Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Production, Particle Sizes, and Preparation
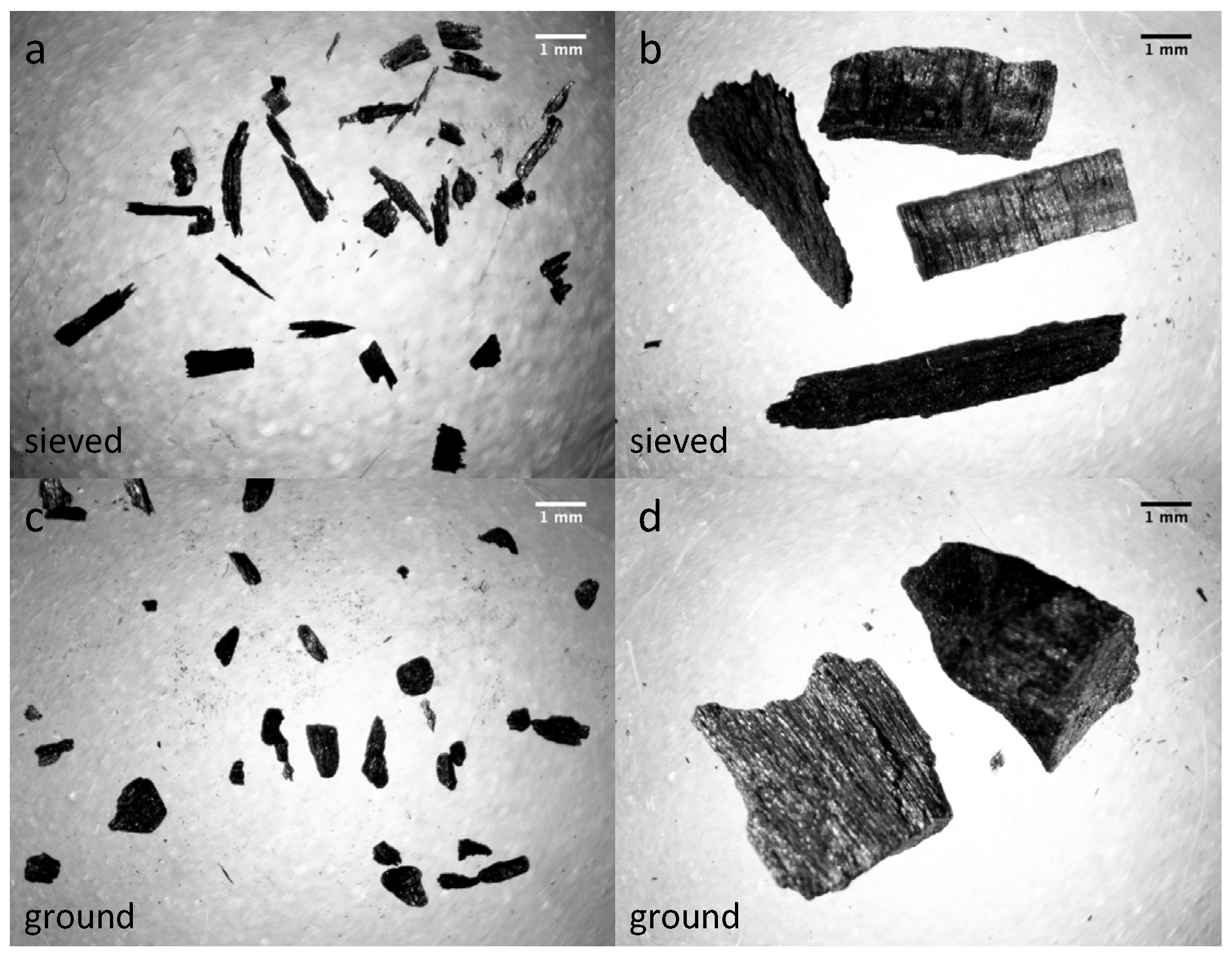
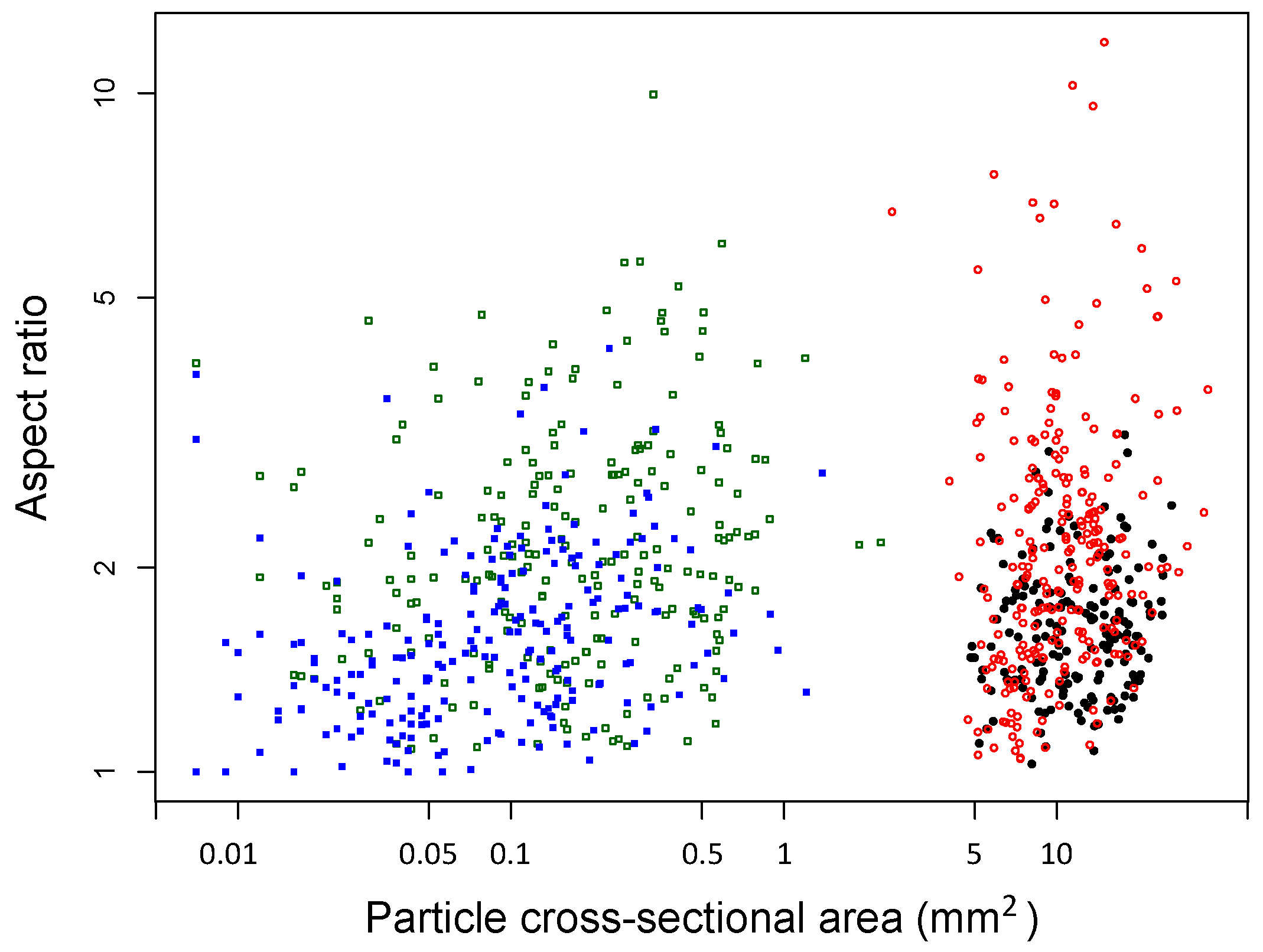
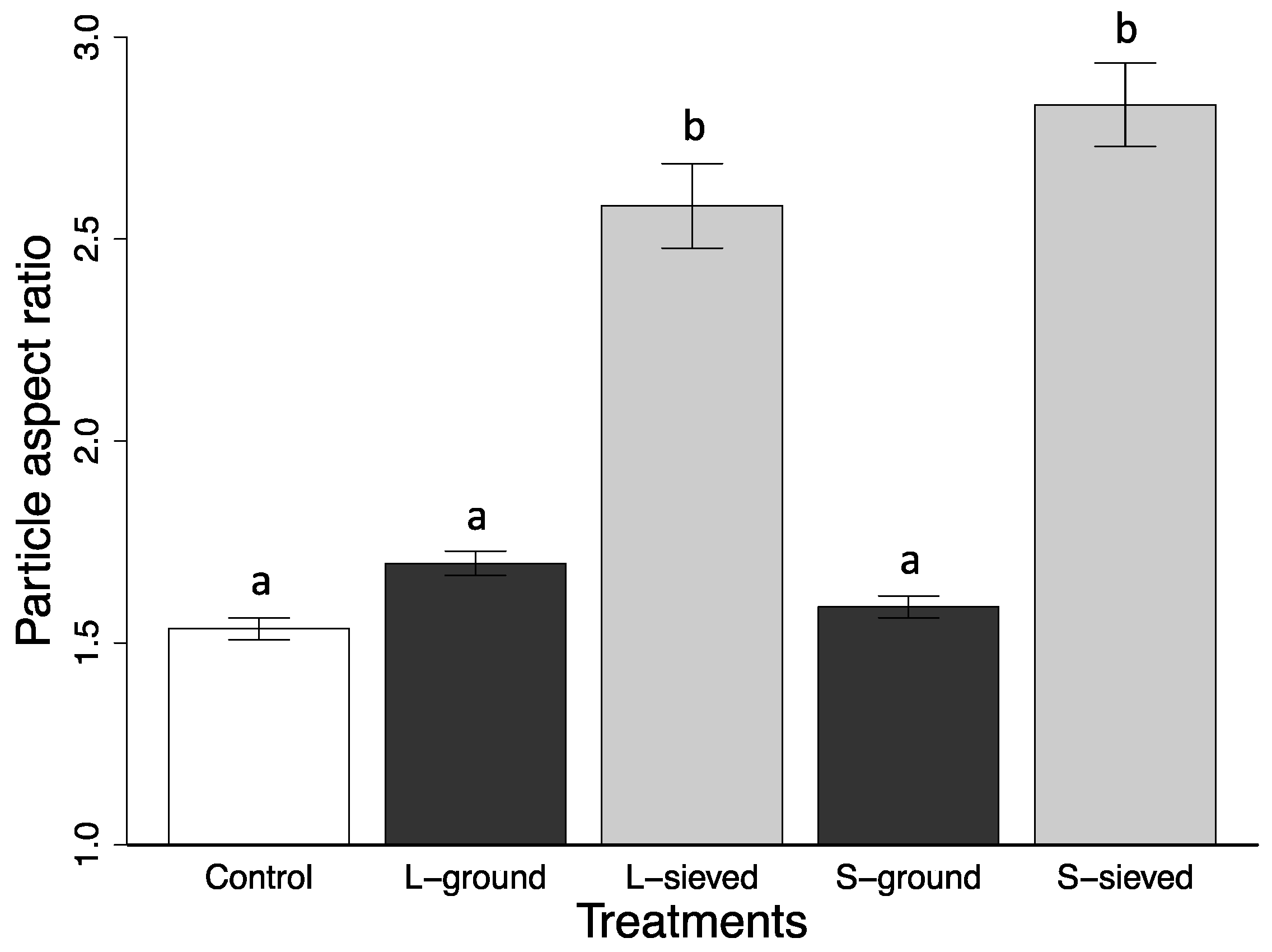
2.2. Characterization of Biochar Particle Size, Shape, Bulk Density, and Tap Density
2.3. Biochar pH, Electrical Conductivity, Ash Content, and Water Retention Capacity
2.4. Growth Conditions and Experimental Design
2.5. Statistical Analysis
3. Results
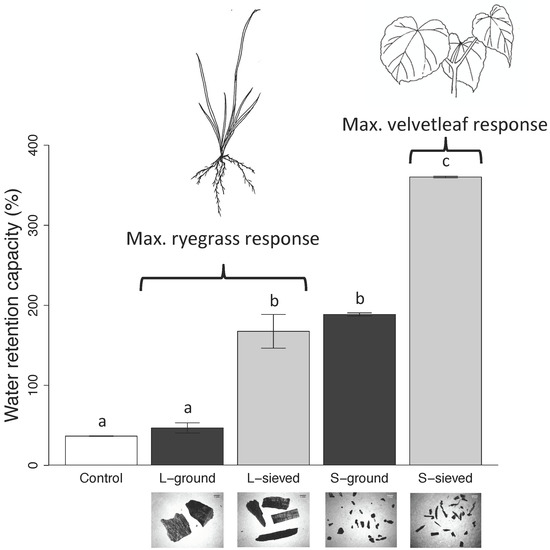
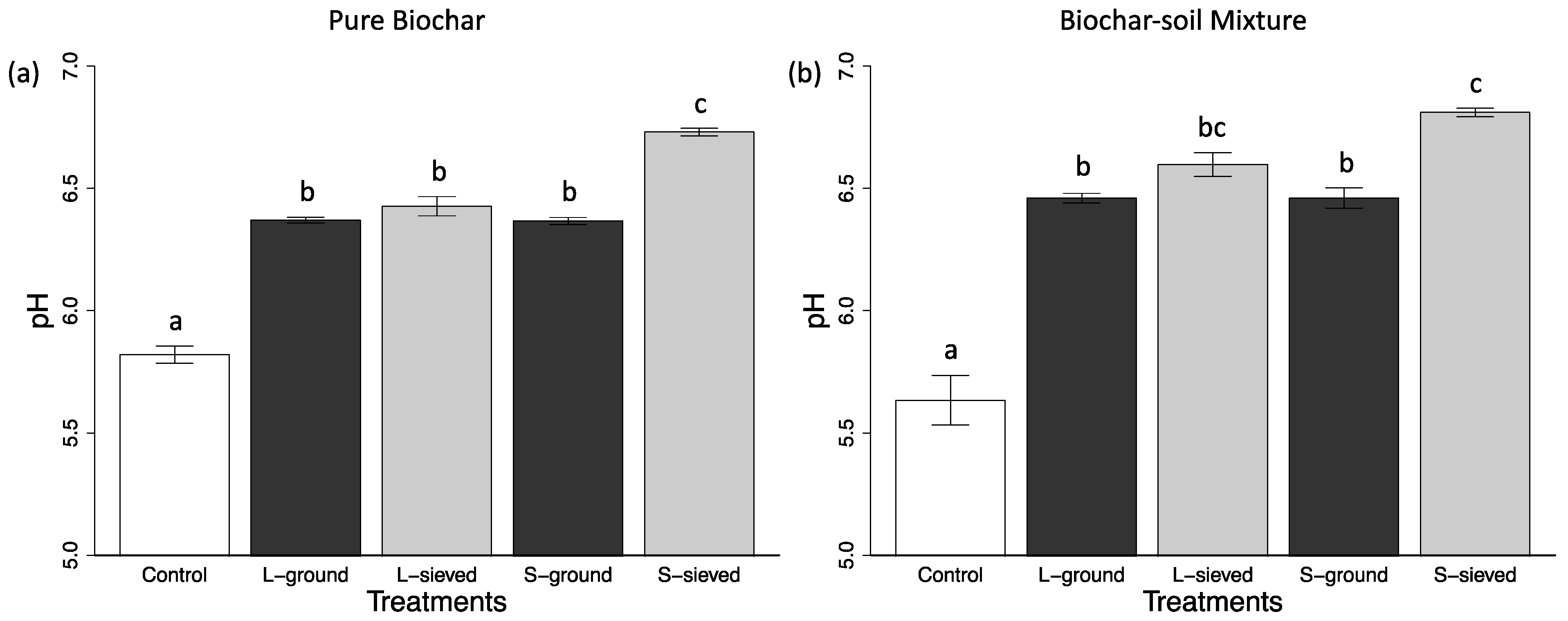
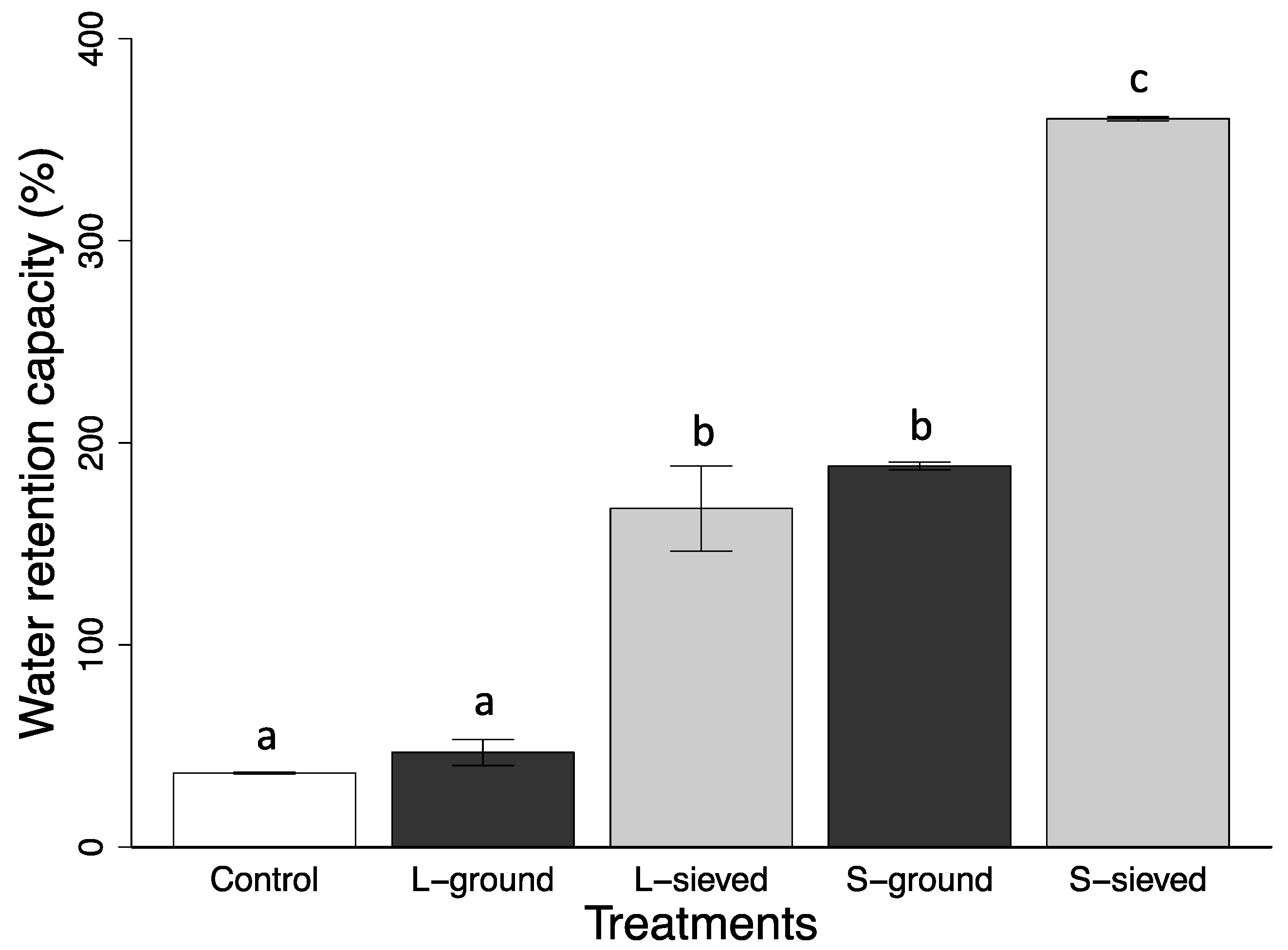
3.1. Properties, pH, and Water Retention Capacity of Biochar and Sand
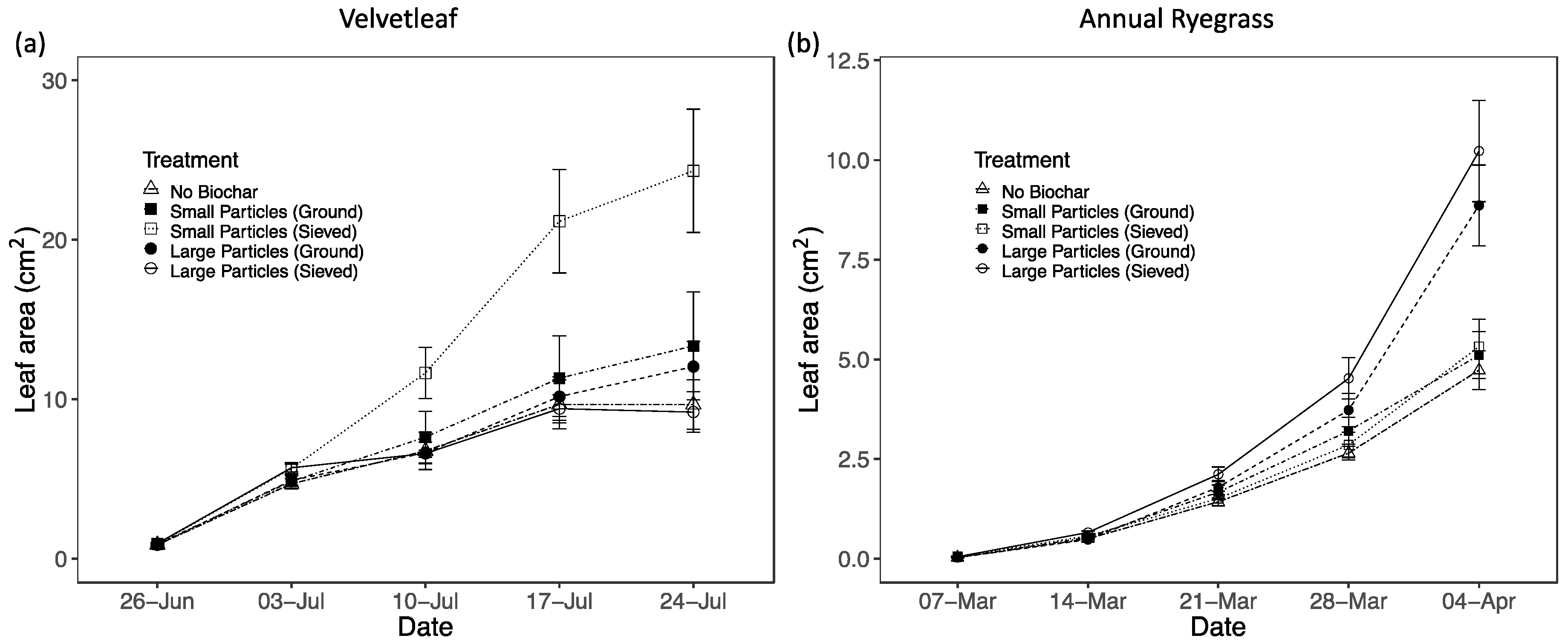
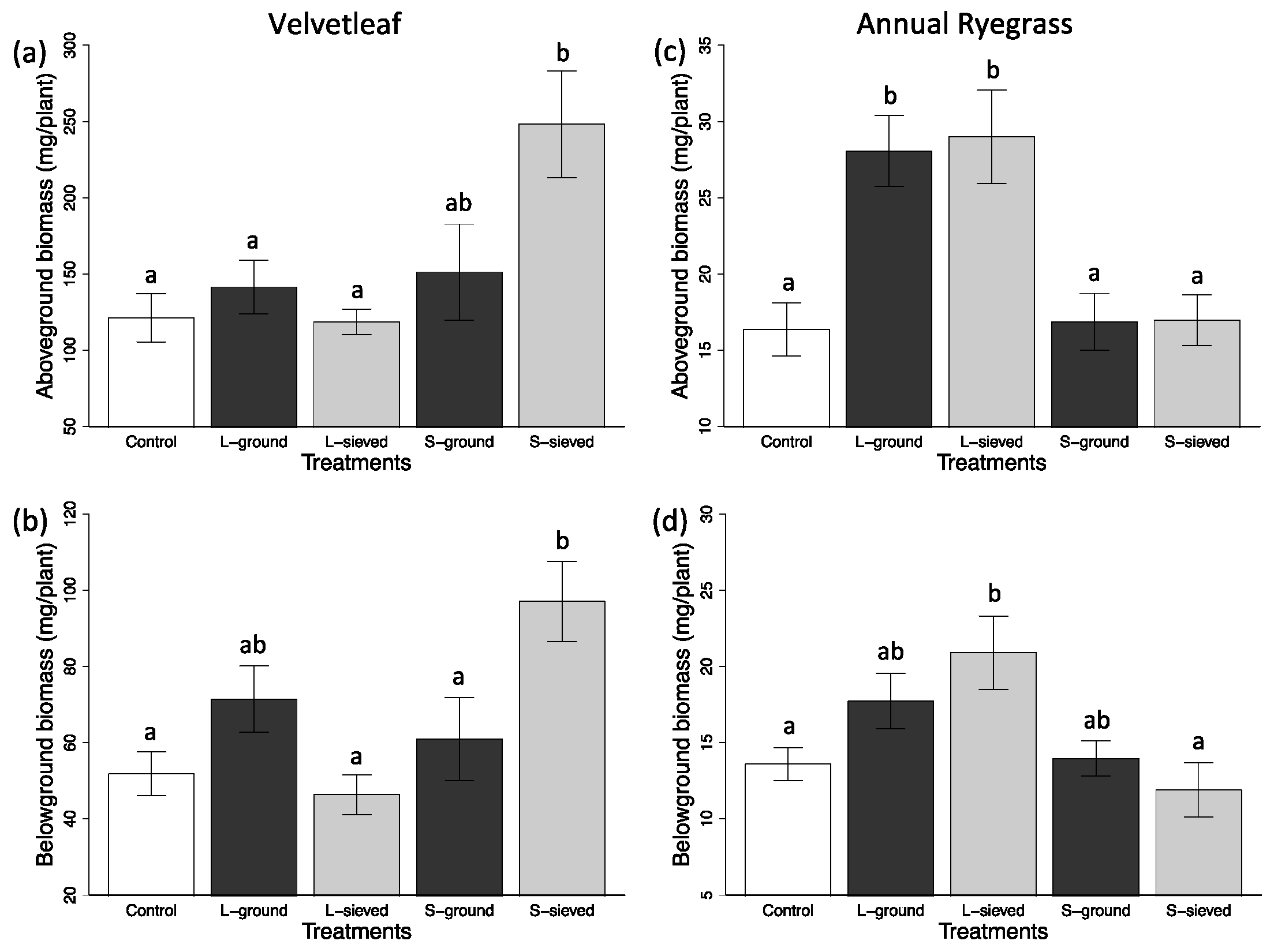
3.2. Plant Performance in Different Biochar Treatments and Sand
3.2.1. Particle Size Effects
3.2.2. Post-Processing Effects
4. Discussion
4.1. Biochar pH and Water Retention Capacity
4.2. Plant Performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in feedstock wood chemistry strongly influences biochar liming potential. Soil Syst. 2019. in review. [Google Scholar]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase: Heavy metal mobility in biochar-amended soils. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Mollinedo, J.; Schumacher, T.E.; Chintala, R. Influence of feedstocks and pyrolysis on biochar’s capacity to modify soil water retention characteristics. J. Anal. Appl. Pyrolysis 2015, 114, 100–108. