Nitrogen Fertilization Reduces the Capacity of Soils to Take up Atmospheric Carbonyl Sulphide
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Microcosm Gas Exchange
2.2. Soil Physico-Chemical Properties Analysis
2.3. Soil Nitrogen Fertilisation Manipulation
2.4. Statistical Analysis
3. Results
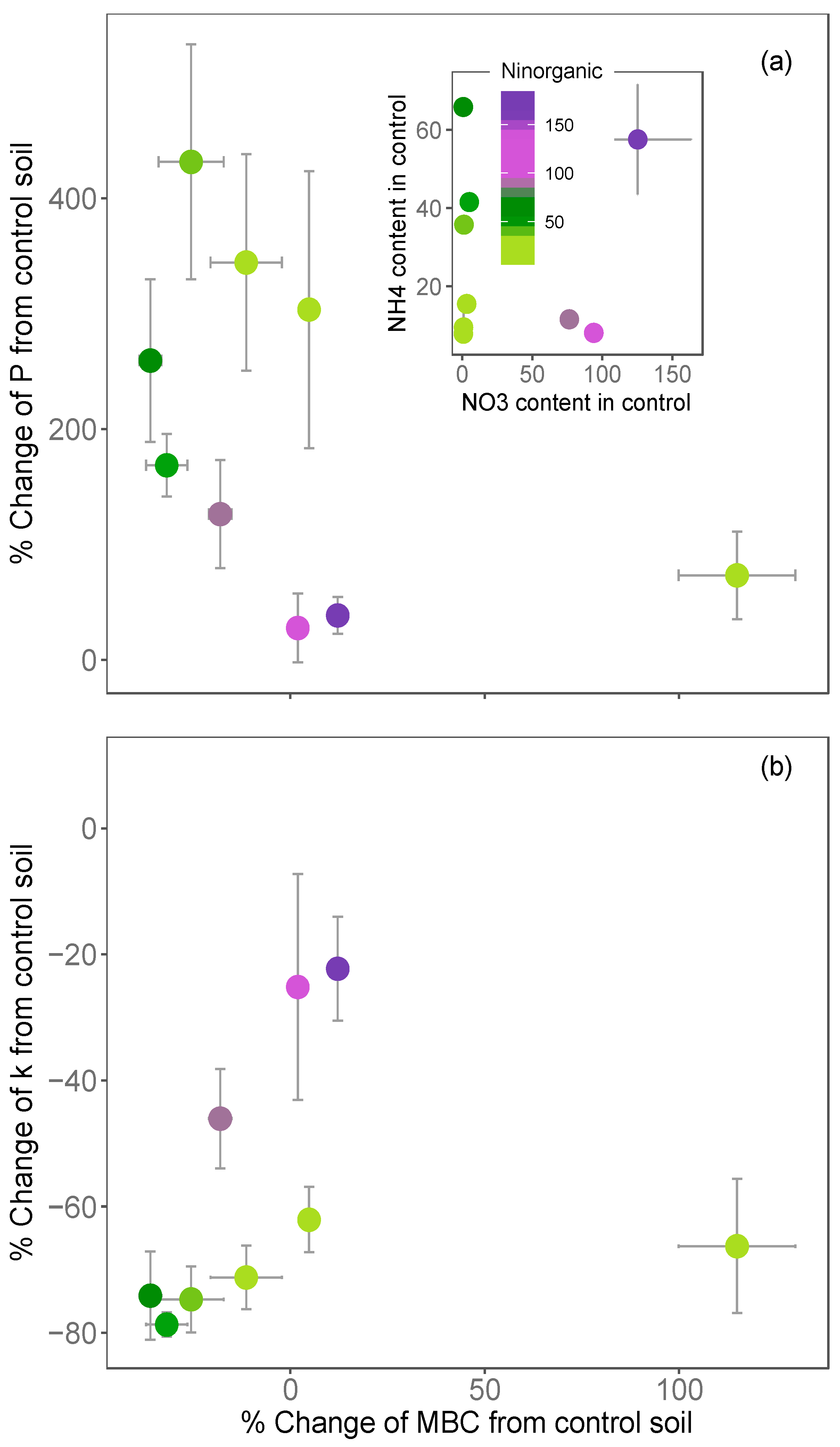
3.1. Variations in Soil COS Fluxes with Inorganic Nitrogen across Europe
3.2. Impact of N Fertilisation on Partitioned Soil COS Fluxes
- larger for soils that were poor in NO3− relative to those rich in NO3− (for the same level of NH4+);
- larger for soils that were poor in NH4+ relative to those rich in NH4+ (for the same level of NO3−) and;
- limited in soil that were initially rich in both NH4+ and NO3− (Figure 5a,b).
