The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review
Abstract
1. Introduction
2. Understanding Soil Properties and Processes and the Dynamics of Chemical Pollutants
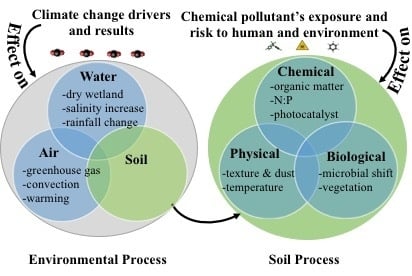
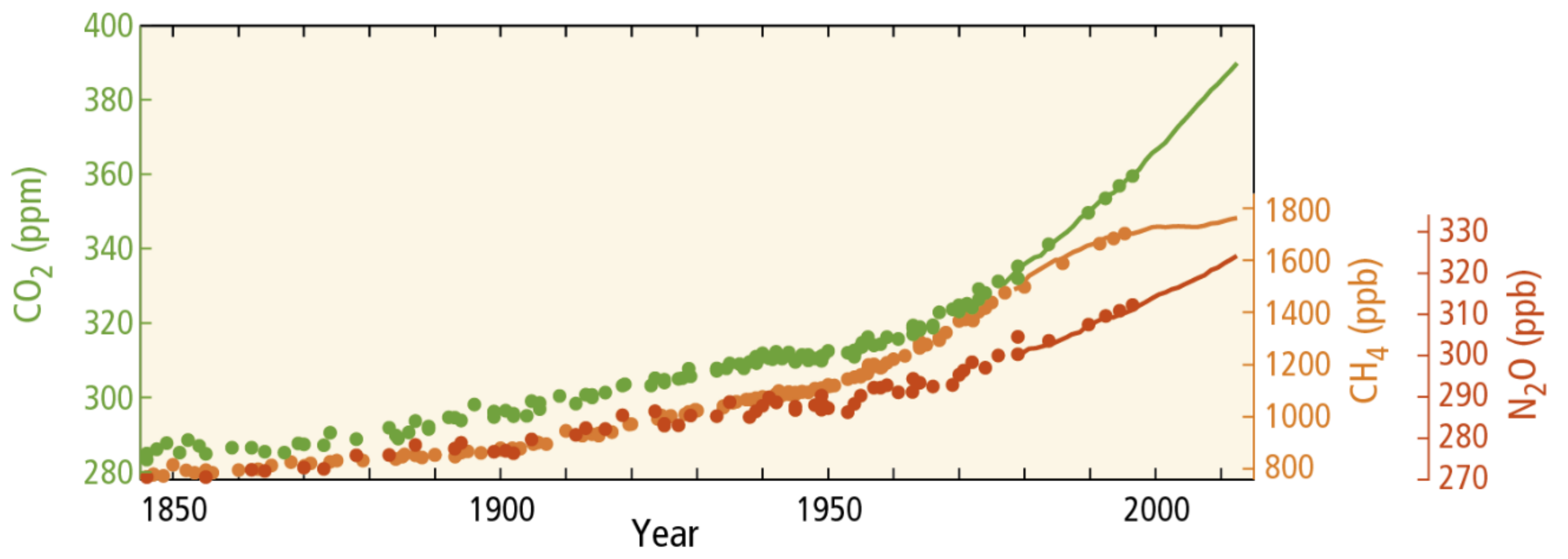
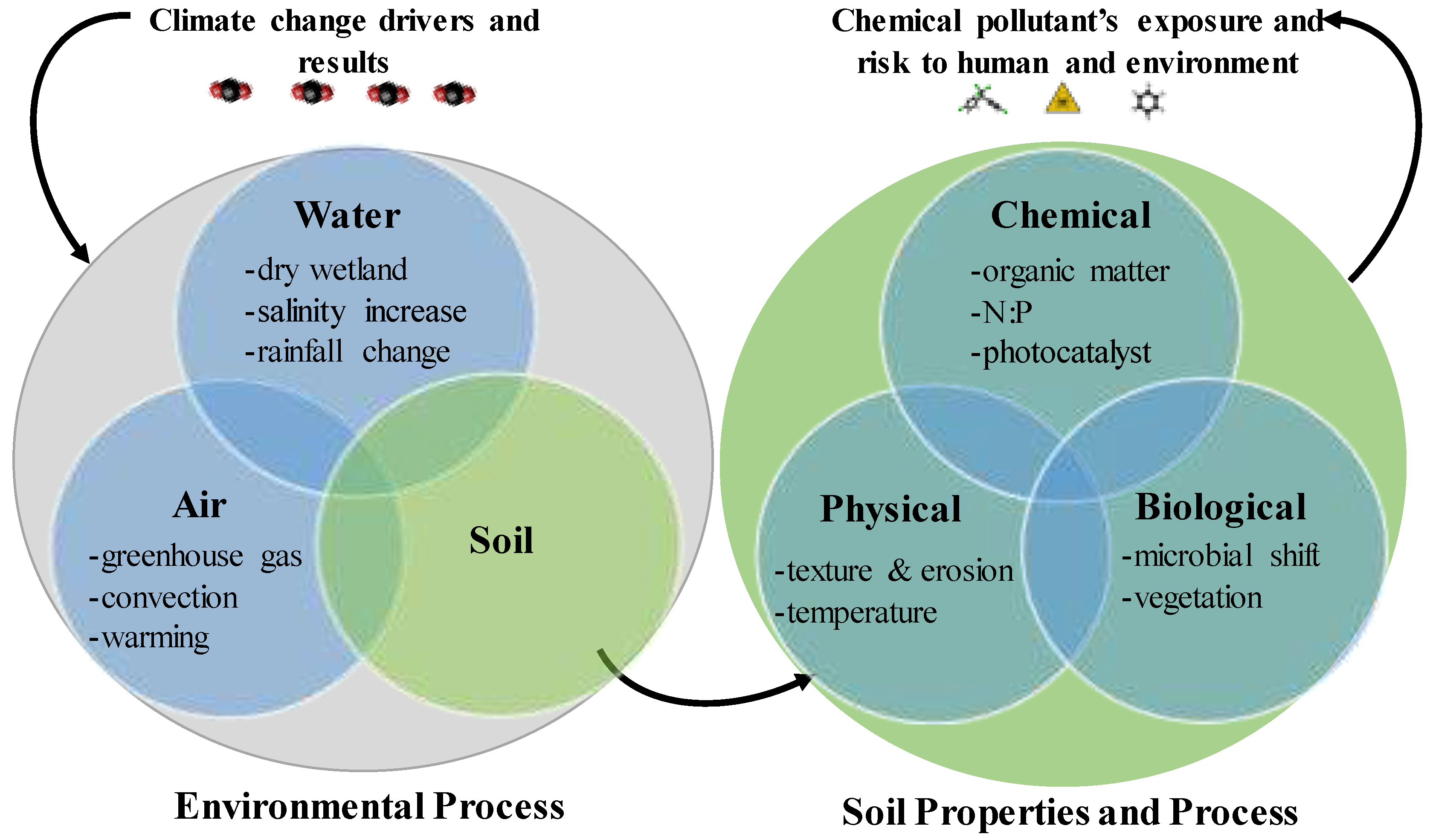
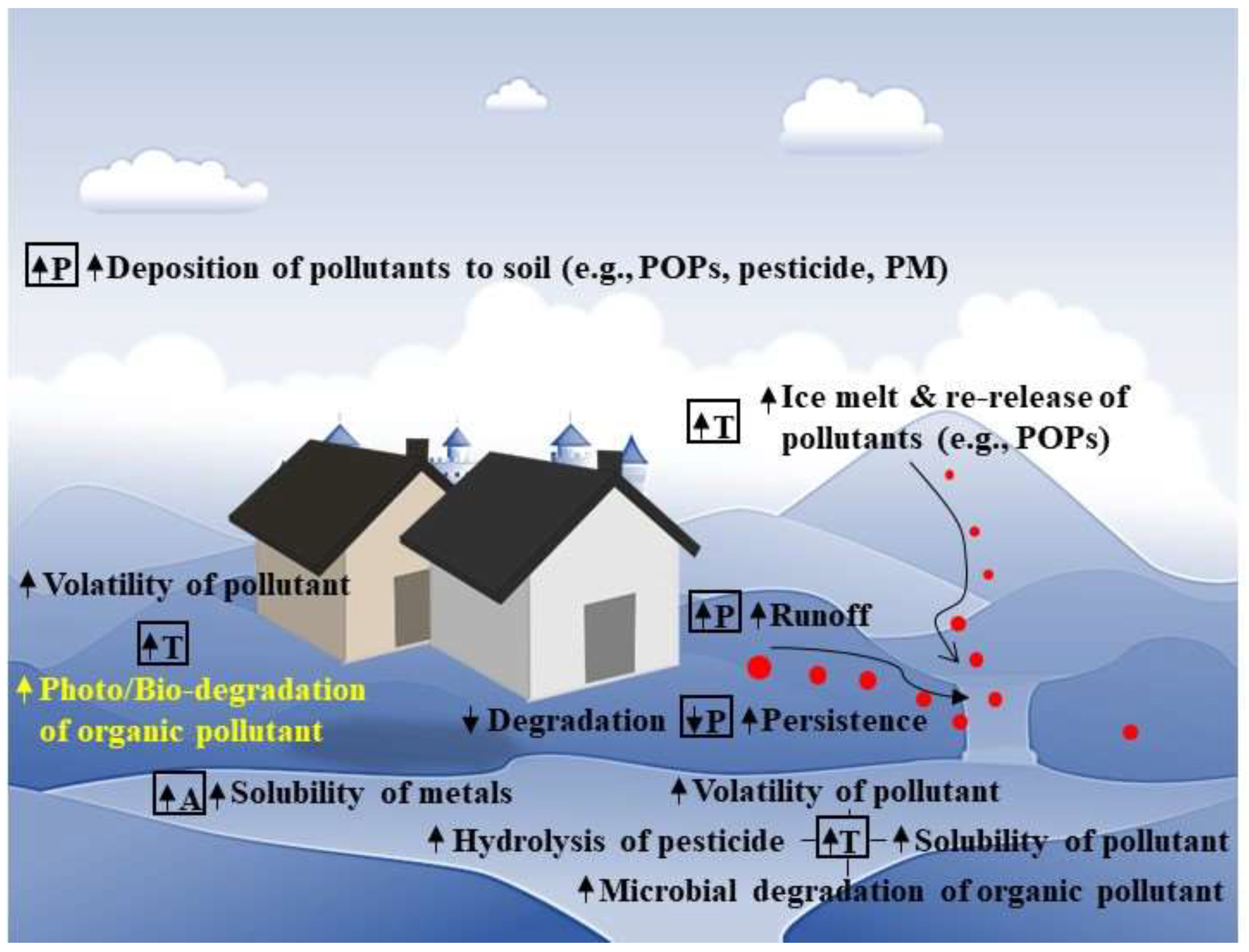
3. Changing Environments–Climate Change and the Soil Properties and Processes
4. Effects of Climate Change on Soil Properties and Processes and the Exposure of Pollutants
4.1. Soil Temperature, Water, and Erosion
4.2. Soil Organic Carbon
4.2.1. SOC for the Exposure of Organic Contaminants
4.2.2. SOC and Release of Metal(loid)s
4.3. Soil Nitrogen and Phosphorus
4.4. Soil Minerals and the Exposure of Chemical Pollutants
4.4.1. Clay Minerals
4.4.2. Non-Clay Minerals
4.5. Soil (Micro)organisms, Enzymes, and Plant Receptors
5. Conclusions, Challenges, and the Way Forward
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landis, W.G.; Yu, M.-H. Introduction to Environmental Toxicology-Impacts of Chemicals upon Ecological Systems; CRC Press: Boca Raton, FL, USA, 2004; p. 484. [Google Scholar]
- Landrigan, P.J.; Schechter, C.B.; Lipton, J.M.; Fahs, M.C.; Schwartz, J. Environmental pollutants and disease in American children: Estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ. Health Perspect. 2002, 110, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Konkel, L. Compound interest: Assessing the effects of chemical mixtures. Environ. Health Perspect. 2017, 125. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Basic Research Opportunities in Earth Science; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Banwart, S.A.; Bernasconi, S.M.; Blum, W.E.H.; de Souza, D.M.; Chabaux, F.; Duffy, C.; Kercheva, M.; Krám, P.; Lair, G.J.; Lundin, L.; et al. Soil functions in earth’s critical zone: Key results and conclusions. In Advances in Agronomy; Banwart, S.A., Sparks, D.L., Eds.; Academic Press: Burlington, VT, USA, 2017; Volume 142, pp. 1–27. [Google Scholar]
- Richter, D.D.; Mobley, M.L. Monitoring earth’s critical zone. Science 2009, 326, 1067–1068. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472. [Google Scholar] [CrossRef] [PubMed]
- Bouskill, N.J.; Wood, T.E.; Baran, R.; Ye, Z.; Bowen, B.P.; Lim, H.; Zhou, J.; Nostrand, J.D.V.; Nico, P.; Northen, T.R.; et al. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front. Microbiol. 2016, 7, 525. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. Soils and climate change. Curr. Opin. Environ. Sustain. 2012, 4, 539–544. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Loza, V.; Marusenko, Y.; Mateo, P.; Potrafka, R.M. Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science 2013, 340, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.-Z.; Zhang, X.-C.; Zheng, F.-L. Assessing the site-specific impacts of climate change on hydrology, soil erosion and crop yields in the loess plateau of China. Clim. Chang. 2011, 105, 223–242. [Google Scholar] [CrossRef]
- Davidson, E.A.; Trumbore, S.E.; Amundson, R. Soil warming and organic carbon content. Nature 2000, 408, 789. [Google Scholar] [CrossRef] [PubMed]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalan, C. Climate change and human health: Impacts, vulnerability and public health. Public Health 2006, 120, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Alava, J.J.; Cheung, W.W.L.; Ross, P.S.; Sumaila, U.R. Climate change–contaminant interactions in marine food webs: Toward a conceptual framework. Glob. Chang. Biol. 2017, 23, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Lipczynska-Kochany, E. Effect of climate change on humic substances and associated impacts on the quality of surface water and groundwater: A review. Sci. Total Environ. 2018, 640, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Agency for Toxic Substances and Disease Registry; Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Naidu, R.; Bolan, N.S.; Megharaj, M.; Juhasz, A.L.; Gupta, S.K.; Clothier, B.E.; Schulin, R. Chemical bioavailability in terrestrial environments. In Developments in Soil Science; Hartemink, A.E., McBratney, A.B., Naidu, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 32, pp. 1–6. [Google Scholar]