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, B.; Gonzales, H.B.; Ravi, S.; Sharratt, B.S.; Mohanty, S.K. Dynamic effects of biochar concentration and particle size on hydraulic properties of sand. Land Degrad. Dev. 2018, 29, 884–893. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef] [PubMed]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Cantrell, K.B.; Shumaker, P.D.; Szögi, A.A.; Johnson, M.G. Carbon mineralization in two ultisols amended with different sources and particle sizes of pyrolyzed biochar. Chemosphere 2014, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Guo, M.; Chow, T.; Bennett, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Sun, H.; Hockaday, W.C.; Masiello, C.A.; Zygourakis, K. Multiple controls on the chemical and physical structure of biochars. Ind. Eng. Chem. Res. 2012, 51, 3587–3597. [Google Scholar] [CrossRef]
- Valenzuela-Calahorro, C.; Bernalte-Garcia, A.; Gómez-Serrano, V.; Bernalte-García, M.J. Influence of particle size and pyrolysis conditions on yield, density and some textural parameters of chars prepared from holm-oak wood. J. Anal. Appl. Pyrolysis 1987, 12, 61–70. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Tang, X.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar increased water holding capacity but accelerated organic carbon leaching from a sloping farmland soil in China. Environ. Sci. Pollut. Res. 2016, 23, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Ulyett, J.; Sakrabani, R.; Kibblewhite, M.