4. Discussion
4.1. Mechanisms Inhibiting the COS Hydrolysis Rate
4.2. COS Production Is Most Closely Linked to Soil Nitrate Availability
4.3. Mechanisms Promoting the Production of COS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crutzen, P.J. The Possible Importance of CSO for the Sulfate Layer of the Stratosphere. Geophys. Res. Lett. 1976, 3, 73–76. [Google Scholar] [CrossRef]
- Andreae, M.O.; Crutzen, P. Atmospheric Aerosol: Biogeochemical Sources and Role in Atmospheric Chemistry. Science 1997, 276, 1052–11058. [Google Scholar] [CrossRef]
- Sandoval-Soto, L.; Stanimirov, M.; von Hobe, M.; Schmitt, V.; Valdes, J.; Wild, A.; Kesselmeier, J. Global Uptake of Carbonyl Sulfide (COS) by Terrestrial Vegetation: Estimates Corrected by Deposition Velocities Normalized to the Uptake of Carbon Dioxide (CO2). Biogeosci. Discuss. 2005, 2, 183–201. [Google Scholar] [CrossRef]
- Montzka, S.A.; Calvert, P.; Hall, B.D.; Elkins, J.W.; Conway, T.J.; Tans, P.P.; Sweeney, C.S. On the Global Distribution, Seasonality, and Budget of Atmospheric Carbonyl Sulfide (COS) and Some Similarities to CO2. J. Geophys. Res. Atmos. 2007, 112, 1–15. [Google Scholar] [CrossRef]
- Liuzzi, G.; Masiello, G.; Serio, C.; Venafra, S.; Camy-Peyret, C. Physical inversion of the full IASI spectra: Assessment of atmospheric parameters retrievals, consistency of spectroscopy and forward modelling. J. Quant. Spectrosc. Radiat. 2016, 182, 128–157. [Google Scholar] [CrossRef]
- Campbell, J.E.; Berry, J.A.; Seibt, U.; Smith, S.J.; Montzka, S.A.; Launois, T.; Belviso, S.; Bopp, L.; Laine, M. Large Historical Growth in Global Terrestrial Gross Primary Production. Nature 2017, 544, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Protoschill-Krebs, G.; Kesselmeier, J. Enzymatic Pathways for the Metabolization of Carbonyl Suphide (COS) by Higher Plants. Bot. Acta 1992, 105, 206–212. [Google Scholar] [CrossRef]
- Protoschill-Krebs, G.; Wilhelm, C.; Kesselmeier, J. Consumption of Carbonyl Sulphide (COS) by Higher Plant Carbonic Anhydrase (CA). Atmos. Environ. 1996, 30, 3151–3156. [Google Scholar] [CrossRef]
- Stimler, K.; Berry, J.A.; Yakir, D. Effects of Carbonyl Sulfide and Carbonic Anhydrase on Stomatal Conductance. Plant Physiol. 2012, 158, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Whelan, M.E.; Lennartz, S.T.; Gimeno, T.E.; Wehr, R.; Wohlfahrt, G.; Wang, Y.; Kooijmans, L.M.J.; Hilton, T.W.; Belviso, S.; Peylin, P.; et al. Reviews and Syntheses: Carbonyl Sulfide as a Multi-Scale Tracer for Carbon and Water Cycles. Biogeosciences 2018, 15, 3625–3657. [Google Scholar] [CrossRef]
- Kettle, A.J.; Kuhn, U.; Von Hobe, M.; Kesselmeier, J.; Andreae, M.O. Global Budget of Atmospheric Carbonyl Sulfide: Temporal and Spatial Variations of the Dominant Sources and Sinks. J. Geophys. Res. Atmos. 2002, 107, 1–16. [Google Scholar] [CrossRef]
- Berry, J.; Wolf, A.; Campbell, J.E.; Baker, I.; Blake, N.; Blake, D.; Denning, A.S.; Kawa, S.R.; Montzka, S.A.; Seibt, U.; et al. A Coupled Model of the Global Cycles of Carbonyl Sulfide and CO2: A Possible New Window on the Carbon Cycle. J. Geophys. Res. Biogeosci. 2013, 118, 842–852. [Google Scholar] [CrossRef]
- Launois, T.; Peylin, P.; Belviso, S.; Poulter, B. A New Model of the Global Biogeochemical Cycle of Carbonyl Sulfide—Part 2: Use of Carbonyl Sulfide to Constrain Gross Primary Productivity in Current Vegetation Models. Atmos. Chem. Phys. 2015, 15, 9285–9312. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Teusch, N.; Kuhn, U. Controlling Variables for the Uptake of Atmospheric Carbonyl Sulfide by Soil. J. Geophys. Res. 1999, 104, 11577–11584. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic Carbonic Anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Wingate, L.; Ogée, J.; Cuntz, M.; Genty, B.; Reiter, I.; Seibt, U.; Yakir, D.; Maseyk, K.; Pendall, E.G.; Barbour, M.M.; et al. The impact of soil microorganisms on the global budget of delta18O in atmospheric CO2. Proc. Natl. Acad. Sci. USA 2009, 106, 22411–22415. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, H.; Kesselmeier, J. Soil Atmosphere Exchange of Carbonyl Sulfide (COS) Regulated by Diffusivity Depending on Water-Filled Pore Space. Biogeosci. Discuss. 2008, 4, 3701–3722. [Google Scholar] [CrossRef]
- Whelan, M.E.; Hilton, T.W.; Berry, J.A.; Berkelhammer, M.; Desai, A.R.; Elliott Campbell, J. Carbonyl Sulfide Exchange in Soils for Better Estimates of Ecosystem Carbon Uptake. Atmos. Chem. Phys. 2016, 16, 3711–3726. [Google Scholar] [CrossRef]
- Meredith, L.K.; Boye, K.; Youngerman, C.; Whelan, M.; Ogée, J.; Sauze, J.; Wingate, L. Coupled Biological and Abiotic Mechanisms Driving Carbonyl Sulfide Production in Soils. Soil Syst. 2018, 2, 37. [Google Scholar] [CrossRef]
- Kaisermann, A.; Ogée, J.; Sauze, J.; Wohl, S.; Jones, S.P.; Gutierrez, A.; Wingate, L. Disentangling the Rates of Carbonyl Sulfide (COS) Production and Consumption and Their Dependency on Soil Properties across Biomes and Land Use Types. Atmos. Chem. Phys. 2018, 18, 9425–9440. [Google Scholar] [CrossRef]