- Poggio, L.; Vrščaj, B.; Schulin, R.; Hepperle, E.; Ajmone Marsan, F. Metals pollution and human bioaccessibility of topsoils in Grugliasco (Italy). Environ. Pollut. 2009, 157, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.I.; Biswas, B.; Smith, E.; Mahmud, S.A.; Hasan, N.A.; Khan, M.A.W.; Naidu, R.; Megharaj, M. Microbial diversity changes with rhizosphere and hydrocarbons in contrasting soils. Ecotoxicol. Environ. Saf. 2018, 156, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Harter, R.D.; Naidu, R. Role of metal-organic complexation in metal sorption by soils. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, VT, USA, 1995; Volume 55, pp. 219–263. [Google Scholar]
- Bolan, N.S.; Adriano, D.C.; Mani, P.A.; Duraisamy, A. Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 2003, 251, 187–198. [Google Scholar] [CrossRef]
- Naidu, R.; Harter, R.D. Effect of different organic ligands on cadmium sorption by and extractability from soils. Soil Sci. Soc. Am. J. 1998, 62, 644–650. [Google Scholar] [CrossRef]
- Hong, C.O.; Chung, D.Y.; Lee, D.K.; Kim, P.J. Comparison of phosphate materials for immobilizing cadmium in soil. Arch. Environ. Contam. Toxicol. 2010, 58, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Bolan, N.S.; Kookana, R.S.; Tiller, K.G. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur. J. Soil Sci. 2006, 45, 419–429. [Google Scholar] [CrossRef]
- Bianchi, S.R.; Miyazawa, M.; Oliveira, E.L.; Pavan, M.A. Relationship between the mass of organic matter and carbon in soil. Braz. Arch. Biol. Technol. 2008, 51, 263–269. [Google Scholar] [CrossRef]
- Qi, F.; Kuppusamy, S.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Lamb, D.; Li, Y.; Yu, L.; Semple, K.T.; Wang, H. Pyrogenic carbon and its role in contaminant immobilization in soils. Crit. Rev. Environ. Sci. Technol. 2017, 47, 795–876. [Google Scholar] [CrossRef]
- Brümmer, G.W. Heavy metal species, mobility and availability in soils. In The Importance of Chemical “Speciation” in Environmental Processes; Bernhard, M., Brinckman, F.E., Sadler, P.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 169–192. [Google Scholar]
- Siripinyanond, A.; Worapanyanond, S.; Shiowatana, J. Field-flow fractionation−inductively coupled plasma mass spectrometry: An alternative approach to investigate metal−humic substances interaction. Environ. Sci. Technol. 2005, 39, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Chemistry of soil organic matter. In Environmental Soil Chemistry, 2nd ed.; Academic Press: Burlington, VT, USA, 2003; pp. 75–113. [Google Scholar]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Bioremediation of PAHs and VOCs: Advances in clay mineral-microbial interaction. Environ. Int. 2015, 85, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- LeMonte, J.J.; Stuckey, J.W.; Sanchez, J.Z.; Tappero, R.; Rinklebe, J.; Sparks, D.L. Sea level rise induced arsenic release from historically contaminated coastal soils. Environ. Sci. Technol. 2017, 51, 5913–5922. [Google Scholar] [CrossRef] [PubMed]
- Stergiadi, M.; van der Perk, M.; de Nijs, A.C.M.; Bierkens, M.F.P. Effects of climate change and land management on soil organic carbon dynamics and carbon leaching in Northwestern Europe. Biogeosci. Discuss. 2015, 12, 19627–19671. [Google Scholar] [CrossRef]
- Vale, C.; Canário, J.; Caetano, M.; Poissant, L.; Ferreira, A.M. Contaminant cycling under climate change: Evidences and scenarios. In Oceans and the Atmospheric Carbon Content; Duarte, P., Santana-Casiano, J.M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 133–156. [Google Scholar]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Serpa, D.; Nunes, J.P.; Keizer, J.J.; Abrantes, N. Impacts of climate and land use changes on the water quality of a small Mediterranean catchment with intensive viticulture. Environ. Pollut. 2017, 224, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Hardy, A.; Beulke, S.; Boucard, T.; Burgin, L.; Falloon, P.D.; Haygarth, P.M.; Hutchinson, T.; Kovats, R.S.; Leonardi, G.; et al. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ. Health Perspect. 