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from two sandy loam soils: Biochar impacts on nitrogen and water dynamics. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, F.; Tang, J.; Yu, L.; Zhang, R. Effects of biochar amendment on soil aggregates and hydraulic properties. J. Soil Sci. Plant Nutr. 2013, 13, 991–1002. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, L.; Shen, Y.; Li, S. Sensitivity of soil water retention and availability to biochar addition in rainfed semi-arid farmland during a three-year field experiment. Field Crops Res. 2016, 196, 284–293. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, J.; Morales, V.L.; Chen, X.; Hay, A.G.; Lehmann, J.; Steenhuis, T.S. Transport and retention of biochar particles in porous media: Effect of pH, ionic strength, and particle size. Ecohydrology 2010, 3, 497–508. [Google Scholar] [CrossRef]
- Sackett, T.E.; Basiliko, N.; Noyce, G.L.; Winsborough, C.; Schurman, J.; Ikeda, C.; Thomas, S.C. Soil and greenhouse gas responses to biochar additions in a temperate hardwood forest. GCB Bioenergy 2015, 7, 1062–1074. [Google Scholar] [CrossRef]
- Barker, D.C.; Knezevic, S.Z.; Martin, A.R.; Walters, D.T.; Lindquist, J.L. Effect of nitrogen addition on the comparative productivity of corn and velvetleaf (Abutilon theophrasti). Weed Sci. 2006, 54, 354–363. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Regnier, E.E.; Harrison, S.K. Compensatory responses of common cocklebur (Xanthium strumarium) and velvetleaf (Abutilon theophrasti) to partial shading. Weed Sci. 1993, 41, 541–547. [Google Scholar]
