Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources
Abstract
:1. Introduction
2. Materials and Methods
2.1 Soil Sampling Sites
2.2 Plant Residues Sampling
2.3. Laboratory Incubation Setup
2.4. Plant Tissue and Soil Analyses
2.5. Statistical Analyses
2.6. Nitrogen Mineralization Kinetics
3. Results
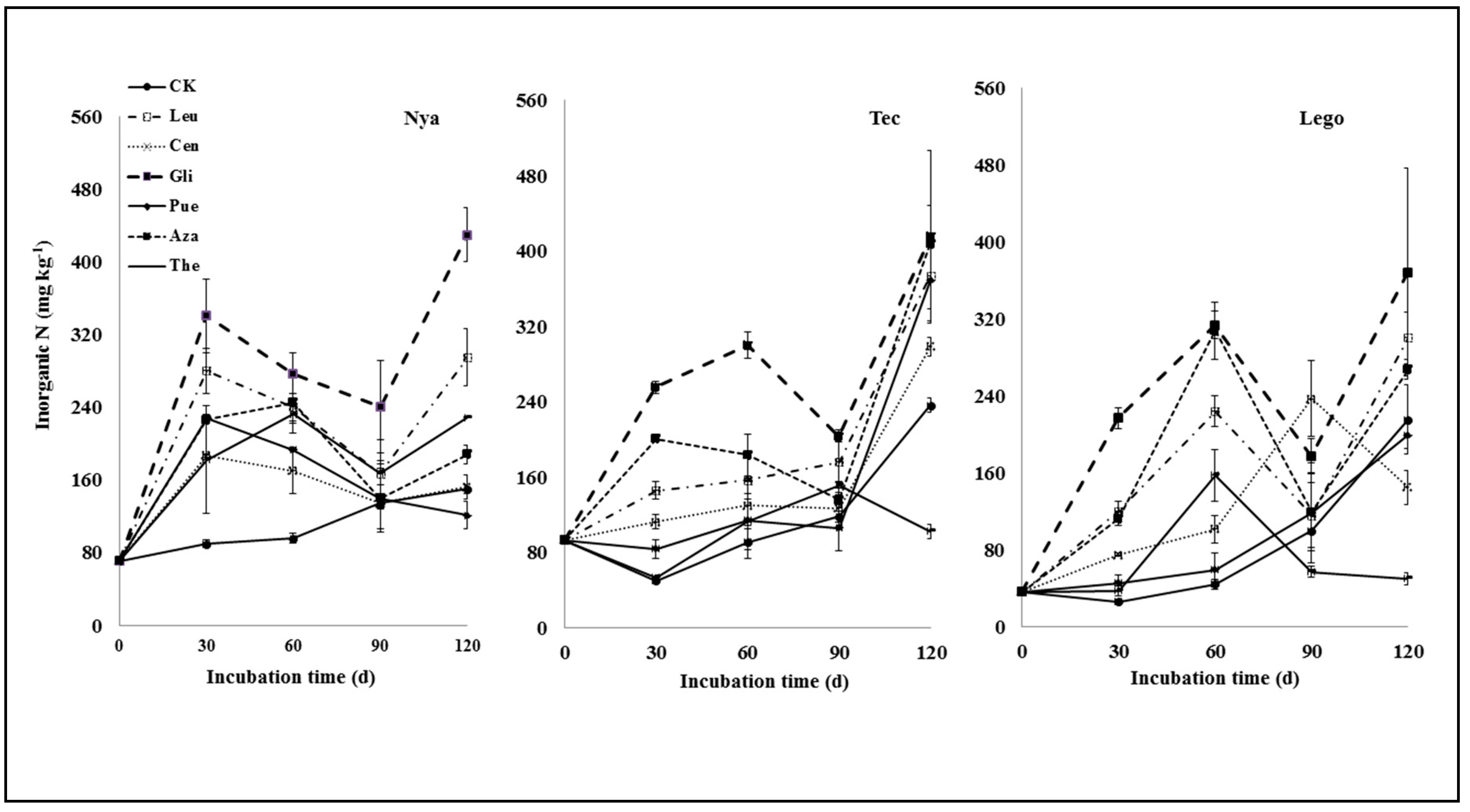
3.1. Nitrogen Mineralization
3.2. Soil pH
3.3 Soluble Organic Carbon and Microbial Biomass Carbon
3.4 Relationships
4. Discussion
4.1 Nitrogen Mineralization and pH Changes in Different Soil Types
4.2 Dissolved Organic Carbon and Microbial Biomass Carbon Contents in Different Soil Types
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez, P.A. Ecology: Soil Fertility and Hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Twomlow, S.J.; Dimes, J.P.; Van Wijk, M.T.; Giller, K.E. Resource Flows, Crops and Soil Fertility Management in Smallholder Farming Systems in Semi-Arid Zimbabwe. Soil Use Manag. 2009, 25, 78–90. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Bationo, A.; Chianu, J.; Giller, K.E.; Merckx, R.; Mokwunye, U.; Ohiokpehai, O.; Pypers, P.; Tabo, R.; Shepherd, K.D.; et al. Integrated Soil Fertility Management: Operational Definition and Consequences for Implementation and Dissemination. Outlook Agric. 2010, 39, 17–24. [Google Scholar] [CrossRef]
- Batjes, N.H. Soil Data Resources for Land Suitability Assessment and Environmental Protection in Central and Eastern Europe: The 1: 2,500,000 Scale SOVEUR Project. Land 2001, 5, 51–68. [Google Scholar]
- Moebius-Clune, B.N.; van Es, H.M.; Idowu, O.J.; Schindelbeck, R.R.; Kimetu, J.M.; Ngoze, S.; Lehmann, J.; Kinyangi, J.M. Long-Term Soil Quality Degradation along a Cultivation Chronosequence in Western Kenya. Agric. Ecosyst. Environ. 2011, 141, 86–99. [Google Scholar] [CrossRef]
- Heve, W.K.; Olesen, J.E.; Chirinda, N.; Adiku, S.G.K. Targeted Management of Organic Resources for Sustainably Increasing Soil Organic Carbon: Observations and Perspectives for Resource Use and Climate Adaptations in Northern Ghana. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 178–190. [Google Scholar] [CrossRef]
- Wood, T.N. Agricultural Development in the Northern Savannah of Ghana. Available online: https://digitalcommons.unl.edu/planthealthdoc/1/ (accessed on 10 September 2018).