- Maseyk, K.; Berry, J.A.; Billesbach, D.; Campbell, J.E.; Torn, M.S.; Zahniser, M.; Seibt, U. Sources and Sinks of Carbonyl Sulfide in an Agricultural Field in the Southern Great Plains. Proc. Natl. Acad. Sci. USA 2014, 111, 9064–9069. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.K.; Ogée, J.; Boye, K.; Singer, E.; Wingate, L.; von Sperber, C.; Sengupta, A.; Whelan, M.; Pang, E.; Keiluweit, M.; et al. Soil Exchange Rates of COS and CO18O Shift with the Diversity of Microbial Communities and Their Carbonic Anhydrase Enzymes. ISME J. 2018. in review. [Google Scholar] [CrossRef] [PubMed]
- Ogée, J.; Sauze, J.; Kesselmeier, J.; Genty, B.; Van Diest, H.; Launois, T.; Wingate, L. A New Mechanistic Framework to Predict OCS Fluxes from Soils. Biogeosciences 2016, 13, 2221–2240. [Google Scholar] [CrossRef]
- Sun, W.; Maseyk, K.; Lett, C.; Seibt, U. Litter Dominates Surface Fluxes of Carbonyl Sulfide in a Californian Oak Woodland. J. Geophys. Res. G Biogeosci. 2016, 121, 438–450. [Google Scholar] [CrossRef]
- Melillo, J.M.; Steudler, P.A. The Effect of Nitrogen Fertilization on the COS and CS2 Emissions from Temperature Forest Soils. J. Atmos. Chem. 1989, 9, 411–417. [Google Scholar] [CrossRef]
- Whelan, M.E.; Rhew, R.C. Carbonyl Sulfide Produced by Abiotic Thermal and Photodegradation of Soil Organic Matter from Wheat Field Substrate. J. Geophys. Res. Biogeosci. 2015, 120, 54–62. [Google Scholar] [CrossRef]
- Smith, N.A.; Kelly, D.P. Oxidation of Carbon Disulphide as the Sole Source of Energy for the Autotrophic Growth of Thiobacillus Thioparus Strain TK-M. Microbiology 1988, 134, 3041–3048. [Google Scholar] [CrossRef]
- Smeulders, M.J.; Barends, T.R.M.; Pol, A.; Scherer, A.; Zandvoort, M.H.; Udvarhelyi, A.; Khadem, A.F.; Menzel, A.; Hermans, J.; Shoeman, R.L.; et al. Evolution of a New Enzyme for Carbon Disulphide Conversion by an Acidothermophilic Archaeon. Nature 2011, 478, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Naraharas, Y.; Inoues, Y.; Amanon, F.; Kanagawa, T.; Kuraishi, H. A Thiocyanate Hydrolase of Thiobacillus Thioparus. A Novel Enzyme Catalyzing the Formation of Carbonyl Sulfide from Thiocyanate. J. Biol. Chem. 1992, 267, 9170–9175. [Google Scholar] [PubMed]
- Welte, C.U.; Rosengarten, J.F.; De Graaf, R.M.; Jetten, M.S.M. SaxA-Mediated Isothiocyanate Metabolism in Phytopathogenic Pectobacteria. Appl. Environ. Microbiol. 2016, 82, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Gleen, H. Microbiological Oxidation of Ammonium and Thiocyanate Ions in Soil. Nature 1951, 168, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Bending, G.D.; Lincoln, S.D. Inhibition of Soil Nitrifying Bacteria Communities and Their Activities by Glucosinolate Hydrolysis Products. Soil Biol. Biochem. 2000, 32, 1261–1269. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Brassicaceae Tissues as Inhibitors of Nitrification in Soil. J. Agric. Food Chem. 2009, 57, 7706–7711. [Google Scholar] [CrossRef] [PubMed]
- Davenport, H. The Inhibition of Carbonic Anhydrase and of Gastric Acid Secretion by Thiocyanate. Am. J. Physiol. 1940, 129, 505–514. [Google Scholar] [CrossRef]
- Mangani, S.; Hakansson, K. Crystallographic Studies of the Binding of Protonated and Unprotonated Inhibitors to Carbonic Anhydrase Using Hydrogen Sulphide and Nitrate Anions. Eur. J. Biochem. 1992, 210, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Peltier, G.; Cournac, L.; Despax, V.; Dimon, B.; Fina, L.; Genty, B.; Rumeau, D. Carbonic Anhydrase Activity in Leaves as Measured in Vivo by 18O Exchange between Carbon Dioxide and Water. Planta 1995, 196, 732–739. [Google Scholar] [CrossRef]
- Vullo, D.; Syrjänen, L.; Kuuslahti, M.; Parkkila, S.; Supuran, C.T. Anion Inhibition Studies of a Beta Carbonic Anhydrase from the Malaria Mosquito Anopheles Gambiae. J. Enzym. Inhib. Med. Chem. 2018, 33, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chem. Rev. 1977, 2, 219–262. [Google Scholar] [CrossRef]
- Moldrup, P.; Yoshikawa, S.; Olesen, T.; Komatsu, T.; Rolston, D.E. Gas Diffusivity in Undisturbed Volcanic Ash Soils: Test of Soil-Water-Charactieristic-Based Prediction Models. Soil Sci. Soc. Am. J. 2003, 67, 41–51. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent Effects of Nitrogen Amendments on Soil Microbial Communities and Processes across Biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.-C. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-131; The R Foundation: Vienna, Austria, 2017. [Google Scholar]
- Nakagawa, S.; Cuthill, I.C. Effect Size, Confidence Interval and Statistical Significance: A Practical Guide for Biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference; R Package Version 1.40.4; The R Foundation: Vienna, Austria, 2018; Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 July 2018).