2009, 117, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Hooper, L.; Abdelhamid, A.; Bentham, G.; Boxall, A.B.A.; Draper, A.; Fairweather-Tait, S.; Hulme, M.; Hunter, P.R.; Nichols, G.; et al. Climate change and food security: Health impacts in developed countries. Environ. Health Perspect. 2012, 120, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Song, S.; Lu, Y.; Liu, S.; Giesy, J.P.; Chen, D.; Jenkins, A.; Sweetman, A.J.; Yvette, B. Potential effects of changes in climate and emissions on distribution and fate of perfluorooctane sulfonate in the Bohai Rim, China. Sci. Total Environ. 2018, 613, 352–360. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Srivastava, P.; Singh, R.; Tripathi, S.; Singh, P.; Singh, S.; Singh, H.; Raghubanshi, A.S.; Mishra, P.K. Soil carbon dynamics under changing climate—A research transition from absolute to relative roles of inorganic nitrogen pools and associated microbial processes: A review. Pedosphere 2017, 27, 792–806. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.J.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 2017, 358, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhou, X.; Zhao, S.; Wang, X.; Zhu, B.; Piao, S.; Fang, J. Quantifying the response of forest carbon balance to future climate change in Northeastern China: Model validation and prediction. Glob. Planet. Chang. 2009, 66, 179–194. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Pearce, F. Lakes of Acid. New Sci. 2018, 238, 38–41. [Google Scholar] [CrossRef]
- Rengel, Z. Soil pH, soil health and climate change. In Soil Health and Climate Change; Singh, B.P., Cowie, A.L., Chan, K.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 69–85. [Google Scholar]
- Zheng, S.; Bian, H.; Quan, Q.; Xu, L.; Chen, Z.; He, N. Effect of nitrogen and acid deposition on soil respiration in a temperate forest in China. Geoderma 2018, 329, 82–90. [Google Scholar] [CrossRef]
- Carré, F.; Caudeville, J.; Bonnard, R.; Bert, V.; Boucard, P.; Ramel, M. Soil contamination and human health: A major challenge for global soil security. In Global Soil Security; Field, D.J., Morgan, C.L.S., McBratney, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 275–295. [Google Scholar]
- Macdonald, R.W.; Harner, T.; Fyfe, J. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci. Total Environ. 2005, 342, 5–86. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Quinn, C.L.; Wania, F. Global climate change and contaminants-an overview of opportunities and priorities for modelling the potential implications for long-term human exposure to organic compounds in the Arctic. J. Environ. Monit. 2011, 13, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Strååt, K.D.; Mörth, C.-M.; Undeman, E. Future export of particulate and dissolved organic carbon from land to coastal zones of the Baltic Sea. J. Mar. Syst. 2018, 177, 8–20. [Google Scholar] [CrossRef]
- McWatters, R.S.; Rutter, A.; Rowe, R.K. Geomembrane applications for controlling diffusive migration of petroleum hydrocarbons in cold region environments. J. Environ. Manag. 2016, 181, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fang, W.; Lu, X.; Sheng, G.-P.; Graham, D.E.; Liang, L.; Wullschleger, S.D.; Gu, B. Warming increases methylmercury production in an Arctic soil. Environ. Pollut. 2016, 214, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Zeller, D. Mercury, food webs, and marine mammals: Implications of diet and climate change for human health. Environ. Health Perspect. 2005, 113, 521–526. [Google Scholar] [CrossRef] [PubMed]
- González-Alcaraz, M.N.; van Gestel, C.A.M. Climate change effects on enchytraeid performance in metal-polluted soils explained from changes in metal bioavailability and bioaccumulation. Environ. Res. 2015, 142, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Klaminder, J.; Hammarlund, D.; Kokfelt, U.; Vonk, J.E.; Bigler, C. Lead contamination of subarctic lakes and its response to reduced atmospheric fallout: Can the recovery process be counteracted by the ongoing climate change? Environ. Sci. Technol. 2010, 44, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Teran, T.; Lamon, L.; Marcomini, A. Climate change effects on POPs’ environmental behaviour: A scientific perspective for future regulatory actions. Atmos. Pollut. Res. 2012, 3, 466–476. [Google Scholar] [CrossRef]
