN 978-0-12-387046-9. [Google Scholar]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Davidson, E.A.; Nepstad, D.C.; Ishida, F.Y.; Brando, P.M. Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2008, 14, 2582–2590. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Van Lent, J.; Hergoualc’h, K.; Verchot, L.V. Reviews and syntheses: Soil N2O and NO emissions from land use and land-use change in the tropics and subtropics: A meta-analysis. Biogeosciences 2015, 12, 7299–7313. [Google Scholar] [CrossRef]
- Schmidt, U.; Thöni, H.; Kaupenjohann, M. Using a boundary line approach to analyze N2O flux data from agricultural soils. Nutr. Cycl. Agroecosyst. 2000, 57, 119–129. [Google Scholar] [CrossRef]
- Laville, P.; Lehuger, S.; Loubet, B.; Chaumartin, F.; Cellier, P. Effect of management, climate and soil conditions on N2O and NO emissions from an arable crop rotation using high temporal resolution measurements. Agric. For. Meteorol. 2011, 151, 228–240. [Google Scholar] [CrossRef]
- Rafique, R.; Hennessy, D.; Kiely, G. Nitrous Oxide Emission from Grazed Grassland under Different Management Systems. Ecosystems 2011, 14, 563–582. [Google Scholar] [CrossRef]
- Ciarlo, E.; Conti, M.; Bartoloni, N.; Rubio, G. The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O+N2) ratio under laboratory conditions. Biol. Fertil. Soils 2007, 43, 675–681. [Google Scholar] [CrossRef]
- Castellano, M.J.; Schmidt, J.P.; Kaye, J.P.; Walker, C.; Graham, C.B.; Lin, H.; Dell, C.J. Hydrological and biogeochemical controls on the timing and magnitude of nitrous oxide flux across an agricultural landscape. Glob. Chang. Biol. 2010, 16, 2711–2720. [Google Scholar] [CrossRef]
- Balaine, N.; Clough, T.J.; Beare, M.H.; Thomas, S.M.; Meenken, E.D.; Ross, J.G. Changes in Relative Gas Diffusivity Explain Soil Nitrous Oxide Flux Dynamics. Soil Sci. Soc. Am. J. 2013, 77, 1496. [Google Scholar] [CrossRef]
- Rabot, E.; Cousin, I.; Hénault, C. A modeling approach of the relationship between nitrous oxide fluxes from soils and the water-filled pore space. Biogeochemistry 2015, 122, 395–408. [Google Scholar] [CrossRef]
- Metay, A.; Oliver, R.; Scopel, E.; Douzet, J.-M.; Aloisio Alves Moreira, J.; Maraux, F.; Feigl, B.J.; Feller, C. N2O and CH4 emissions from soils under conventional and no-till management practices in Goiânia (Cerrados, Brazil). Geoderma 2007, 141, 78–88. [Google Scholar] [CrossRef]
- Jantalia, C.P.; dos Santos, H.P.; Urquiaga, S.; Boddey, R.M.; Alves, B.J.R. Fluxes of nitrous oxide from soil under different crop rotations and tillage systems in the South of Brazil. Nutr. Cycl. Agroecosyste. 2008, 82, 161–173. [Google Scholar] [CrossRef]
- Li, C.; Frolking, S.; Butterbach-Bahl, K. Carbon Sequestration in Arable Soils is Likely to Increase Nitrous Oxide Emissions, Offsetting Reductions in Climate Radiative Forcing. Clim. Chang. 2005, 72, 321–338. [Google Scholar] [CrossRef]