- Murtaugh, P.A. In Defense of P Values. Ecology 2014, 95, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Baty, F.; Ritz, C.; Charles, S.; Flandrois, J.-P.; Delignette-Muller, M.-L. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- Simmons, J.S.; Klemedtsson, L.; Hultberg, H.; Hines, M.E.; Banwart, K. Consumption of Atmospheric Carbonyl Sulfide by Coniferous Boreal Forest Soils. J. Geophys. Res. 1999, 104, 11569–11576. [Google Scholar] [CrossRef]
- Ogawa, T.; Noguchi, K.; Saito, M.; Nagahata, Y.; Kato, H.; Ohtaki, A.; Nakayama, H.; Dohmae, N.; Matsushita, Y.; Odaka, M.; et al. Carbonyl Sulfide Hydrolase from Thiobacillus thioparus Strain THI115 Is One of the β-Carbonic Anhydrase Family Enzymes. J. Am. Chem. Soc. 2013, 135, 3818–3825. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, L.J.; Yuan, D.X.; Wu, Y.; Zeng, X.D. A Study of the Activity and Ecological Significance of Carbonic Anhydrase from Soil and Its Microbes from Different Karst Ecosystems of Southwest China. Plant Soil 2005, 272, 133–141. [Google Scholar] [CrossRef]
- Del Prete, S.; Vullo, D.; Osman, S.M.; Alothman, Z.; Capasso, C.; Supuran, C.T. Anion Inhibition Studies of the Dandruff-Producing Fungus Malassezia Globosa β-Carbonic Anhydrase MgCA. Bioorg. Med. Chem. Lett. 2015, 25, 5194–5198. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Zimmerman, S.; Ferry, J.; Scozzafava, A.; Supuran, C. Carbonic Anhydrase Inhibitors. Inhibition of the Beta-Class Enzyme from the Methanoarchaeon Methanobacterium Thermoautotrophicum (Cab) with Anions. Bioorg. Med. Chem. Lett. 2004, 14, 4563–4567. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, R.S. Structure and Catalytic Mechanism of the Beta-Carbonic Anhydrases. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of Forest Soil Respiration in Response to Nitrogen Deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L. Consistent Responses of Soil Microbial Communities to Elevated Nutrient Inputs in Grasslands across the Globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Ozawa, R.; Kageyama, K.; Katayama, Y. Degradation and Emission of Carbonyl Sulfide, an Atmospheric Trace Gas, by Fungi Isolated from Forest Soil. FEMS Microbiol. Lett. 2016, 363, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bunk, R.; Behrendt, T.; Yi, Z.; Andreae, M.O.; Kesselmeier, J. Exchange of Carbonyl Sulfide (OCS) between Soils and Atmosphere under Various CO2 Concentrations. J. Geophys. Res. Biogeosci. 2017, 122, 1343–1358. [Google Scholar] [CrossRef]
- Sauze, J.; Ogée, J.; Maron, P.-A.; Crouzet, O.; Nowak, V.; Wohl, S.; Kaisermann, A.; Jones, S.P.; Wingate, L. The Interaction of Soil Phototrophs and Fungi with pH and Their Impact on Soil CO2, CO18O and OCS Exchange. Soil Biol. Biochem. 2017, 115, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, R.S.; Tu, C.; McKay, M.M.; Preiss, J.R.; Loomis, R.J.; Hicks, K.A.; Marchione, R.J.; Strong, J.A.; Donovan, G.S.; Chamberlin, J.E. Kinetic Characterization of Wild-Type and Proton Transfer-Impaired Variants of β-Carbonic Anhydrase from Arabidopsis Thaliana. Arch. Biochem. Biophys. 2002, 404, 197–209. [Google Scholar] [CrossRef]
- Cronk, J.D.; Endrizzi, J.A.; Cronk, M.R.; Neill, J.W.O. Crystal Structure of E. Coli B-Carbonic Anhydrase, an Enzyme with an Unusual pH-Dependent Activity. Protein Sci. 2001, 10, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal Structure and Kinetic Studies of a Tetrameric Type II β-Carbonic Anhydrase from the Pathogenic Bacterium Vibrio Cholerae. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular Aspects of Bacterial pH Sensing and Homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Kitz, F.; Gerdel, K.; Hammerle, A.; Laterza, T.; Spielmann, F.M.; Wohlfahrt, G. In Situ Soil COS Exchange of a Temperate Mountain Grassland under Simulated Drought. Oecologia 2017, 183, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Dick, W. Production of Thiocysteine (Sulfide) in Cysteine Amended Soils. Soil Sci. Soc. Am. J. 1985, 49, 882–886. [Google Scholar] [CrossRef]
