Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Information and Sample Collection
2.2. Radiolabel Addition and Incubation
2.3. Extractions and P Determination
2.4. 33P Label Recovery
2.5. Statistics
3. Results
3.1. Biologically Available P Pools Across Crust Type
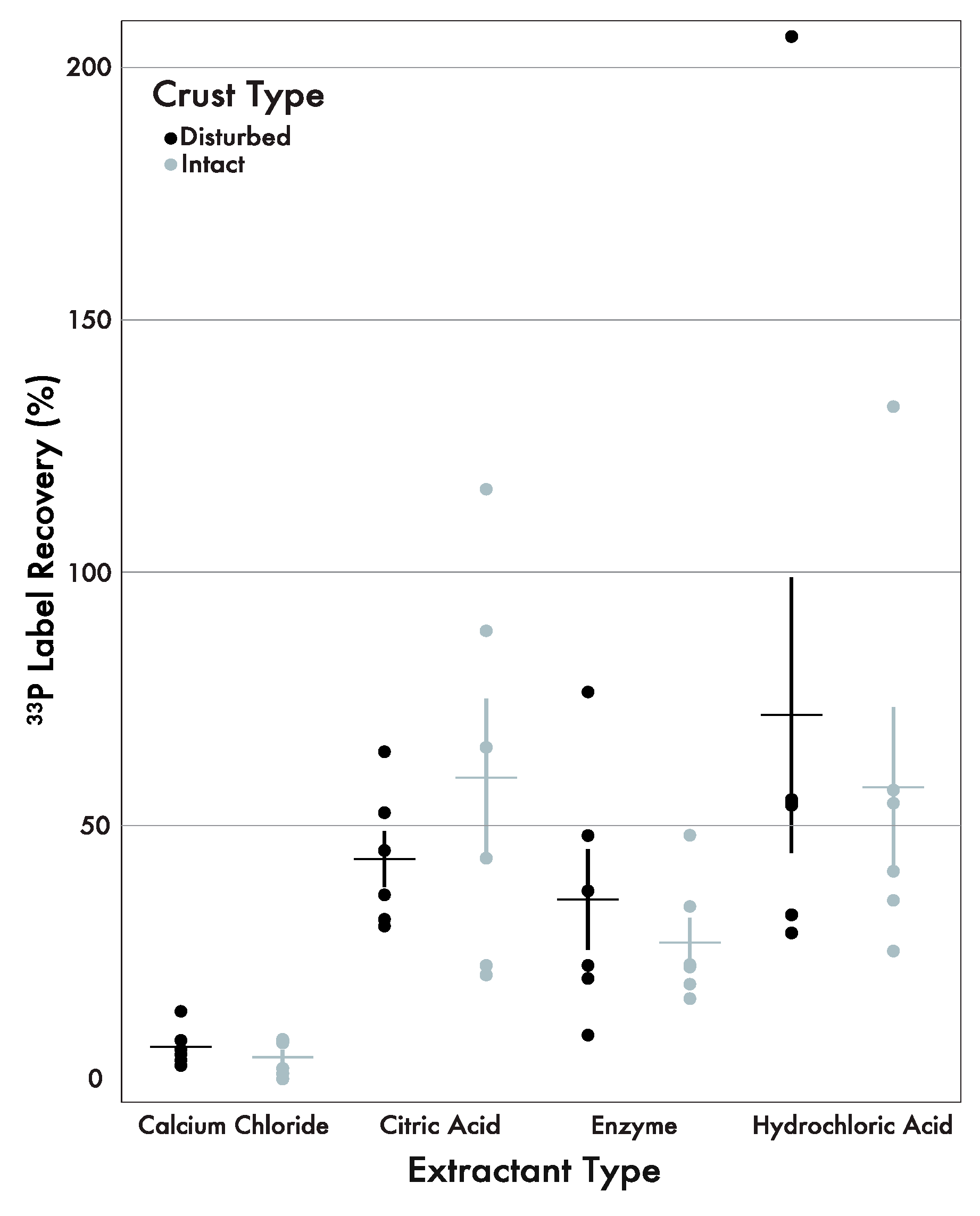
3.2. 33P Label Recovery
4. Discussion
4.1. Biologically Available P in Drylands
4.2. Short-Term Fate of PO43− Added to Biocrust-Dominated Soils
4.3. Bioavailable P in Intact Crusts versus Areas of Disturbance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Post, W.M. Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef]
- Lajtha, K.; Schlesinger, H. The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 1988, 69, 24–39. [Google Scholar] [CrossRef]
- Porder, S.; Ramachandran, S. The phosphorus concentration of common rocks-a potential driver of ecosystem P status. Plant Soil 2013, 367, 41–55. [Google Scholar] [CrossRef]
- Belnap, J. Biological phosphorus cycling in dryland regions. Phosphorus Action 2011, 26, 371–406. [Google Scholar]
- Dalal, R.C. Soil organic phosphorus. Adv. Agron. 1977, 29, 83–117. [Google Scholar]
- Cross, A.F.; Schlesinger, W.H. Biological and chemical controls on phosphorus fraction in semiarid soils. Biogeochemistry 2001, 52, 155–172. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.T.; Schlesinger, W.H. A comparison of fractionation methods for forms of phosphorus in soils. Biogeochemistry 1999, 47, 25–38. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil P in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. Soil Sampl. Methods Anal. 1993, 7, 5–229. [Google Scholar]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.R.; Miller, B.W.; Rubilar, R.; Stape, J.L.; Albaugh, T.J. Phosphorus Nutrition of Forest Plantations: The Role of Inorganic and Organic Phosphorus. Phosphorus Action 2011, 26, 317–338. [Google Scholar]
- Bowman, R.A.; Cole, C.V. An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978, 2, 94–101. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.A.; de Sosa, L.L.; Jones, D.L. A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Darch, T.; Blackwell, M.S.A.; Chadwick, D.; Haygarth, P.M.; Hawkins, J.M.B.; Turner, B.L. Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 2016, 284, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, J.C. Dynamics of phosphate in soils. An isotopic outlook. Fertil. Res. 1995, 45, 91–100. [Google Scholar] [CrossRef]
- Di, H.J.; Condron, L.M.; Frossard, E. Isotope techniques to study phosphorus cycling in agricultural and forest soils: A review. Biol. Fertil. Soils 1997, 24, 1–12. [Google Scholar] [CrossRef]