- Rochette, P. No-till only increases N2O emissions in poorly-aerated soils. Soil Tillage Res. 2008, 101, 97–100. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267. [Google Scholar] [CrossRef]
- Palma, R.M.; Rímolo, M.; Saubidet, M.I.; Conti, M.E. Influence of tillage system on denitrification in maize-cropped soils. Biol. Fertil. Soils 1997, 25, 142–146. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Keller, M.; Reiners, W.A. Soil-atmosphere exchange of nitrous oxide, nitric oxide, and methane under secondary succession of pasture to forest in the Atlantic lowlands of Costa Rica. Glob. Biogeochem. Cycles 1994, 8, 399–409. [Google Scholar] [CrossRef]
- Kiese, R.; Wochele, S.; Butterbach-Bahl, K. Site specific and regional estimates of methane uptake by tropical rainforest soils in north eastern Australia. Plant Soil 2008, 309, 211–226. [Google Scholar] [CrossRef]
- Keller, M.; Mitre, M.E.; Stallard, R.F. Consumption of atmospheric methane in soils of central Panama: Effects of agricultural development. Glob. Biogeochem. Cycles 1990, 4, 21–27. [Google Scholar] [CrossRef]
- Keller, M.; Jacob, D.J.; Wofsy, S.C.; Harriss, R.C. Effects of tropical deforestation on global and regional atmospheric chemistry. Clim. Chang. 1991, 19, 139–158. [Google Scholar] [CrossRef]
- Steudler, P.A.; Bowden, R.D.; Melillo, J.M.; Aber, J.D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 1989, 341, 314–316. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Ineson, P. Methane oxidation in a temperate coniferous forest soil: Effects of inorganic N. Soil Biol. Biochem. 2003, 35, 427–433. [Google Scholar] [CrossRef]
- Reay, D.S.; Nedwell, D.B. Methane oxidation in temperate soils: Effects of inorganic N. Soil Biol. Biochem. 2004, 36, 2059–2065. [Google Scholar] [CrossRef]
- De Visscher, A.; Cleemput, O.V. Induction of enhanced CH4 oxidation in soils: NH4+ inhibition patterns. Soil Biol. Biochem. 2003, 35, 907–913. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Wanek, W.; Ripka, K.; Hackl, E.; Sessitsch, A.; Strauss, J.; Zechmeister-Boltenstern, S. Greenhouse gas fluxes respond to different N fertilizer types due to altered plant-soil-microbe interactions. Plant Soil 2011, 343, 17–35. [Google Scholar] [CrossRef]
- Sitaula, B.K.; Hansen, S.; Bonilla, J.I.; Bakken, L.R. Methane oxidation potentials and fluxes in agricultural soil: Effects of fertilisation and soil compaction. Biogeochemistry 2000, 48, 323–339. [Google Scholar] [CrossRef]
- Klüber, H.D.; Conrad, R. Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. Fems Microbiol. Ecol. 1998, 25, 301–318. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; ISBN 978-1-107-05821-7. [Google Scholar]
- Dimassi, B.; Mary, B.; Wylleman, R.; Labreuche, J.; Couture, D.; Piraux, F.; Cohan, J.-P. Long-term effect of contrasted tillage and crop management on soil carbon dynamics during 41 years. Agric. Ecosyst. Environ. 2014, 188, 134–146. [Google Scholar] [CrossRef]