- Banerjee, M.; Chapman, S.J. The Significance of Microbial Biomass Sulphur in Soil. Biol. Fertil. Soils 1996, 22, 116–125. [Google Scholar] [CrossRef]
- Bezsudnova, E.Y.; Sorokin, D.Y.; Tikhonova, T.V.; Popov, V.O. Thiocyanate Hydrolase, the Primary Enzyme Initiating Thiocyanate Degradation in the Novel Obligately Chemolithoautotrophic Halophilic Sulfur-Oxidizing Bacterium Thiohalophilus thiocyanoxidans. Biochim. Biophys. Acta Proteins Proteom. 2007, 1774, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Tourova, T.P.; Bezsoudnova, E.Y.; Pol, A.; Muyzer, G. Denitrification in a Binary Culture and Thiocyanate Metabolism in Thiohalophilus thiocyanoxidans Gen. Nov. Sp. Nov.—A Moderately Halophilic Chemolithoautotrophic Sulfur-Oxidizing Gammaproteobacterium from Hypersaline Lakes. Arch. Microbiol. 2007, 187, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ogawa, T.; Saito, M.; Sekine, T.; Nameki, M.; Matsushita, Y.; Hayashi, T.; Katayama, Y. Cloning and Expression of a Gene Encoding a Novel Thermostable Thiocyanate-Degrading Enzyme from a Mesophilic alphaproteobacteria Strain THI201. Microbiology 2013, 159, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Kantor, R.S.; van Zyl, A.W.; van Hille, R.P.; Thomas, B.C.; Harrison, S.T.L.; Banfield, J.F. Bioreactor Microbial Ecosystems for Thiocyanate and Cyanide Degradation Unravelled with Genome-Resolved Metagenomics. Environ. Microbiol. 2015, 17, 4929–4941. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.P.; Moreau, J.W. New Insights into the Genetic and Metabolic Diversity of Thiocyanate-Degrading Microbial Consortia. Appl. Microbiol. Biotechnol. 2016, 100, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Baker, S.C. The Organosulfur Cycle: Aerobic and Anaerobic Processes Leading to Turnover of C1-Sulfur Compounds. FEMS Microbiol. Rev. 1990, 87, 241–246. [Google Scholar] [CrossRef]