- Todd, A.S.; Manning, A.H.; Verplanck, P.L.; Crouch, C.; McKnight, D.M.; Dunham, R. Climate-change-driven deterioration of water quality in a mineralized watershed. Environ. Sci. Technol. 2012, 46, 9324–9332. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Anawar, H.M. Impact of climate change on acid mine drainage generation and contaminant transport in water ecosystems of semi-arid and arid mining areas. Phys. Chem. Earth Parts A/B/C 2013, 58, 13–21. [Google Scholar] [CrossRef]
- Velki, M.; Ečimović, S. Changes in exposure temperature lead to changes in pesticide toxicity to earthworms: A preliminary study. Environ. Toxicol. Pharmacol. 2015, 40, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.C.; Schmelz, R.M.; Niva, C.C.; Correia, M.E.F.; Silva-Filho, E.V. Mercury critical concentrations to Enchytraeus crypticus (Annelida: Oligochaeta) under normal and extreme conditions of moisture in tropical soils—Reproduction and survival. Environ. Res. 2017, 155, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zobeck, T.M.; Van Pelt, R.S. Wind-induced dust generation and transport mechanics on a bare agricultural field. J. Hazard. Mater. 2006, 132, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.W.; Mackay, D.; Li, Y.F.; Hickie, B. How will global climate change affect risks from long-range transport of persistent organic pollutants? Hum. Ecol. Risk Assess. Int. J. 2003, 9, 643–660. [Google Scholar] [CrossRef]
- Giardina, C.P.; Ryan, M.G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 2000, 404, 858. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Xing, B.; Pignatello, J.J. Dual-mode sorption of low-polarity compounds in glassy poly (vinyl chloride) and soil organic matter. Environ. Sci. Technol. 1997, 31, 792–799. [Google Scholar] [CrossRef]
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Marquès, M.; Mari, M.; Audí-Miró, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Climate change impact on the PAH photodegradation in soils: Characterization and metabolites identification. Environ. Int. 2016, 89–90, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardena, M.A.A.; Naidu, R.; Megharaj, M.; Lamb, D.; Thavamani, P.; Kuchel, T. Using soil properties to predict in vivo bioavailability of lead in soils. Chemosphere 2015, 138, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Temminghoff, E.J.; Van der Zee, S.E.; de Haan, F.A. Copper mobility in a copper-contaminated sandy soil as affected by pH and solid and dissolved organic matter. Environ. Sci. Technol. 1997, 31, 1109–1115. [Google Scholar] [CrossRef]
- Sauvé, S.; McBride, M.B.; Norvell, W.A.; Hendershot, W.H. Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut. 1997, 100, 133–149. [Google Scholar] [CrossRef]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. J. Hazard. Mater. 2009, 171, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.B.; Jensen, D.L.; Christensen, T.H. Effect of dissolved organic carbon on the mobility of cadmium, nickel and zinc in leachate polluted groundwater. Water Res. 1996, 30, 3037–3049. [Google Scholar] [CrossRef]
- Lewis, D.B.; Brown, J.A.; Jimenez, K.L. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuar. Coast. Shelf Sci. 2014, 139, 11–19. [Google Scholar] [CrossRef]
- Hofmockel, K.S.; Zak, D.R.; Moran, K.K.; Jastrow, J.D. Changes in forest soil organic matter pools after a decade of elevated CO2 and O3. Soil Biol. Biochem. 2011, 43, 1518–1527. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry 2000, 48, 21–51. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kashem, M.; Singh, B. Metal availability in contaminated soils: I. Effects of floodingand organic matter on changes in Eh, Ph and solubility of Cd, Ni and Zn. Nutr. Cycl. Agroecosyst. 2001, 61, 247–255. [Google Scholar] [CrossRef]