- Barrow, N.J. A mechanistic model for describing the sorption and desorption of phosphate by soil. J. Soil Sci. 1983, 34, 733–750. [Google Scholar] [CrossRef]
- Buehler, S.; Oberson, A.R.; Rao, I.M.; Friesen, D.K.; Frossard, E. Sequential phosphorus extraction of a 33P-labeled Oxisol under contrasting agricultural systems. Soil Sci. Soc. Am. J. 2002, 66, 868–877. [Google Scholar] [CrossRef]
- Joner, E.; Jakobsen, I. Uptake of 32P from labelled organic matter by mycorrhizal and non-mycorrhizal subterranean clover (Tri-folium subterraneum L.). Plant Soil 1985, 172, 221–227. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Thomas, A.D.; Dougill, A.J. Spatial and temporal distribution of cyanobacterial soil crusts in the Kalahari: Implications for soil surface properties. Geomorphology 2007, 85, 17–29. [Google Scholar] [CrossRef]
- Murmann, R.P.; Peech, M. Relative significance of labile and crystalline phosphates in soil. Soil Sci. 1969, 107, 249–255. [Google Scholar] [CrossRef]
- Belnap, J.; Lange, O.L. Structure and functioning of biological soil crusts: A synthesis. Biol. Soil Crusts Struct. Funct. Manag. 2001, 150, 471–479. [Google Scholar]
- Reed, S.C.; Townsend, A.R.; Taylor, P.G.; Cleveland, C.C. Phosphorus cycling in tropical forests growing on highly weathered soils. Phosphorus Action 2011, 26, 339–369. [Google Scholar]
- Jones, D.L.; Oburger, E. Solubilization of phosphorus by soil microorganisms. Phosphorus 2011, 26, 169–198. [Google Scholar]
- Whitton, B.A. Soils and rice-fields. Ecology Cyanobacteria 2000, 257–259. [Google Scholar]
- Bolton, H.J.; Smith, J.L.; Link, S.O. Soil microbial biomass and activity of a disturbed and undisturbed shrub-steppe ecosystem. Soil Biol. Biochem. 1993, 25, 545–552. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Loza, V.; Marusenko, Y.; Mateo, P.; Potrafka, R.M. Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science 2013, 28, 1574. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Crutchfield, J.; Vandiviere, M. Rapid sensitive, microscale determination of phosphate in water and soil. J. Environ. Qual. 2001, 30, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Steinebrunner, F.; Smithson, P.C.; Frossard, E.; Oberson, A. Phosphorus dynamics in a highly weathered soil as revealed by isotopic labeling techniques. Soil Sci. Soc. Am. J. 2004, 68, 1645–1655. [Google Scholar] [CrossRef]
- Carpenter, J.; Bithell, J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Chien, S.H.; Sikora, F.J.; Gilkes, R.J.; McLaughlin, M.J. Comparing of the difference and balance methods to calculate percent recovery of fertilizer phosphorus applied to soils: A critical discussion. Nutrient Cycl. Agroecosyst. 2012, 92, 1–8. [Google Scholar] [CrossRef]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: http://www.R-project.org (accessed on 21 May 2018).