- Omari, R.A.; Bellingrath-Kimura, S.D.; Sarkodee-Addo, E.; Oikawa, Y.; Fujii, Y. Exploring farmers’ indigenous knowledge of soil quality and fertility management practices in selected farming communities of the guinea savannah agro-ecological zone of Ghana. Sustainability 2018, 10, 1034. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Giller, K.E. Popular Myths around Soil Fertility Management in Sub-Saharan Africa. Agric. Ecosyst. Environ. 2006, 116, 34–46. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Ameliorating Soil Acidity of Tropical Oxisols by Liming for Sustainable Crop Production. Adv. Agron. 2008, 99, 345–399. [Google Scholar]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic Inputs for Soil Fertility Management in Tropical Agro Ecosystems: Application of an Organic Resource Database. Agric. Ecosyst. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Nitrogen Release from Crop Residues and Organic Amendments as Affected by Biochemical Composition. Commun. Soil Sci. Plant Anal. 2003, 34, 2441–2460. [Google Scholar] [CrossRef]
- Bayorbor, T.B.; Addai, I.K.; Lawson, I.Y.D.; Dogbe, W.; Djabletey, D. Evaluation of some herbaceous legumes for use as green manure crops in the rain-fed rice based cropping system in northern Ghana. J. Agron. 2006, 5, 137–141. [Google Scholar]
- Adiku, S.G.K.; Jones, J.W.; Kumaga, F.K.; Tonyigah, A. Effects of Crop Rotation and Fallow Residue Management on Maize Growth, Yield and Soil Carbon in a Savannah-Forest Transition Zone of Ghana. J. Agric. Sci. 2009, 147, 313–322. [Google Scholar] [CrossRef]
- Nyalemegbe, K.K.; Asuming-Brempong, S.; Danso, S.K.A. Evaluation of Sesbania Sesban L. (Merr) and Mimosa Invisa L. (Fabaceae) as Sources of Nitrogen in Irrigated Rice on the Vertisols of the Accra Plains of Ghana. J. Ecol. Nat. Environ. 2012, 4, 88–93. [Google Scholar] [CrossRef]
- Amato, M.; Ladd, J.N. Decomposition of 14C-Labelled Glucose and Legume Material in Soils: Properties Influencing the Accumulation of Organic Residue C and Microbial Biomass C. Soil Biol. Biochem. 1992, 24, 455–464. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Mapfumo, P.; and Kirchmann, H. Decomposition of organic matter in soil as influenced by texture and pore space distribution. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Bationo, A., Ed.; Academy Science Publishers/TSBF-CIAT: Nairobi, Kenya, 2004; pp. 261–275. [Google Scholar]