- Natali, S.M.; Sañudo-Wilhelmy, S.A.; Norby, R.J.; Zhang, H.; Finzi, A.C.; Lerdau, M.T. Increased mercury in forest soils under elevated carbon dioxide. Oecologia 2008, 158, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Prasad, M.N.V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pandey, S.D.; Misra, V. Stability constants of metal–humic acid complexes and its role in environmental detoxification. Ecotoxicol. Environ. Saf. 2000, 47, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Hughes, L.; Alexander, D.; Rice, M. Cranking up the Intensity: Climate Change and Extreme Weather Events; Climate Council: Australia, 2017. [Google Scholar]
- Hoyle, F.C.; Baldock, J.A.; Murphy, D.V. Soil organic carbon—Role in rainfed farming systems. In Rainfed Farming Systems; Tow, P., Cooper, I., Partridge, I., Birch, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 339–361. [Google Scholar]
- Zhang, Q.; Jiang, X.; Tong, D.; Davis, S.J.; Zhao, H.; Geng, G.; Feng, T.; Zheng, B.; Lu, Z.; Streets, D.G.; et al. Transboundary health impacts of transported global air pollution and international trade. Nature 2017, 543, 705. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, S.; Uno, I.; Osada, K.; Kamiguchi, Y.; Yamamoto, S.; Tamura, K.; Wang, Z.; Kurosaki, Y.; Kanaya, Y. Nitrate transboundary heavy pollution over East Asia in winter. Atmos. Chem. Phys. 2017, 17, 3823–3843. [Google Scholar] [CrossRef]
- Casazza, M.; Lega, M.; Liu, G.; Ulgiati, S.; Endreny, T.A. Aerosol pollution, including eroded soils, intensifies cloud growth, precipitation, and soil erosion: A review. J. Clean. Prod. 2018, 189, 135–144. [Google Scholar] [CrossRef]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Santo Domingo, J.W. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.C.; Pan, Y.; Shyla, F.S.; Tam, N.F.-Y. Effects of polybrominated diphenyl ethers and plant species on nitrification, denitrification and anammox in mangrove soils. Sci. Total Environ. 2016, 553, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- FOG, K. The effect of added nitrogen on the rate of decomposition of organic matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Wedin, D.A.; Tilman, D. Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science 1996, 274, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Thurston, J.M.; Williams, E.; Johnston, A. Modern developments in an experiment on permanent grassland started in 1856: Effects of fertilisers and lime on botanical composition and crop and soil analyses. Ann. Agron. (Fr.) 1976, 27, 1043–1082. [Google Scholar]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, N.; van Dijk, H.F.G. Ecosystem effects of atmospheric deposition of nitrogen in The Netherlands. Environ. Pollut. 1988, 54, 249–274. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Lee, D.K.; Chung, D.Y.; Kim, P.J. Liming effects on cadmium stabilization in upland soil affected by gold mining activity. Arch. Environ. Contam. Toxicol. 2007, 52, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Naidu, R.; Kookana, R.S.; Sumner, M.E.; Harter, R.D.; Tiller, K.G. Cadmium sorption and transport in variable charge soils: A Review. J. Environ. Qual. 1997, 26, 602–617. [Google Scholar] [CrossRef]
- McGowen, S.L.; Basta, N.T.; Brown, G.O. Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J. Environ. Qual. 2001, 30, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, B.; Bolan, N.; Wijesekara, H.; Kunhikrishnan, A.; Thangarajan, R.; Qi, F.; Matheyarasu, R.; Rocco, C.; Mbene, K.; Naidu, R. Phosphorus–cadmium interactions in paddy soils. Geoderma 2016, 270, 43–59. [Google Scholar] [CrossRef]
- Ge, L.-Q.; Cang, L.; Liu, H.; Zhou, D.-M. Effects of warming on uptake and translocation of cadmium (Cd) and copper (Cu) in a contaminated soil-rice system under Free Air Temperature Increase (FATI). Chemosphere 2016, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. The origin and formation of clay minerals in soils: Past, present and future perspectives. Clay Miner. 1999, 34, 7. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 1–19. [Google Scholar]
- Bergaya, F.; Lagaly, G. Introduction on modified clays and clay minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, p. 383. [Google Scholar]