- Broman, E.; Jawad, A.; Wu, X.; Christel, S.; Ni, G.; Lopez-Fernandez, M.; Sundkvist, J.E.; Dopson, M. Low Temperature, Autotrophic Microbial Denitrification Using Thiosulfate or Thiocyanate as Electron Donor. Biodegradation 2017, 28, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Tegetmeyer, H.E.; Sharma, R.; Klotz, M.G.; Ferdelman, T.G.; Hettich, R.L.; Geelhoed, J.S.; Strous, M. The Environmental Controls That Govern the End Product of Bacterial Nitrate Respiration. Science 2014, 345, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Tourova, T.P.; Antipov, A.N.; Muyzer, G.; Kuenen, J.G. Anaerobic Growth of the Haloalkaliphilic Denitrifying Sulfur-Oxidizing Bacterium Thialkalivibrio thiocyanodenitrificans sp. Nov. with Thiocyanate. Microbiology 2004, 150, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Berben, T.; Overmars, L.; Sorokin, D.Y.; Muyzer, G. Comparative Genome Analysis of Three Thiocyanate Oxidizing Thioalkalivibrio Species Isolated from Soda Lakes. Front. Microbiol. 2017, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, T.; Catão, E.C.; Bunk, R.; Yi, Z.; Schwer, E.; Trumbore, S. Microbial Community Responses Determine How Soil-Atmosphere Exchange of Carbonyl Sulfide, Carbon Monoxide and Nitric Oxide Respond to Soil Moisture. SOIL Discuss. 2018, 1–42. [Google Scholar] [CrossRef]
- Kumar, R.; Saha, S.; Dhaka, S.; Kurade, M.B.; Kang, C.U.; Baek, S.H.; Jeon, B.H. Remediation of Cyanide-Contaminated Environments through Microbes and Plants: A Review of Current Knowledge and Future Perspectives. Geosyst. Eng. 2017, 20, 28–40. [Google Scholar] [CrossRef]
- Cipollone, R.; Ascenzi, P.; Tomao, P.; Imperi, F.; Visca, P. Enzymatic Detoxification of Cyanide: Clues from Pseudomonas aeruginosa Rhodanese. J. Mol. Microbiol. Biotechnol. 2008, 15, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Enzymatic Mechanism and Biochemistry for Cyanide Degradation: A Review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