- Adjei-Gyapong, T.; Asiamah, R. The Interim Ghana Soil Classification System and Its Relation with the World Reference Base for Soil Resources; FAO Report on Soil Resources: Abomey, Republic of Benin, 2002; pp. 9–13. [Google Scholar]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Wakelin, S.A.; Macdonald, L.M.; Rogers, S.L.; Gregg, A.L.; Bolger, T.P.; Baldock, J.A. Habitat Selective Factors Influencing the Structural Composition and Functional Capacity of Microbial Communities in Agricultural Soils. Soil Biol. Biochem. 2008, 40, 803–813. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture Is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Docherty, K.M.; Borton, H.M.; Espinosa, N.; Gebhardt, M.; Gil-Loaiza, J.; Gutknecht, J.L.M.; Maes, P.W.; Mott, B.M.; Parnell, J.J.; Purdy, G.; et al. Key Edaphic Properties Largely Explain Temporal and Geographic Variation in Soil Microbial Communities across Four Biomes. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Issaka, N.R.; Mohammed, M.; Tobita, S.; Nakamura, S.; Owusu-Adjei, E. Indigenous Fertilizing Materials to Enhance Soil Productivity in Ghana. In Soil Fertility Improvement and Integrated Nutrient Management—A Global Perspective; Whalen, K.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 119–134. [Google Scholar]
- Omari, R.A.; Aung, H.P.; Hou, M.; Yokoyama, T.; Onwona-Agyeman, S.; Oikawa, Y.; Fujii, Y.; Bellingrath-Kimura, S.D. Influence of Different Plant Materials in Combination with Chicken Manure on Soil Carbon and Nitrogen Contents and Vegetable Yield. Pedosphere 2016, 26, 510–521. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-meskel, E.; Bakken, L.R. Carbon and Nitrogen Mineralization Dynamics in Different Soils of the Tropics Amended with Legume Residues and Contrasting Soil Moisture Contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s No Place like Home? An Exploration of the Mechanisms behind Plant Litter-Decomposer Affinity in Terrestrial Ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Brammer, H. Classification of Ghanaian soils. In Agriculture and Land Use in Ghana; Wills, J.B., Ed.; Oxford University Press: London, UK, 1962; pp. 90–126. [Google Scholar]
- Flavel, T.C.; Murphy, D.V. Carbon and Nitrogen Mineralization Rates after Application of Organic Amendments to Soil. J. Environ. Qual. 2006, 35, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. The phenolics constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Sci. Food Agr. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis, 1st ed.; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- US Environmental Protection Agency. Methods for Chemical Analysis of Water and Wastes; Environmental Protection Agency: Washington, DC, USA, 1983; p. 491.