- Thomas, S.C.; Jasienski, M.; Bazzaz, F.A. Early vs. asymptotic growth responses of herbaceous plants to elevated CO2. Ecology 1999, 80, 1552–1567. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G.W.; Weis, R.; Koskinen, W.C. Influence of biochar particle size and shape on soil hydraulic properties. J. Environ. Sci. Eng. 2017, 5, 8–15. [Google Scholar]
- Ibrahim, A.; Usman, A.R.A.; Al-Wabel, M.I.; Nadeem, M.; Ok, Y.S.; Al-Omran, A. Effects of conocarpus biochar on hydraulic properties of calcareous sandy soil: Influence of particle size and application depth. Arch. Agron. Soil Sci. 2017, 63, 185–197. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil microbial responses to biochars varying in particle size, surface and pore properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Architectural plasticity, photosynthesis and growth responses of velvetleaf (Abutilon theophrasti Medicus) plants to water stress in a semi-arid environment. Aust. J. Crop Sci. 2011, 5, 369–374. [Google Scholar]
- Patterson, D.T.; Highsmith, M.T. Competition of spurred anoda (Anoda cristata) and velvetleaf (Abutilon theophrasti) with cotton (Gossypium hirsutum) during simulated drought and recovery. Weed Sci. 1989, 37, 658–664. [Google Scholar]