- McAllister, L.E.; Semple, K.T. Role of clay and organic matter in the biodegradation of organics in soil. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 367–384. [Google Scholar]
- Zhu, D.; Zhong, H. Potential bioavailability of mercury in humus-coated clay minerals. J. Environ. Sci. 2015, 36, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardena, M.A.A.; Megharaj, M.; Naidu, R. Exposure, toxicity, health impacts, and bioavailability of heavy metal mixtures. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, VT, USA, 2016; Volume 138, pp. 175–234. [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V.; et al. An assessment of the global impact of 21st century land use change on soil erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Munro, S.; Barthold, F.; Steffens, M.; Schad, P.; Kögel-Knabner, I. Carbon storage capacity of semi-arid grassland soils and sequestration potentials in northern China. Glob. Chang. Biol. 2015, 21, 3836–3845. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Kuzyakov, Y.; Stahr, K. Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J. Soils Sediments 2005, 5, 245–252. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; McClure, S.; Naidu, R. Modified osmium tracer technique enables precise microscopic delineation of hydrocarbon-degrading bacteria in clay aggregates. Environ. Technol. Innov. 2017, 7, 12–20. [Google Scholar] [CrossRef]
- Mandal, A.; Biswas, B.; Sarkar, B.; Patra, A.K.; Naidu, R. Surface tailored organobentonite enhances bacterial proliferation and phenanthrene biodegradation under cadmium co-contamination. Sci. Total Environ. 2016, 550, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Mild acid and alkali treated clay minerals enhance bioremediation of polycyclic aromatic hydrocarbons in long-term contaminated soil: A14C-tracer study. Environ. Pollut. 2017, 223, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sarkar, B.; Mandal, A.; Naidu, R. Heavy metal-immobilizing organoclay facilitates polycyclic aromatic hydrocarbon biodegradation in mixed-contaminated soil. J. Hazard. Mater. 2015, 298, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, U.C.; Fialips, C.I. Removal of crude oil polycyclic aromatic hydrocarbons via organoclay-microbe-oil interactions. Chemosphere 2017, 174, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Megharaj, M.; Gates, W.P.; Churchman, G.J.; Anderson, J.; Kookana, R.S.; Naidu, R.; Chen, Z.; Slade, P.G.; Sethunathan, N. Bioavailability of an organophosphorus pesticide, fenamiphos, sorbed on an organo clay. J. Agric. Food Chem. 2003, 51, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Xiaozhen, F.; Bo, L.; Aijun, G. Dynamics of solar light photodegradation behavior of atrazine on soil surface. J. Hazard. Mater. 2005, 117, 75–79. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Fan, J.; Hart, M.M.; Siddique, K.H.M.; Solaiman, Z.M.; Jenkins, S.N. Application of compost and clay under water-stressed conditions influences functional diversity of rhizosphere bacteria. Biol. Fertil. Soils 2018, 54, 55–70. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Sarkar, B.; Singh, M.; Mandal, S.; Churchman, G.J.; Bolan, N.S. Clay minerals—Organic matter interactions in relation to carbon stabilization in soils. In The Future of Soil Carbon; Nannipieri, P., Hernandez, T., Eds.; Academic Press: Burlington, VT, USA, 2018; pp. 71–86. [Google Scholar]
- Singh, B.; Schulze, D.G. Soil minerals and plant nutrition. Nat. Educ. Knowl. 2015, 6, 1. [Google Scholar]
- Gislason, S.R.; Oelkers, E.H.; Eiriksdottir, E.S.; Kardjilov, M.I.; Gisladottir, G.; Sigfusson, B.; Snorrason, A.; Elefsen, S.; Hardardottir, J.; Torssander, P.; et al. Direct evidence of the feedback between climate and weathering. Earth Planet. Sci. Lett. 2009, 277, 213–222. [Google Scholar] [CrossRef]
- Brady, P.V.; Carroll, S.A. Direct effects of CO2 and temperature on silicate weathering: Possible implications for climate control. Geochim. Cosmochim. Acta 1994, 58, 1853–1856. [Google Scholar] [CrossRef]