- Mohanty, M.; Reddy, K.S.; Probert, M.E.; Dalal, R.C.; Rao, A.S.; Menzies, N.W. Modelling N Mineralization from Green Manure and Farmyard Manure from a Laboratory Incubation Study. Ecol. Model. 2011, 222, 719–726. [Google Scholar] [CrossRef]
- Sistani, K.R.; Adeli, A.; McGowen, S.L.; Tewolde, H.; Brink, G.E. Laboratory and Field Evaluation of Broiler Litter Nitrogen Mineralization. Bioresour. Technol. 2008, 99, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analyses, Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Hu, S.; Coleman, D.C.; Carroll, C.R.; Hendrix, P.F.; Beare, M.H. Labile Soil Carbon Pools in Subtropical Forest and Agricultural Ecosystems as Influenced by Management Practices and Vegetation Types. Agric. Ecosyst. Environ. 1997, 65, 69–78. [Google Scholar] [CrossRef]
- Hobbie, S.E. Chloroform Fumigation Direct Extraction (CFDE) Protocol for Microbial Biomass Carbon and Nitrogen. Available online: https://web.stanford.edu/group/Vitousek/chlorofume.html (accessed on 10 September 2018).
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the KEC factor. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen Mineralization Dynamics of Different Valuable Organic Amendments Commonly Used in Agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon and Nitrogen Mineralization in Different Upland Soils of the Subtropics Treated with Organic Materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Li, L.J.; Han, X.Z.; You, M.Y.; Yuan, Y.R.; Ding, X.L.; Qiao, Y.F. Carbon and Nitrogen Mineralization Patterns of Two Contrasting Crop Residues in a Mollisol: Effects of Residue Type and Placement in Soils. Eur. J. Soil Biol. 2013, 54, 1–6. [Google Scholar] [CrossRef]
- De Neve, S.; Hofman, G. Influence of Soil Compaction on Carbon and Nitrogen Mineralization of Soil Organic Matter and Crop Residues. Biol. Fertil. Soils. 2000, 30, 544–549. [Google Scholar] [CrossRef]
- Kaleem Abbasi, M.; Mahmood Tahir, M.; Sabir, N.; Khurshid, M. Impact of the Addition of Different Plant Residues on Nitrogen Mineralization-Immobilization Turnover and Carbon Content of a Soil Incubated under Laboratory Conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Paul, K.I.; Black, A.S.; Conyers, M.K. Effect of Plant Residue Return on the Development of Surface Soil pH Gradients. Biol. Fertil. Soils. 2001, 33, 75–82. [Google Scholar] [CrossRef]
- Sakala, G.M.; Rowell, D.L.; Pilbeam, C.J. Acid-Base Reactions between an Acidic Soil and Plant Residues. Geoderma 2004, 123, 219–232. [Google Scholar] [CrossRef]