- Aghaie, P.; Kazemeini, S.A.; Majd, R.; Alebrahim, M.T. Role of phosphorus in maize (Zea mays L.) competitiveness against velvetleaf (Abutilon theophrasti). Int. J. Agron. Plant Prod. 2013, 4, 2323–2329. [Google Scholar]
- Nagy, V.; Szabo, R.; Budai, P.; Nadasy, E. Sprouting of velvetleaf (Abutilon theohrasti Medic.) influenced by N, P and K fertilizers. 13th Alps-Adria Sci. Workshop 2014, 63, 275–278. [Google Scholar]
- Warwick, S.I.; Black, L.D. The biology of Canadian weeds. 90. Abutilon theophrasti. Can. J. Plant Sci. 1988, 68, 1069–1085. [Google Scholar] [CrossRef]
- Evers, G.W.; Smith, G.R.; Hoveland, C.S. Ecology and production of annual ryegrass. In Ecology, Production, and Management of Lolium for Forage in the USA; Crop Science Society of America: Medison, WI, USA, 1997; pp. 29–43. [Google Scholar]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Esmaeelnejad, L.; Shorafa, M.; Gorji, M.; Hosseini, S.M. Impacts of woody biochar particle size on porosity and hydraulic conductivity of biochar-soil mixtures: An incubation study. Commun. Soil Sci. Plant Anal. 2017, 48, 1710–1718. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and mineral-nutrition properties of sand-based turfgrass root zones amended with biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Houlbrooke, D.J.; Thom, E.R.; Chapman, R.; McLay, C.D.A. A study of the effects of soil bulk density on root and shoot growth of different ryegrass lines. N. Z. J. Agric. Res. 1997, 40, 429–435. [Google Scholar] [CrossRef]