- Hoang, K.T.K.; Marschner, P. Plant and microbial-induced changes in P pools in soil amended with straw and inorganic P. J. Soil Sci. Plant Nutr. 2017, 17, 1088–1101. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.D.; Voroney, R.P.; Lynch, D.H.; Oberson, A.; Frossard, E.; Bünemann, E.K. Microbially-mediated P fluxes in calcareous soils as a function of water-extractable phosphate. Soil Biol. Biochem. 2017, 106, 51–60. [Google Scholar] [CrossRef]
- Tate, K.R. The biological transformation of P in soil. Biol. Process. Soil Fertil. 1984, 11, 245–256. [Google Scholar]
- Stewart, J.W.; Tiessen, H. Dynamics of soil organic phosphorus. Biogeochemistry 1987, 4, 41–60. [Google Scholar] [CrossRef]
- Gaffney, A.M.; Markov, S.A.; Gunasekaran, M. Utilization of cyanobacteria in photobioreactors for orthophosphate removal from water. Appl. Biochem. Biotechnol. 2001, 91, 185–193. [Google Scholar] [CrossRef]
- Knelman, J.E.; Schmidt, S.K.; Lynch, R.C.; Darcy, J.L.; Castle, S.C.; Cleveland, C.C.; Nemergut, D.R. Nutrient addition dramatically accelerates microbial community succession. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.L.; Schmidt, S.K.; Knelman, J.E.; Cleveland, C.C.; Castle, S.C.; Nemergut, D.R. Phosphorus, not nitrogen, limits plants and microbial primary producers following glacial retreat. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Glaser, K.; Mutz, J.E.; Karsten, U.; MacLennan, A.; Hu, Y.; Leinweber, P. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]


| Extractant Type | Form of P Accessed | Biotic System Mimicked by Extraction Method |
|---|---|---|
| 0.01 M CaCl2 | Weakly adsorbed inorganic P | P accessed by root interception & diffusion |
| 0.01 M citrate | Active inorganic P sorbed to clay particles or weakly bound in inorganic precipitates | Organic acid release by plants and microorganisms |
| 0.2 enzyme unit (wheat germ phosphatase) | Organic P readily attached by acid phosphatase and phytase enzymes | Enzyme release by plants and microorganisms to access labile organic P |
| 1 M HCl | Soluble, active and moderately stable inorganic P adsorbed to mineral surfaces or present in inorganic precipitates. | Proton release by plants and microorganisms to access adsorbed and precipitated P. |
| Ratio | High (>1) | Similar (≈1) | Low (<1) |
|---|---|---|---|
| Citric acid/CaCl2 Enzyme/CaCl2 HCl/CaCl2 | Acids or enzymes may be effective P acquisition strategies beyond taking up soil pore water. | Most P is readily available in soil pore water | Unlikely to observe since CaCl2 pool should be a subset of other pools |