- Xu, J.M.; Tang, C.; Chen, Z.L. The Role of Plant Residues in pH Change of Acid Soils Differing in Initial pH. Soil Biol. Biochem. 2006, 38, 709–719. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. The Potential of Using Cocoa Pod Husks as Green Solid Base Catalysts for the Transesterification of Soybean Oil into Biodiesel: Effects of Biodiesel on Engine Performance. Chem. Eng. J. 2013, 220, 395–401. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Voroney, P.; Kachanoski, R.G. Turnover of Carbon through the Microbial Biomass in Soils with Different Textures. Soil Biol. Biochem. 1991, 23, 1–3. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and Negative Carbon Mineralization Priming Effects among a Variety of Biochar-Amended Soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Mandal, B.; Majumder, B.; Bandyopadhyay, P.K.; Hazra, G.C.; Gangopadhyay, A.; Samantaray, R.N.; Mishra, A.K.; Chaudhury, J.; Saha, M.N.; Kundu, S. The Potential of Cropping Systems and Soil Amendments for Carbon Sequestration in Soils under Long-Term Experiments in Subtropical India. Glob. Chang. Biol. 2007, 13, 357–369. [Google Scholar] [CrossRef]
- Kukal, S.S.; Rehana-Rasool; Benbi, D.K. Soil Organic Carbon Sequestration in Relation to Organic and Inorganic Fertilization in Rice-Wheat and Maize-Wheat Systems. Soil Tillage Res. 2009, 102, 87–92. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. A Review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Saber, M.; Hejazi, M.J.; Hassan, A.S. Effects of azadirachtin/neemazal on different stages and adult life table parameters of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2004, 97, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Elzaawely, A.A.; Deba, F.; Fukuta, M.; Tawata, S. Mimosine in Leucaena as a Potent Bio-Herbicide. Agron. Sustain. Dev. 2006, 26, 89–97. [Google Scholar] [CrossRef]
- Takemura, T.; Kamo, T.; Sakuno, E.; Hiradate, S.; Fujii, Y. Discovery of Coumarin as the Predominant Allelochemical in Gliricidia sepium. J. Trop. Sci. 2013, 25, 268–272. [Google Scholar]
| Soil Parameter | Nya | Tec | Lego |
|---|---|---|---|
| Sand (g kg−1) | 790 | 750 | 340 |
| Silt (g kg−1) | 180 | 130 | 590 |
| Clay (g kg−1) | 30 | 120 | 70 |
| Total C (g kg−1) | 3.2 ± 0.0 | 6.6 ± 0.2 | 7.2 ± 0.1 |
| Total N (g kg−1) | 1.8 ± 0.1 | 2.6 ± 0.1 | 2.3 ± 0.0 |
| CN ratio | 1.8 | 2.5 | 3.2 |
| NO3 (mg N kg−1) | 57.8 ± 0.5 | 81.3 ± 0.2 | 18.8 ± 1.3 |
| NH4 (mg N kg−1) | 13.5 ± 0.0 | 11.3 ± 0.2 | 17.8 ± 0.1 |
| SOM (%) | 0.7 | 2.1 | 1.7 |
| DOC (mg C kg−1) | 47.4 ± 0.2 | 45.9 ± 0.0 | 61.6 ± 0.4 |
| pH (1:2 H2O) | 6.1 | 7.1 | 6.0 |
| Organic Residues | TC (g kg−1) | TN (g kg−1) | CN Ratio | PP (mg GAE g−1) |
|---|---|---|---|---|
| Leucaena Leucaena leucocephala | 463.8 ± 21.7 ab | 41.1 ± 1.3 a | 11 | 11.6 ± 1.8 b |
| Centrosema Centrosema pubescence | 443.3 ± 8.0 bcd | 35.2 ± 0.8 b | 13 | 2.6 ± 0.1 e |
| Gliricidia Gliricidia sepium | 446.2 ± 8.8 abc | 30.7 ± 0.9 c | 15 | 8.8 ± 0.4 c |