- Yusof, J.M.; Salleh, M.A.M.; Rashid, S.A.; Ismail, I.; Adam, S.N. Characterisation of carbon particles (CPs) derived from dry milled kenaf biochar. J. Eng. Sci. Technol. 2014, 125–131. [Google Scholar]
- Hlabangana, N.; Danha, G.; Muzenda, E. Effect of ball and feed particle size distribution on the milling efficiency of a ball mill: An attainable region approach. S. Afr. J. Chem. Eng. 2018, 25, 79–84. [Google Scholar] [CrossRef]
- Khumalo, N.; Glasser, D.; Hildebrandt, D.; Hausberger, B.; Kauchali, S. The application of the attainable region analysis to comminution. Chem. Eng. Sci. 2006, 61, 5969–5980. [Google Scholar] [CrossRef]
- Kaudal, B.B.; Chen, D.; Madhavan, D.B.; Downie, A.; Weatherley, A. An examination of physical and chemical properties of urban biochar for use as growing media substrate. Biomass Bioenergy 2016, 84, 49–58. [Google Scholar] [CrossRef]
- Dwari, R.K.; Hanumantha Rao, K. Fine coal preparation using novel tribo-electrostatic separator. Miner. Eng. 2009, 22, 119–127. [Google Scholar] [CrossRef]
- Higashiyama, Y.; Asano, K. Recent progress in electrostatic separation technology. Part. Sci. Technol. 1998, 16, 77–90. [Google Scholar] [CrossRef]
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle size dependence of the physicochemical properties of biochar. Chemosphere 2018, 212, 385–392. [Google Scholar] [CrossRef] [PubMed]
| Treatment | |||||
|---|---|---|---|---|---|
| Attribute | S-sieved | L-sieved | S-ground | L-ground | Sand |
| Pyrolysis temperature (°C) | 374 | 374 | 363 | 363 | N/A |