- De Oliveira Silva, B.; Moitinho, M.R.; de Araújo Santos, G.A.; Teixeira, D.D.; Fernandes, C.; La Scala, N., Jr. Soil CO2 emission and short-term soil pore class distribution after tillage operations. Soil Tillage Res. 2019, 186, 224–232. [Google Scholar] [CrossRef]
- Hickman, J.E.; Tully, K.L.; Groffman, P.M.; Diru, W.; Palm, C.A. A potential tipping point in tropical agriculture: Avoiding rapid increases in nitrous oxide fluxes from agricultural intensification in Kenya: Non-linear N2O in tropical agriculture. J. Geophys. Res. Biogeosci. 2015, 120, 938–951. [Google Scholar] [CrossRef]
- Arora, B.; Wainwright, H.M.; Dwivedi, D.; Vaughn, L.J.S.; Curtis, J.B.; Torn, M.S.; Dafflon, B.; Hubbard, S.S. Evaluating temporal controls on greenhouse gas (GHG) fluxes in an Arctic tundra environment: An entropy-based approach. Sci. Total Environ. 2019, 649, 284–299. [Google Scholar] [CrossRef]
- Courtois, E.A.; Stahl, C.; Burban, B.; Van den Berge, J.; Berveiller, D.; Bréchet, L.; Soong, J.L.; Arriga, N.; Peñuelas, J.; Janssens, I.A. Automatic high-frequency measurements of full soil greenhouse gas fluxes in a tropical forest. Biogeosciences 2019, 16, 785–796. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Grace, P.; Mosier, A.R. N2O emissions from agricultural lands: A synthesis of simulation approaches. Plant Soil 2008, 309, 169–189. [Google Scholar] [CrossRef]
- Dwivedi, D.; Arora, B.; Steefel, C.I.; Dafflon, B.; Versteeg, R. Hot Spots and Hot Moments of Nitrogen in a Riparian Corridor. Water Resour. Res. 2018, 54, 205–222. [Google Scholar] [CrossRef]
- Lugato, E.; Zuliani, M.; Alberti, G.; Vedove, G.D.; Gioli, B.; Miglietta, F.; Peressotti, A. Application of DNDC biogeochemistry model to estimate greenhouse gas emissions from Italian agricultural areas at high spatial resolution. Agric. Ecosyst. Environ. 2010, 139, 546–556. [Google Scholar] [CrossRef]



| System | N (kg ha−1 year−1) | P2O5 (kg ha−1 year−1) | K2O (kg ha−1 year−1) | Dolomite (t ha−1 year−1) | Aboveground Biomass (t C ha−1 year−1) | ||
|---|---|---|---|---|---|---|---|
| G | 50–60 | 50–60 | 50–60 | 1 | Export | 4.76 ± 0.68 | |
| Restitution | 1.85 ± 0.34 | ||||||
| DT | Maize | 140–173 | 73–80 | 78–80 | 1 | Yield | 2.10 ± 0.08 |
| Residues | 3.43 ± 0.10 | ||||||
| Soybean | 0 | 72–80 | 78–80 | Yield | 1.50 ± 0.06 | ||
| Residues | 2.29 ± 0.21 | ||||||
| NT | Maize | 140–173 | 73–80 | 78–80 | 1 | Yield | 2.10 ± 0.12 |
| Residues | 3.37 ± 0.17 | ||||||
| Soybean | 0 | 72–80 | 78–80 | Yield | 1.60 ± 0.07 | ||
| Residues | 2.34 ± 0.11 | ||||||
| Date | System | 0–5 cm | 5–10 cm |
|---|---|---|---|
| T2—October 2010 | Grassland | 2.00 ± 0.08 a | 2.05 ± 0.08 a |
| Disc tillage | 2.03 ± 0.08 a | 1.91 ± 0.07 ab | |
| No-tillage | 1.93 ± 0.08 a | 1.72 ± 0.06 b | |
| T3—October 2011 | Grassland | 2.16 ± 0.07 a | 1.97 ± 0.06 a |
| Disc tillage | 1.84 ± 0.06 b | 1.86 ± 0.05 a | |
| No-tillage | 1.84 ± 0.06 b | 1.60 ± 0.04 b | |