- Frohne, T.; Rinklebe, J.; Diaz-Bone, R.A. Contamination of floodplain soils along the Wupper River, Germany, with As, Co, Cu, Ni, Sb, and Zn and the impact of pre-definite redox variations on the mobility of these elements. Soil Sediment Contam. Int. J. 2014, 23, 779–799. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shand, P.; Grocke, S.; Creeper, N.L.; Baker, A.K.; Fitzpatrick, R.W.; Love, A.J. Impacts of climate change, climate variability and management on soil and water quality in wetlands of south australia. Procedia Earth Planet. Sci. 2017, 17, 456–459. [Google Scholar] [CrossRef]
- Waring, B.; Hawkes, C.V. Ecological mechanisms underlying soil bacterial responses to rainfall along a steep natural precipitation gradient. FEMS Microbiol. Ecol. 2018, 94, fiy001. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Romero-Olivares, A.L.; Lu, Y.; Taylor, J.W.; Treseder, K.K. Temperature sensitivities of extracellular enzyme Vmax and Km across thermal environments. Glob. Chang. Biol. 2018, 24, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Hicks Pries, C.E.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liao, S.; Guo, J.; Song, Z.; Wang, R.; Zhou, X. Growth and cesium uptake responses of Phytolacca americana Linn. and Amaranthus cruentus L. grown on cesium contaminated soil to elevated CO2 or inoculation with a plant growth promoting rhizobacterium Burkholderia sp. D54, or in combination. J. Hazard. Mater. 2011, 198, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, H. Effects of elevated CO2 and Pb on phytoextraction and enzyme activity. Water Air Soil Pollut. 2011, 219, 365–375. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, Y.; Ling, W.; Kong, H.; Zhu, X. Gradient Distribution of root exudates and polycyclic aromatic hydrocarbons in rhizosphere soil. Soil Sci. Soc. Am. J. 2011, 75, 1694–1703. [Google Scholar] [CrossRef]
- Ling, W.; Dang, H.; Liu, J. In situ gradient distribution of polycyclic aromatic hydrocarbons (PAHs) in contaminated rhizosphere soil: a field study. J. Soils Sediments 2013, 13, 677–685. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A beneficial plant-microbe interaction. Mol. Plant-Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schöftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of petroleum hydrocarbon-contaminated soils: Improvement opportunities and field applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Anderson-Furgeson, J.; Estera, K.Y.; Pett-Ridge, J.; de Valpine, P.; Brodie, E.L.; Firestone, M.K. Climate and edaphic controllers influence rhizosphere community assembly for a wild annual grass. Ecology 2016, 97, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Cook, C.W.; Pippen, J.S.; Palmer, S.M. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 2009, 90, 3352–3366. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.H.; Richter, D.D., Jr. Soil acidification induced by elevated atmospheric CO2. Glob. Chang. Biol. 2004, 10, 1936–1946. [Google Scholar]
- Gray, C.W.; McLaren, R.G.; Roberts, A.H.C.; Condron, L.M. Effect of soil pH on cadmium phytoavailability in some New Zealand soils. N. Z. J. Crop Hortic. Sci. 1999, 27, 169–179. [Google Scholar] [CrossRef]
- Öborn, I.; Jansson, G.; Johnsson, L. A field study on the influence of soil pH on trace element levels in spring wheat (Triticum aestivum), potatoes (Solanum tuberosum) and carrots (Daucus carota). Water Air Soil Pollut. 1995, 85, 835–840. [Google Scholar] [CrossRef]
- Khalvati, M.; Dincer, I. Environmental impact of soil microorganisms on global change. In Causes, Impacts and Solutions to Global Warming; Dincer, I., Colpan, C.O., Kadioglu, F., Eds.; Springer: New York, NY, USA, 2013; pp. 233–250. [Google Scholar]
- Krishnan, A.; Convey, P.; Gonzalez, M.; Smykla, J.; Alias, S.A. Effects of temperature on extracellular hydrolase enzymes from soil microfungi. Polar Biol. 2018, 41, 537–551. [Google Scholar] [CrossRef]
- Tan, X.; Machmuller, M.B.; Wang, Z.; Li, X.; He, W.; Cotrufo, M.F.; Shen, W. Temperature enhances the affinity of soil alkaline phosphatase to Cd. Chemosphere 2018, 196, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Warming and drought change trace element bioaccumulation patterns in a Mediterranean shrubland. Chemosphere 2008, 70, 874–885. [Google Scholar] [CrossRef] [PubMed]