| Particle cross-sectional area (mm2) | 0.26 (0.02) | 11.31 (0.34) | 0.12 (0.01) | 12.76 (0.32) | 0.07 (0.00) |
| Particle aspect ratio | 2.83 (0.10) | 2.58 (0.10) | 1.59 (0.03) | 1.70 (0.03) | 1.53 (0.03) |
| pH of pure biochar or sand | 6.73 (0.02) | 6.43 (0.04) | 6.37 (0.03) | 6.37 (0.01) | 5.82 (0.04) |
| pH of Biochar-sand mixture or sand * | 6.81 (0.02) | 6.60 (0.05) | 6.46 (0.04) | 6.46 (0.02) | 5.63 (0.10) |
| Electrical conductivity (uS/cm) | 23.9 (1.0) | 28.5 (1.1) | 23.9 (0.6) | 25.7 (1.5) | 45.3 (7.8) |
| Water retention capacity (%) | 360 (1) | 168 (21) | 189 (1) | 47 (6) | 37 (0) |
| Carbon (%) | 68.3 | 68.9 | 68.2 | 68.9 | 0.240 |
| Nitrogen (%) | 0.350 | 0.335 | 0.253 | 0.205 | ND |
| Ash content (%) | 3.28 (0.35) | 3.74 (1.00) | 1.26 (0.16) | 1.58 (0.17) | N/A |
| Bulk density (g/cm3) | 0.16 (0.00) | 0.14 (0.00) | 0.29 (0.00) | 0.22 (0.02) | 1.38 (0.01) |
| Tap density (g/cm3) | 0.23 (0.00) | 0.20 (0.00) | 0.41 (0.01) | 0.34 (0.04) | 1.69 (0.01) |
| Treatment | Leaf Area (cm2) | Aboveground biomass (mg) | Belowground biomass (mg) | Chlorophyll conc. index | Fv/Fm | Leaf N (%) |
|---|---|---|---|---|---|---|
| Annual Ryegrass | ||||||
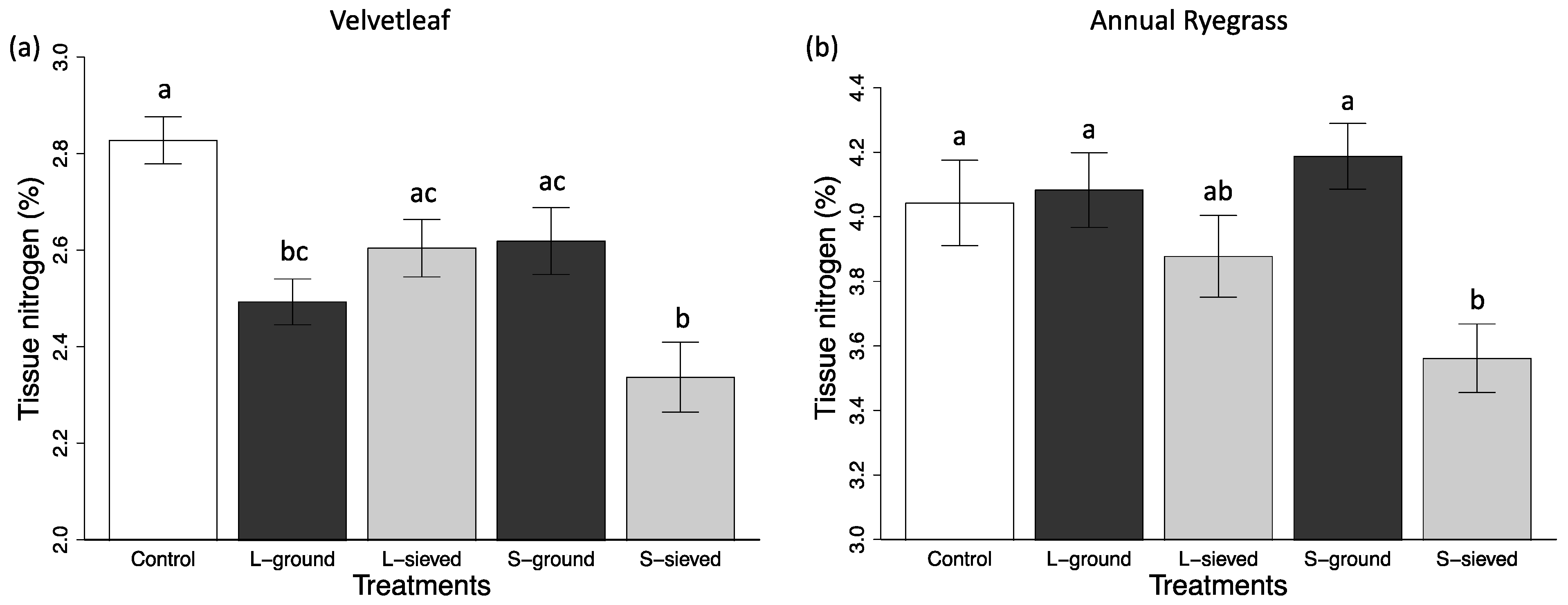
| S-sieved | 5.3 (0.7) | 16.96 (1.66) | 11.90 (1.77) | N/A | N/A | 3.56 (0.11) |
| L-sieved | 10.2 (1.3) | 28.99 (3.07) | 20.90 (2.42) | N/A | N/A | 3.88 (0.13) |
| S-ground | 5.1 (0.6) | 16.87 (1.86) | 13.96 (1.15) | N/A | N/A | 4.19 (0.10) |