| Pueraria Pueraria phaseoloides | 415.1 ± 8.9 d | 24.4 ± 0.9 d | 17 | 4.4 ± 0.2 d |
| Azadirachta Azadirachta indica | 467.9 ± 2.5 a | 24.5 ± 0.4 d | 19 | 17.1 ± 1.8 a |
| Theobroma Theobroma cacao | 423.9 ± 15.0 cd | 10.8 ± 0.3 e | 39 | 1.6 ± 0.0 e |
| LSD0.05 | 30.8 | 2.1 | 1.6 | |
| p-value | 0.002** | 0.01** | 0.01** |
| OR/ST | Days of Incubation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30D | 60D | 90D | 120D | |||||||||
| Nya | Tec | Lego | Nya | Tec | Lego | Nya | Tec | Lego | Nya | Tec | Lego | |
| Leucaena | 190.5 ± 25.7 ab | 95.9 ± 10.8 c | 94.4 ± 4.8 b | 144.7 ± 18.6 ab | 65.9 ± 17.5 b | 179.5 ± 12.6 b | 32.1 ± 12.2 ab | 57.8 ± 26.3ab | 16.3 ± 13.2 bc | 144.7 ± 36.5 b | 137.6 ± 30.1 a | 84.8 ± 63.4 ab |
| Centrosema | 97.3 ± 7.3 bc | 62.5 ± 7.8 d | 48.7 ± 0.9 c | 74.8 ± 31.7 c | 39.2 ± 8.8 b | 57.0 ± 20.2cd | −0.6 ± 9.3 b | 8.9 ± 8.7 cd | 137.2 ± 55.7 a | 2.3 ± 7.5 de | 62.3 ± 7.4 a | −70.7 ± 56.1 cd |
| Gliricidia | 251.0 ± 36.9 a | 205.4 ± 5.1 a | 190.9 ± 16.2 a | 181.5 ± 14.0 a | 209.2 ± 32.8a | 269.7 ± 24.2 a | 105.2 ± 64.9 a | 85.4 ± 10.4a | 77.7 ± 22.2 ab | 279.5 ± 25.5a | 179.7 ± 86.8 a | 153.1 ± 118.5 a |
| Pueraria | 137.7 ± 7.7 bc | 33.6 ± 6.9 e | 19.3 ± 9.4 d | 97.3 ± 5.0 bc | 23.1 ± 4.2 b | 14.7 ± 23.8 d | 4. 9 ± 15.7 b | −12.0 ± 18.5d | 18.5 ± 26.5 bc | −29.0 ± 4.7 e | 132.5 ± 52.5 a | −16.1 ± 12.5 bc |
| Azadirachta | 136.6 ± 6.5 bc | 150.5 ± 5.5 b | 86.0 ± 5.9 b | 149.8 ± 24.3 ab | 92.4 ± 46.9 b | 263.6 ± 30.4 a | 4.4 ± 13.7 b | 17.4 ± 14.9bcd | 19.7 ± 22.8 bc | 38.2 ± 2.0 dc | 171.3 ± 39.8 a | 51.8 ± 42.1 abc |
| Theobroma | 92.4 ± 74.0 c | 3.3 ± 0.6 f | 11.1 ± 5.1 d | 136.6 ± 7.4 ab | 21.8 ± 12.9 b | 113.3 ± 21.6 bc | 32.5 ± 14.6 ab | 33.6 ± 3.9 bc | −42.6 ± 22.9 c | 78. 8 ± 10.9c | −134.3 ± 3.9 b | −165.6 ± 31.0 d |
| Mean | 129.4 A | 78.7 B | 64.3 B | 112.1 B | 64.5 C | 128.3 A | 25.5 A | 27.3 A | 32.4 A | 73.5 A | 78.5 A | 5.3 B |
| p-value (ST) | <0.01 | <0.01 | 0.74 ns | <0.01 | ||||||||
| p-value (OR) | <0.03 | <0.01 | <0.01 | <0.03 | <0.01 | <0.01 | <0.04 | <0.03 | <0.021 | <0.01 | <0.01 | <0.01 |
| ST x OR | <0.02 | <0.01 | <0.01 | <0.01 | ||||||||
| OR/ST | Mineralization at t = 0 (intercept) (mg N kg−1 soil ) | Mineralization rate at (K) (at 25 °C) (mg N kg−1 soil day−1) | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nya | Tec | Lego | Nya | Tec | Lego | Nya | Tec | Lego | |
| Control | 67.9 ± 1.6 b | 46.3 ± 1.3 cd | −1.9 ± 5.7 d | 0.7 ± 0.1cd | 1.2 ± 0.1 bc | 1.4 ± 0.3 a | 0.9 | 0.6 | 0.8 |
| Leucaena | 144.1 ± 3.0 a | 70.3 ± 8.9 bc | 54.8 ± 4.7 b | 1.1 ± 0.2 b | 2.0 ± 0.3 a | 1.7 ± 0.2 a | 0.3 | 0.8 | 0.6 |
| Centrosema | 121.4 ± 7.5 a | 66.7 ± 2.9 bcd | 43.2 ± 2.5 bc | 0.4 ± 0.1 de | 1.4 ± 0.1 ab | 1.3 ± 0.2 a | 0.2 | 0.7 | 0.6 |