| Citric acid/Enzyme | Weak acids may have more potential than phosphatases for P acquisition. | Weak acids have similar potential to phosphatases for P acquisition. | Weak acids may have less potential than phosphatases for P acquisition |
| HCl/Citric acid | Weak acids are not sufficient to release P from the soil matrix; inorganic P strongly occluded. | Weak acids are sufficient to release P from the soil matrix. | Unlikely to observe since citric acid pool is likely a subset of the HCl (a stronger acid) pool |
| HCl/Enzyme | Most P in the soil is inorganic. | There are substantial organic and inorganic P pools | Most P in the soil is organic |
| Crust Type | Comparison | Effectiveness Ratio | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Intact | Citric acid/CaCl2 | 374 | 211 | 966 |
| Enzyme/CaCl2 | 62.2 | 32.4 | 245 | |
| HCl/CaCl2 | 293 | 124 | 667 | |
| Citric acid/enzyme | 6.02 | 4.17 | 7.63 | |
| HCl/citric acid | 0.782 | 0.483 | 1.39 | |
| HCl/enzyme | 4.71 | 3.21 | 7.69 | |
| Disturbed | Citric acid/CaCl2 | 107 | 65.6 | 324 |
| Enzyme/CaCl2 | 41.3 | 21.8 | 136 | |
| HCl/CaCl2 | 121 | 51.1 | 422 | |
| Citric acid/enzyme | 2.60 | 2.17 | 3.13 | |
| HCl/citric acid | 1.13 | 0.794 | 1.44 | |
| HCl/enzyme | 2.93 | 2.31 | 3.77 |
| Crust Type | Comparison | Effectiveness Ratio | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Intact | Citric acid/CaCl2 | 12.1 | 7.54 | 31 |
| Enzyme/CaCl2 | 5.56 | 3.29 | 11.7 | |
| HCl/CaCl2 | 11.7 | 6.69 | 19.7 | |
| Citric acid/enzyme | 2.18 | 1.48 | 2.61 | |
| HCl/citric acid | 0.969 | 0.491 | 1.57 | |
| HCl/enzyme | 2.11 | 1.07 | 3.12 | |
| Disturbed | Citric acid/CaCl2 | 6.29 | 4.43 | 11.5 |
| Enzyme/CaCl2 | 5.15 | 2.68 | 9.84 | |
| HCl/CaCl2 | 10.4 | 5.13 | 29.8 | |
| Citric acid/enzyme | 1.22 | 0.76 | 1.73 | |
| HCl/citric acid | 1.65 | 1.06 | 2.81 | |
| HCl/enzyme | 2.01 | 1.05 | 3.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crain, G.M.; McLaren, J.R.; Brunner, B.; Darrouzet-Nardi, A. Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert. Soil Syst. 2018, 2, 56. https://doi.org/10.3390/soilsystems2040056
Crain GM, McLaren JR, Brunner B, Darrouzet-Nardi A. Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert. Soil Systems. 2018; 2(4):56. https://doi.org/10.3390/soilsystems2040056
Chicago/Turabian StyleCrain, Grace M., Jennie R. McLaren, Benjamin Brunner, and Anthony Darrouzet-Nardi. 2018. "Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert" Soil Systems 2, no. 4: 56. https://doi.org/10.3390/soilsystems2040056
APA StyleCrain, G. M., McLaren, J. R., Brunner, B., & Darrouzet-Nardi, A. (2018). Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert. Soil Systems, 2(4), 56. https://doi.org/10.3390/soilsystems2040056