| L-ground | 8.9 (1.0) | 28.06 (2.33) | 17.72 (2.33) | N/A | N/A | 4.08 (0.12) |
| Control | 4.7 (0.5) | 16.37 (13.59) | 13.59 (1.08) | N/A | N/A | 4.04 (0.13) |
| Velvetleaf | ||||||
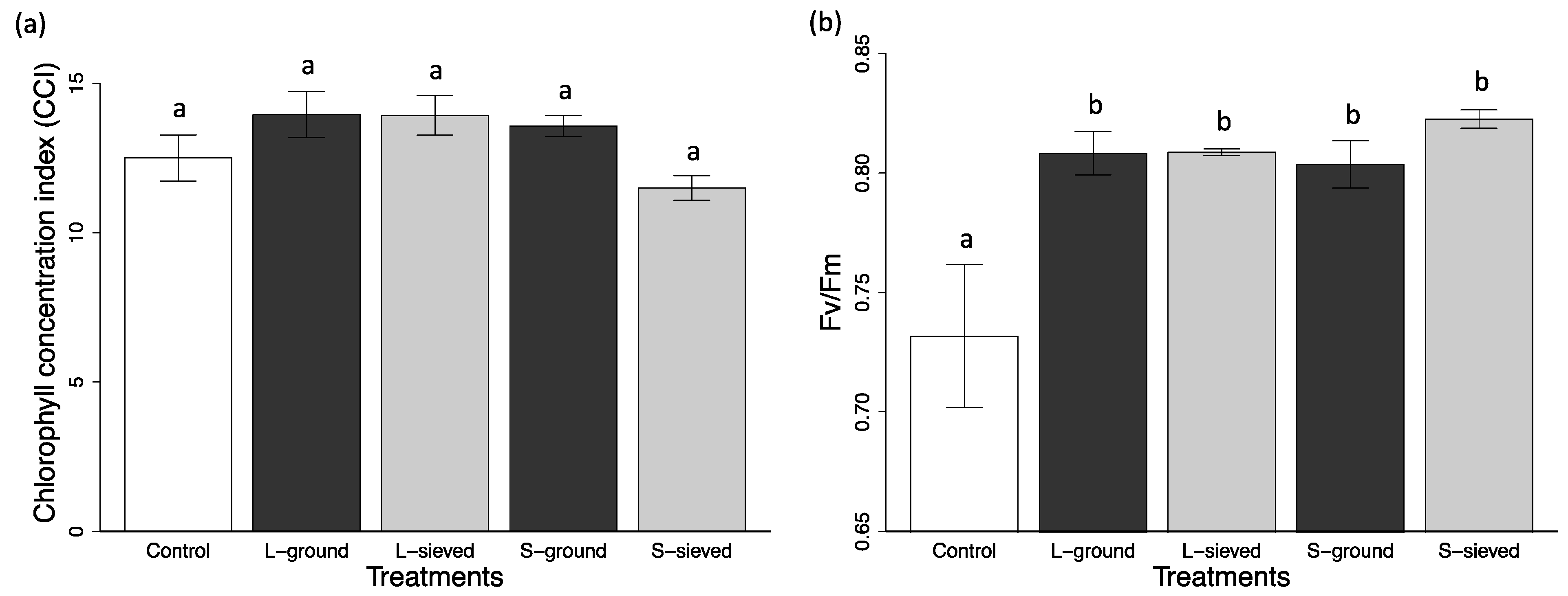
| S-sieved | 24.3 (3.9) | 248.28 (35.05) | 97.11 (10.52) | 11.5 (0.4) | 0.823 (0.004) | 2.34 (0.07) |
| L-sieved | 9.2 (1.3) | 118.67 (8.31) | 46.35 (5.20) | 13.9 (0.7) | 0.809 (0.001) | 2.60 (0.06) |
| S-ground | 13.3 (3.4) | 151.31 (31.46) | 60.89 (10.88) | 13.6 (0.4) | 0.804 (0.010) | 2.62 (0.07) |
| L-ground | 12.0 (1.6) | 141.51 (17.60) | 71.44 (8.69) | 14.0 (0.8) | 0.808 (0.009) | 2.49 (0.05) |
| Control | 9.7 (1.6) | 121.27 (15.85) | 51.82 (5.79) | 12.5 (0.9) | 0.732 (0.030) | 2.83 (0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Thomas, S.C. Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance. Soil Syst. 2019, 3, 14. https://doi.org/10.3390/soilsystems3010014
Liao W, Thomas SC. Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance. Soil Systems. 2019; 3(1):14. https://doi.org/10.3390/soilsystems3010014
Chicago/Turabian StyleLiao, Wenxi, and Sean C. Thomas. 2019. "Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance" Soil Systems 3, no. 1: 14. https://doi.org/10.3390/soilsystems3010014
APA StyleLiao, W., & Thomas, S. C. (2019). Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil pH, Water Retention Capacity, and Plant Performance. Soil Systems, 3(1), 14. https://doi.org/10.3390/soilsystems3010014