| Gliricidia | 148.7 ± 11.2 a | 134.4 ± 24.9 a | 97.8 ± 28.8 a | 2.1 ± 0.1 a | 2.0 ± 0.6 a | 2.1 ± 0.8 a | 0.5 | 0.6 | 0.6 |
| Pueraria | 148.3 ± 3.9 a | 37.9 ± 10.4 c | 12.0 ± 4.3 cd | 0.1 ± 0.2 e | 1.9 ± 0.3 a | 1.3 ± 0.1 a | 0.1 | 0.6 | 0.9 |
| Azadirachta | 144.7 ± 7.8 a | 90.7 ± 5.9 b | 74.9 ± 6.8 ab | 0.5 ± 0.1 d | 1.9 ± 0.2 a | 1.6 ± 0.2 a | 0.1 | 0.5 | 0.4 |
| Theobroma | 116.4 ± 27.4 a | 78.9 ± 3.9 b | 58.3 ± 4.8 b | 1.0 ± 0.3 bc | 0.4 ± 0.1 c | 0.2 ± 0.0 b | 0.5 | 0.3 | 0.1 |
| Mean | 127.4 A | 75.0 B | 48.5 C | 0.8 B | 1.5 A | 1.4 A | |||
| p-value (ST) | <0.01 | <0.01 | |||||||
| p-value (OR) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| ST x OR | <0.01 | <0.01 | |||||||
| SOV | DF | pH | DOC (mg C kg−1) | MBC (mg C kg−1) | |||
|---|---|---|---|---|---|---|---|
| Means of Squares | p-Value | Means of Squares | p-Value | Means of Squares | p-Level | ||
| Site | 2 | 4.0 | ** | 18,359.9 | ** | 579,554.8 | ** |
| OR | 6 | 4.2 | ** | 7,032.1 | ** | 198,137.2 | ** |
| Site x OR | 12 | 0.05 | ** | 1449.8 | ** | 101,523.8 | ** |
| Error | 40 | 0.00 | 25.6 | 108.3 | |||
| Total | 60 | ||||||
| R2 | 0.9 | 0.9 | 0.9 |
| OR/ST | Soil pH | ||
|---|---|---|---|
| Nya | Tec | Lego | |
| Control | 6.8 ± 0.01 e | 6.4 ± 0.02 f | 6.1 ± 0.01 g |
| Leucaena | 7.3 ± 0.01 d | 6.9 ± 0.01 d | 6.3 ± 0.01 f |
| Centrosema | 7.5 ± 0.02 c | 7.0 ± 0.01 c | 6.8 ± 0.01 b |
| Gliricidia | 7.5 ± 0.02 c | 6.9 ± 0.02 d | 6.7 ± 0.01 c |
| Pueraria | 7.9 ± 0.01 b | 7.1 ± 0.01 b | 6.6 ± 0.01 d |
| Azadirachta | 7.3 ± 0.02 d | 6.8 ± 0.01 e | 6.5 ± 0.01 e |
| Theobroma | 9.0 ± 0.01 a | 8.7 ± 0.01 a | 8.1 ± 0.01 a |
| Mean | 7.6 A | 7.1 B | 6.8 C |
| p-value (ST) | <0.01 | <0.01 | <0.01 |
| p-value (OR) | <0.01 | <0.01 | <0.01 |
| ST x OR | <0.01 | <0.01 | <0.01 |
| OR/ST | DOC (mg C kg−1) | MBC (mg C kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Nya | Tec | Lego | Mean | Nya | Tec | Lego | Mean | |
| Control | 34.0 ± 1.4 g | 18.6 ± 0.3 e | 32.1 ± 0.1 f | 28.2 | 686.3 ± 4.8 b | 594.9 ± 17.9 b | 677.2 ± 8.6 f | 652.8 |
| Leucaena | 133.8 ± 1.6 c | 64.8 ± 0.3 c | 67.8 ± 0.7 c | 88.8 | 65.4 ± 13.3 e | 648.9 ± 8.0 a | 714.3 ± 15.3 e | 476.2 |
| Centrosema | 91.6 ± 0.5 f | 59.3 ± 0.2 d | 52.4 ± 1.4 d | 67.8 | 565.2 ± 10.9 c | 563.8 ± 9.6 c | 890.7 ± 18.1 b | 673.3 |
| Gliricidia | 101.4 ± 0.3 d | 94.6 ± 0.6 b | 74.3 ± 0.3 b | 90.1 | 572.6 ± 10.6 c | 224.8 ± 2.5 d | 753.1 ± 17.7 d | 516.8 |
| Pueraria | 98.5 ± 0.1 e | 64.7 ± 0.3 c | 53.3 ± 0.1 d | 72.2 | 862.7 ± 33.3 a | 668.8 ± 12.0 a | 971.6 ± 16.3 a | 834.3 |
| Azadirachta | 166.4 ± 0.1 b | 100.0 ± 1.1 a | 86.8 ± 0.4 a | 117.7 | 427.7 ± 14.6 d | 35.8 ± 0.3 e | 734.7 ± 12.3 de | 399.4 |