| T4—October 2012 | Grassland | 2.30 ± 0.07 a | 2.07 ± 0.08 a |
| Disc tillage | 1.86 ± 0.06 b | 1.75 ± 0.04 b | |
| No-tillage | 2.12 ± 0.07 a | 1.68 ± 0.06 b | |
| T5—October 2013 | Grassland | 2.17 ± 0.07 a | 2.02 ± 0.08 a |
| Disc tillage | 1.82 ± 0.05 b | 1.75 ± 0.05 b | |
| No-tillage | 1.99 ± 0.07 ab | 1.66 ± 0.05 b |
| Forest | Grassland | Cropland Disc Tillage | Cropland No-Tillage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | All | Dry | Wet | All | Dry | Wet | All | Dry | Wet | All | ||
| Soil GWC (%) | Mean ± SE | 21.3 ± 1.0 | 26.9 ± 0.9 | 24.8 ± 0.8 | 15.2 ± 0.5 | 20.8 ± 0.3 | 18.7 ± 0.3 | 14.2 ± 0.4 | 19.2 ± 0.3 | 17.3 ± 0.3 | 14.0 ± 0.4 | 18.3 ± 0.2 | 16.7 ± 0.2 |
| Range | 15.0–32.0 | 19.0–40.0 | 15.0–40.0 | 9.0–30.0 | 12.0–29.0 | 9.0–30.0 | 8.0–25.0 | 8.0–32.0 | 8.0–32.0 | 8.0–23.3 | 13.0–25.0 | 8.0–25.0 | |
| n | 18 | 30 | 48 | 75 | 123 | 198 | 72 | 122 | 194 | 76 | 123 | 199 | |
| Soil Temp. (°C) | Mean ± SE | 25.4 ± 0.1 | 25.2 ± 0.1 | 25.3 ± 0.1 | 28.2 ± 0.2 | 27.2 ± 0.1 | 27.6 ± 0.1 | 28.8 ± 0.2 | 27.3 ± 0.1 | 27.9 ± 0.1 | 29.0 ± 0.2 | 27.5 ± 0.1 | 28.1 ± 0.1 |
| Range | 24.9–26.0 | 24.5–25.8 | 24.5–26.0 | 25.8–32.2 | 25.4–29.0 | 25.4–32.2 | 25.2–32.6 | 24.5–31.9 | 24.5–32.6 | 25.5–32.5 | 24.6–31.5 | 24.6–32.5 | |
| n | 17 | 17 | 34 | 75 | 123 | 198 | 72 | 122 | 194 | 72 | 123 | 195 | |
| NH4+ (mg N kg−1 soil) | Mean ± SE | 3.5 ± 0.7 | 3.6 ± 0.4 | 3.6 ± 0.3 | 4.3 ± 0.3 | 3.7 ± 0.2 | 4.0 ± 0.2 | 4.1 ± 0.3 | 3.6 ± 0.4 | 3.8 ± 0.3 | 3.2 ± 0.3 | 2.9 ± 0.4 | 3.0 ± 0.3 |
| Range | 0.0–11.7 | 0.5–9.0 | 0.0–11.7 | 0.3–12.0 | 0.3–17.6 | 0.3–17.6 | 0.4–17.9 | 0.1–33.5 | 0.1–33.5 | 0.1–16.8 | 0.1–39.9 | 0.1–39.9 | |
| n | 17 | 30 | 47 | 87 | 123 | 210 | 84 | 122 | 206 | 88 | 123 | 211 | |
| NO3− (mg N kg−1 soil) | Mean ± SE | 2.4 ± 0.3 | 2.3 ± 0.3 | 2.4 ± 0.2 | 3.1 ± 0.4 | 0.8 ± 0.1 | 1.8 ± 0.2 | 16.2 ± 1.3 | 4.0 ± 0.5 | 8.9 ± 0.7 | 16.5 ± 1.4 | 3.8 ± 0.4 | 9.1 ± 0.8 |
| Range | 0.4–4.7 | 0.7–10.9 | 0.4–10.9 | 0.1–19.8 | 0.1–4.9 | 0.1–19.8 | 0.6–51.9 | 0.2–22.5 | 0.2–51.9 | 1.1–54.7 | 0.3–17.8 | 0.3–54.7 | |
| n | 17 | 30 | 47 | 87 | 123 | 210 | 84 | 122 | 206 | 88 | 123 | 211 | |
| N2O Fluxes (g N ha−1 day−1) | Mean ± SE | 2.0 ± 0.5 | 3.1 ± 0.4 | 2.7 ± 0.3 | 3.5 ± 1.3 | 3.2 ± 0.9 | 3.3 ± 0.8 a | 7.4 ±1.0 | 11.6 ± 1.9 | 10.0 ± 1.3 b | 6.0 ± 0.9 | 11.5 ± 2.7 | 9.4 ± 1.7 b |
| Range | 0.0–8.2 | 0.2–9.4 | 0.0–9.4 | −0.2 to 55.0 | −1.0 to 75.8 | −1.0 to 75.8 | 0.1–56.6 | 0.0–139.0 | 0.0–139.0 | 1.0–47.4 | 0.5–231.8 | 0.5–231.8 | |
| n | 18 | 30 | 48 | 75 | 123 | 198 | 76 | 121 | 197 | 76 | 123 | 199 | |
| CH4 Fluxes (g C ha−1 day−1) | Mean ± SE | −3.1 ± 2.6 | −2.9 ± 2.2 | −3.0 ± 1.7 | 3.3 ± 1.0 | 10.0 ± 1.0 | 7.5 ± 0.8 a | −0.8 ± 0.6 | 2.7 ± 1.0 | 1.4 ± 0.7 b | −2.2 ± 1.2 | 1.5 ± 0.5 | 0.2 ± 0.5 b |
| Range | −11.8 to 29.8 | −26.2 to 45.9 | −26.2 to 45.9 | −12.5 to 24.3 | −5.6 to 57.5 | −12.5 to 57.5 | −10.6 to 15.7 | −58.6 to 44.7 | −58.6 to 44.7 | −58.6 to 35.2 | −11.1 to 24.0 | −58.6 to 35.2 | |