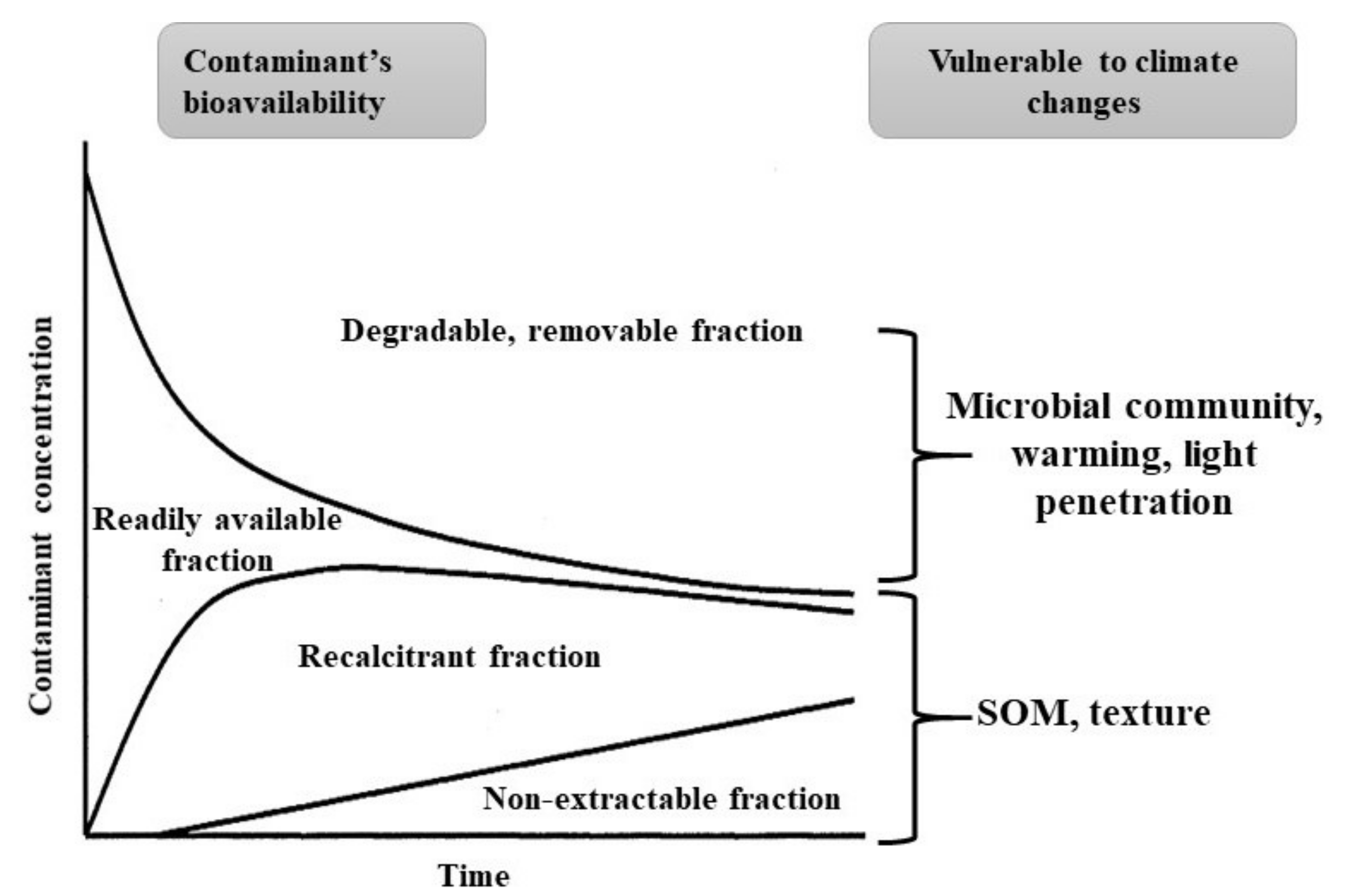
| Properties and Processes | Potential Impact of Climate Changes | Generalized Toxicological Results | Details in Section |
|---|---|---|---|
| pH | Warming: pH can drop due to formation of sulfate and rhizosphere acidification; pH can raise due to presence of calcite, dolomite or dissolution/weathering of gypsum and aluminosilicates | Soil acidification could increase desorption of heavy metal(loid)s from their mineral-bound complex or favor re-mobilization | Section 3, Section 4.1 and Section 4.4 |
| Inundation: pH can raise (if pyrite is formed in the initially during inundation; pH can drop when flood recedes or water level drops due to dissolution of pyrite. Atmospheric deposition: N coupling with acid species increase soil acidity | |||
| Temperature | Global warming: Increase of soil temperature; Degradation of SOC increases/more labile fractions to microorganisms; microbial feedback to temperature might be positive | More bioavailability of chemical pollutants; Biodegradation of organic pollutants might increase; Dissolution of metals from its substrate | Section 3, Section 4.1 |
| SOC | Warming: Degradation of SOC increases/both persistent and labile fractions are vulnerable | More bioavailability of chemical pollutants | Section 3, Section 4.2 |
| Erosion: Loss of SOC from soil | Mobility of chemical pollutants | ||
| Moisture/rainfall | Water repellence: Growth of microorganism decreases; less vegetation | Longer residence of pollutants | Section 3, Section 4.1, Section 4.4.2 |
| Inundation: Anoxic environment in soil | Redox controls the mobility of chemical pollutants; mineral’s dissolution can release toxic metals, such arsenic | ||
| Extreme rainfall pattern: Soil inundation, surface runoff and salt imbalance in soil | |||
| N and P | Deposition of atmospheric N and load of P from land-use practice: Increase of N and P in soils; acidification of soil | (Im)mobilization of metals (e.g., Cd) in P-supplemented soils; nutrient pollution and surface runoff | Section 4.3 |
| Clay minerals | Erosion: Loss of surface soils | Clay-organic matter disintegration might release heavy metals; loss of clay could reduce microbial function in rhizosphere; partial photodegradation could result in a more toxic metabolite of organic pollutants and thus increased bioavailability of them | Section 4.4.1 |
| Warming: Increase of soil temperature | |||
| Intensity of light: Light penetration in soil is high | |||
| Other minerals (e.g., oxides) | Extreme rainfall pattern: Inundation of soils affects redox of soils | Redox controls the mobility of chemical pollutants; mineral’s dissolution can release toxic metals, such as arsenic | Section 4.4.2 |
| Temperature: Increase of soil temperature | |||
| Microorganisms, enzyme and plants | Warming: Microbial activity may increase but community structure changes | Biodegradation of organic pollutants may be increased but the contaminant-specific microbial functions could be affected; plant uptake of metal(loid)s is affected due to climatic influence in rhizosphere | Section 4.4.2 |
| GHG in soil: Community structure changes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, B.; Qi, F.; Biswas, J.K.; Wijayawardena, A.; Khan, M.A.I.; Naidu, R. The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review. Soil Syst. 2018, 2, 51. https://doi.org/10.3390/soilsystems2030051
Biswas B, Qi F, Biswas JK, Wijayawardena A, Khan MAI, Naidu R. The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review. Soil Systems. 2018; 2(3):51. https://doi.org/10.3390/soilsystems2030051
Chicago/Turabian StyleBiswas, Bhabananda, Fangjie Qi, Jayanta Kumar Biswas, Ayanka Wijayawardena, Muhammad Atikul Islam Khan, and Ravi Naidu. 2018. "The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review" Soil Systems 2, no. 3: 51. https://doi.org/10.3390/soilsystems2030051
APA StyleBiswas, B., Qi, F., Biswas, J. K., Wijayawardena, A., Khan, M. A. I., & Naidu, R. (2018). The Fate of Chemical Pollutants with Soil Properties and Processes in the Climate Change Paradigm—A Review. Soil Systems, 2(3), 51. https://doi.org/10.3390/soilsystems2030051