| Theobroma | 170.1 ± 0.7 a | 65.1 ± 2.4 c | 47.0 ± 0.4 e | 94.1 | 658.8 ± 7.6 b | 584.8 ± 7.2 bc | 802.2 ± 20.7 c | 681.9 |
| Mean | 113.7 A | 66.7 B | 59.1 C | 548.4 B | 474.5 C | 791.7 A | ||
| p-value (ST) | <0.01 | <0.01 | ||||||
| p-value(OR) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| ST x OR | <0.01 | <0.01 | ||||||
| Parameter | Resultant soil property | ||||
|---|---|---|---|---|---|
| Organic Residue Quality | MBC | DOC | pH | CIN | K |
| TC | 0.21* (−5.4) | 0.02ns | 0.21* (−0.02) | 0.12ns | 0.05ns |
| TN | 0.01ns | 0.04ns | 0.44** (−0.05) | 0.48* (8.5) | 0.14ns |
| CN ratio | 0.01ns | 0.02ns | 0.79** (0.06) | 0.20* (−11.6) | 0.22* (−0.03) |
| PP | 0.08ns | 0.03ns | 0.20* (−0.06) | 0.11ns | 0.02ns |
| Interaction (TC x TN x CN ratio x PP) (A) | ns | ns | ** | ns | ns |
| Initial soil property | |||||
| TN | 0.01ns | 0.38* (−66.8) | 0.09ns | 0.02ns | 0.20ns |
| TC | 0.08ns | 0.61** (−15.9) | 0.24* (−0.2) | 0.09ns | 0.17ns |
| CN ratio | 0.22* (200.5) | 0.54** (−45.2) | 0.26* (−0.6) | 0.12ns | 0.09ns |
| SOM | 0.06ns | 0.21* (−27.4) | 0.03ns | 0.00ns | 0.17ns |
| Interaction (TC x TN x CN ratio x SOM) (B) | * | ** | ns | ns | ns |
| Soil texture | |||||
| Sand | 0.34* (−7.3) | 0.30* (0.9) | 0.20* (0.01) | 0.11ns | 0.02ns |
| Silt | 0.36* (7.5) | 0.20* (−0.7) | 0.15ns | 0.09ns | 0.002ns |
| Clay | 0.02ns | 0.32* (−5.5) | 0.07ns | 0.01ns | 0.20* (0.08) |
| Interaction (sand x silt x clay) (C) | * | ** | ns | ns | ns |
| Interaction (A*B*C) | * | ** | ** | NS | NS |
| Parameter | K | CIN | DOC | MBC | pH |
|---|---|---|---|---|---|
| K | |||||
| CIN | 0.63** | ||||
| DOC | −0.17ns | 0.44* | |||
| MBC | −0.20ns | −0.53* | −0.48* | ||
| pH | −0.63** | −0.26ns | 0.37ns | 0.04ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansong Omari, R.; Bellingrath-Kimura, D.S.; Fujii, Y.; Sarkodee-Addo, E.; Appiah Sarpong, K.; Oikawa, Y. Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources. Soil Syst. 2018, 2, 63. https://doi.org/10.3390/soilsystems2040063
Ansong Omari R, Bellingrath-Kimura DS, Fujii Y, Sarkodee-Addo E, Appiah Sarpong K, Oikawa Y. Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources. Soil Systems. 2018; 2(4):63. https://doi.org/10.3390/soilsystems2040063
Chicago/Turabian StyleAnsong Omari, Richard, Dorothea Sonoko Bellingrath-Kimura, Yoshiharu Fujii, Elsie Sarkodee-Addo, Kwame Appiah Sarpong, and Yosei Oikawa. 2018. "Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources" Soil Systems 2, no. 4: 63. https://doi.org/10.3390/soilsystems2040063
APA StyleAnsong Omari, R., Bellingrath-Kimura, D. S., Fujii, Y., Sarkodee-Addo, E., Appiah Sarpong, K., & Oikawa, Y. (2018). Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources. Soil Systems, 2(4), 63. https://doi.org/10.3390/soilsystems2040063