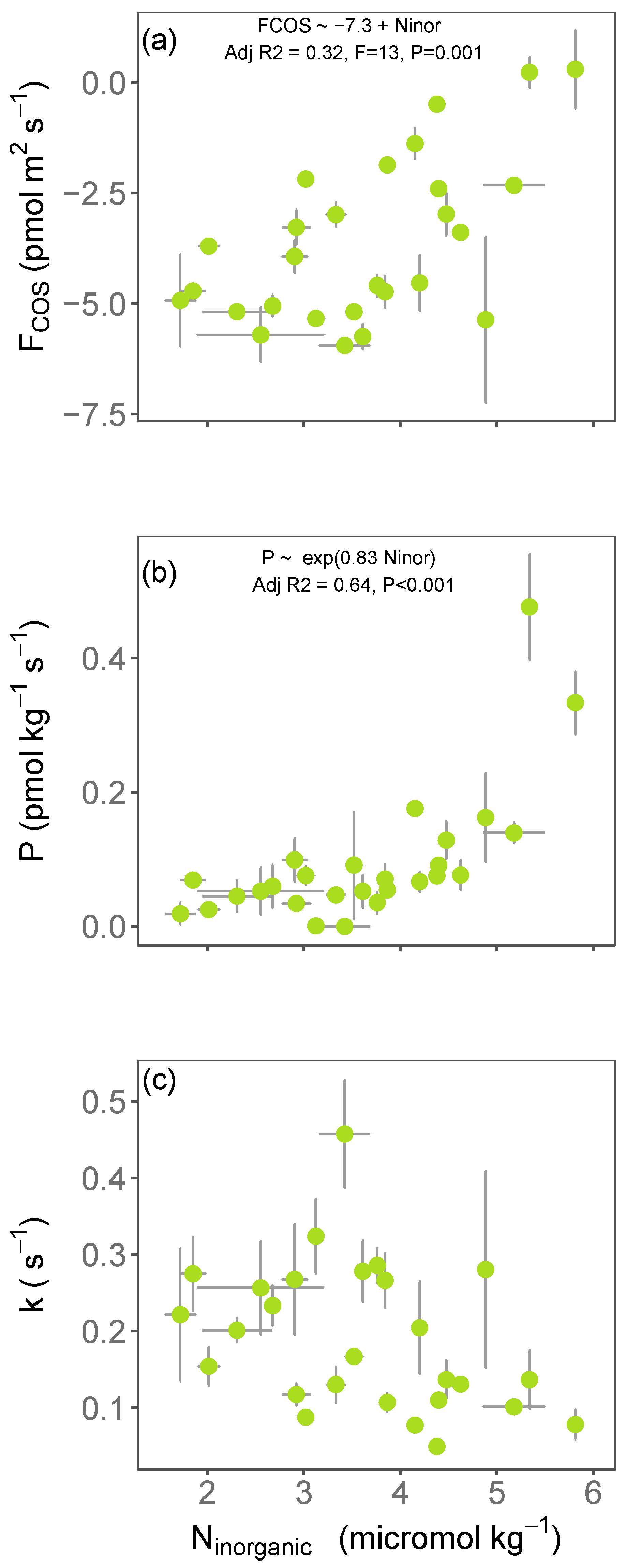
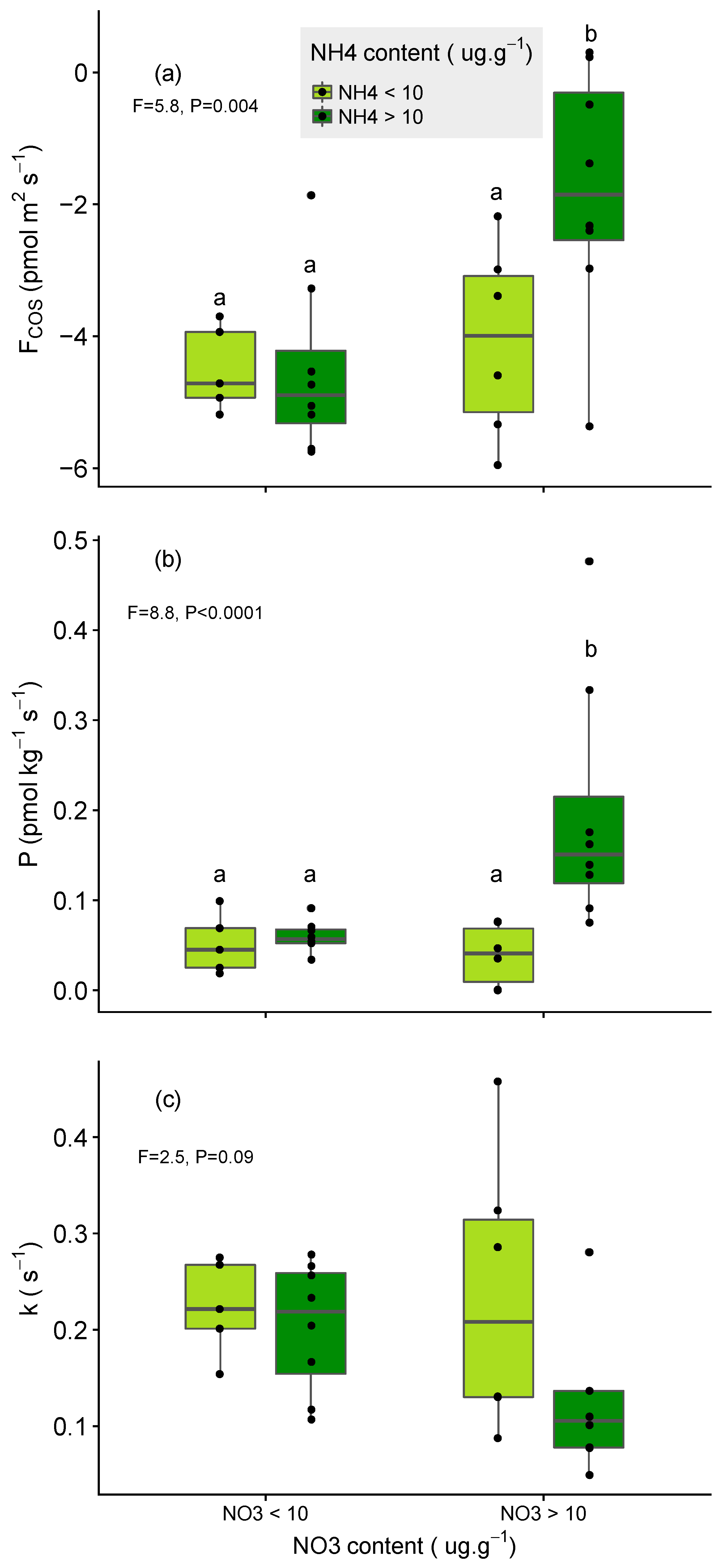
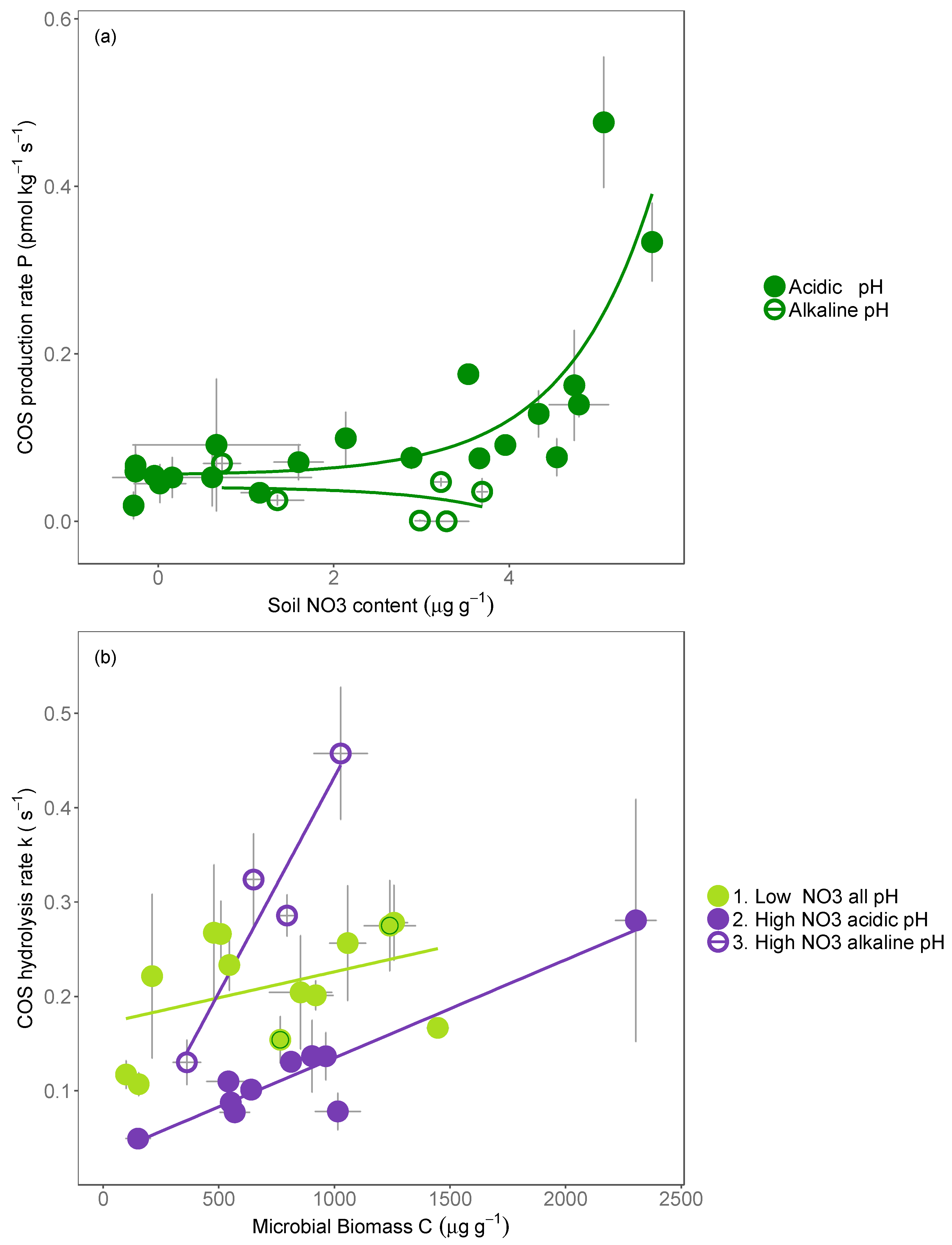
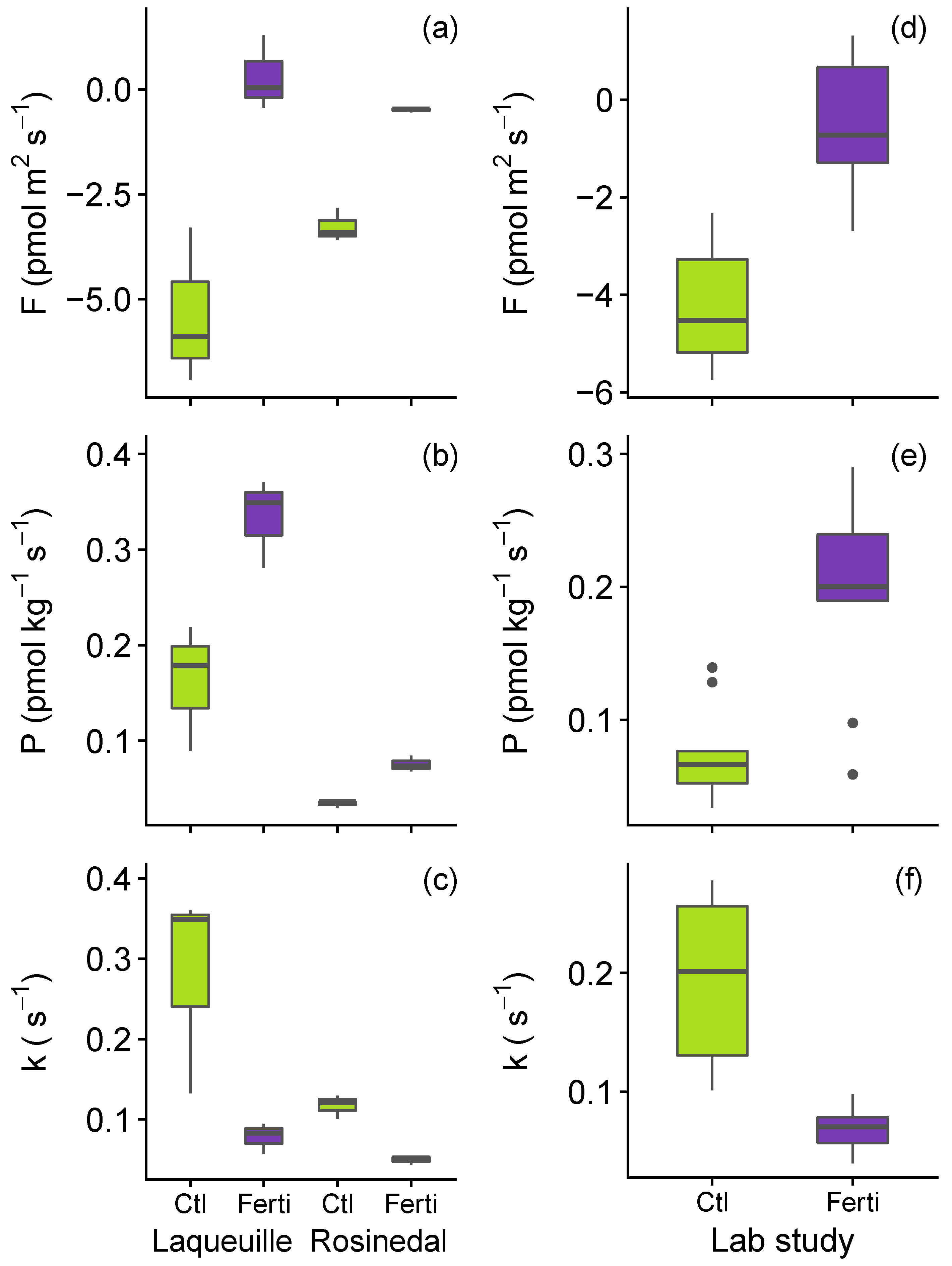
| Predictors | F | P | k | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imp | Coef | p-Value | R2 | Imp | Coef | p-Value | R2 | Imp | Coef | p-Value | R2 | |
| MBC | 1 | 0.61 | <0.0001 | 0.69 | 0.18 | 0.05 | 0.49 | 0.66 | 1 | −0.57 | <0.0001 | 0.64 |
| NO3 | 1 | −0.63 | <0.0001 | 1 | 0.47 | <0.0001 | 1 | 0.54 | <0.0001 | |||
| NH4 | 0.55 | −0.13 | 0.1 | 0.15 | 0.14 | 0.18 | ||||||
| pH | 0.13 | −0.1 | 0.2 | 1 | 0.39 | <0.0001 | 1 | 0.31 | <0.0001 | |||
| MBC:NO3 | 0.48 | 0.15 | 0.06 | 0.58 | −0.21 | 0.02 | ||||||
| MBC:NH4 | 0.13 | 0.13 | 0.17 | |||||||||
| NO3:pH | 0.13 | −0.22 | 0.04 | 1 | 0.29 | <0.0001 | 0.3 | 0.23 | 0.049 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaisermann, A.; Jones, S.P.; Wohl, S.; Ogée, J.; Wingate, L. Nitrogen Fertilization Reduces the Capacity of Soils to Take up Atmospheric Carbonyl Sulphide. Soil Syst. 2018, 2, 62. https://doi.org/10.3390/soilsystems2040062
Kaisermann A, Jones SP, Wohl S, Ogée J, Wingate L. Nitrogen Fertilization Reduces the Capacity of Soils to Take up Atmospheric Carbonyl Sulphide. Soil Systems. 2018; 2(4):62. https://doi.org/10.3390/soilsystems2040062
Chicago/Turabian StyleKaisermann, Aurore, Sam P. Jones, Steven Wohl, Jérôme Ogée, and Lisa Wingate. 2018. "Nitrogen Fertilization Reduces the Capacity of Soils to Take up Atmospheric Carbonyl Sulphide" Soil Systems 2, no. 4: 62. https://doi.org/10.3390/soilsystems2040062
APA StyleKaisermann, A., Jones, S. P., Wohl, S., Ogée, J., & Wingate, L. (2018). Nitrogen Fertilization Reduces the Capacity of Soils to Take up Atmospheric Carbonyl Sulphide. Soil Systems, 2(4), 62. https://doi.org/10.3390/soilsystems2040062