| n | 17 | 30 | 47 | 71 | 123 | 194 | 72 | 121 | 193 | 72 | 123 | 195 | |
| CO2 Fluxes (kg C ha−1 day−1) | Mean ± SE | 23.9 ± 1.9 | 26.7 ± 1.2 | 25.6 ± 1.0 | 47.3 ± 1.9 | 52.3 ± 1.3 | 50.4 ± 1.1 | 32.8 ± 1.4 | 42.0 ± 1.4 | 38.4 ± 1.1 a | 29.1 ± 1.4 | 34.8 ± 1.0 | 32.6 ± 0.8 b |
| Range | 13.6–42.8 | 18.2–45.2 | 13.6–45.2 | 20.4–95.9 | 20.9–112.1 | 20.4–112.1 | 14.3–66.9 | 15.0–98.0 | 14.3–98.0 | 12.2–65.6 | 16.6–66.3 | 12.2–66.3 | |
| n | 18 | 30 | 48 | 75 | 123 | 198 | 76 | 121 | 197 | 76 | 123 | 199 | |
| GHG | Models | R2 | ||||
|---|---|---|---|---|---|---|
| N2O | log(N2O+1.1) ~ s(NH4+) + s(NO3−) + s(GWC) + s(temperature) | 0.35 | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | |||
| intercept | 1.47 | 0.03 | 43.66 | <0.001 | ||
| edf | Ref.df | F | p-value | |||
| s(NH4+) | 2.48 | 3.09 | 2.54 | 0.06 | ||
| s(NO3−) | 7.99 | 8.73 | 25.35 | <0.001 | ||
| s(GWC) | 8.28 | 8.82 | 10.62 | <0.001 | ||
| s(temperature) | 1.00 | 1.00 | 6.75 | <0.01 | ||
| CH4 | log(CH4 + 60) ~ s(NH4+) + s(NO3−) + s(GWC) | 0.31 | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | |||
| intercept | 4.13 | 0.01 | 457.60 | <0.001 | ||
| edf | Ref.df | F | p-value | |||
| s(NH4+) | 8.94 | 9.00 | 21.53 | <0.001 | ||
| s(NO3−) | 7.13 | 8.18 | 3.71 | <0.001 | ||
| s(GWC) | 1.00 | 1.00 | 7.22 | <0.01 | ||
| CO2 | log(CO2) ~ s(NO3−) + s(GWC) + s(temperature) | 0.29 | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | |||
| intercept | 3.63 | 0.01 | 270.90 | <0.001 | ||
| edf | Ref.df | F | p-value | |||
| s(NO3−) | 5.05 | 6.15 | 15.69 | <0.001 | ||
| s(GWC) | 1.57 | 1.96 | 7.81 | <0.001 | ||
| s(temperature) | 5.39 | 6.56 | 10.35 | <0.001 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petitjean, C.; Le Gall, C.; Pontet, C.; Fujisaki, K.; Garric, B.; Horth, J.-C.; Hénault, C.; Perrin, A.-S. Soil N2O, CH4, and CO2 Fluxes in Forest, Grassland, and Tillage/No-Tillage Croplands in French Guiana (Amazonia). Soil Syst. 2019, 3, 29. https://doi.org/10.3390/soilsystems3020029
Petitjean C, Le Gall C, Pontet C, Fujisaki K, Garric B, Horth J-C, Hénault C, Perrin A-S. Soil N2O, CH4, and CO2 Fluxes in Forest, Grassland, and Tillage/No-Tillage Croplands in French Guiana (Amazonia). Soil Systems. 2019; 3(2):29. https://doi.org/10.3390/soilsystems3020029
Chicago/Turabian StylePetitjean, Caroline, Cécile Le Gall, Célia Pontet, Kenji Fujisaki, Bernard Garric, Jean-Claude Horth, Catherine Hénault, and Anne-Sophie Perrin. 2019. "Soil N2O, CH4, and CO2 Fluxes in Forest, Grassland, and Tillage/No-Tillage Croplands in French Guiana (Amazonia)" Soil Systems 3, no. 2: 29. https://doi.org/10.3390/soilsystems3020029
APA StylePetitjean, C., Le Gall, C., Pontet, C., Fujisaki, K., Garric, B., Horth, J.-C., Hénault, C., & Perrin, A.-S. (2019). Soil N2O, CH4, and CO2 Fluxes in Forest, Grassland, and Tillage/No-Tillage Croplands in French Guiana (Amazonia). Soil Systems, 3(2), 29. https://doi.org/10.3390/soilsystems3020029




