Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture
Abstract
:1. Introduction
2. Methods
3. Results
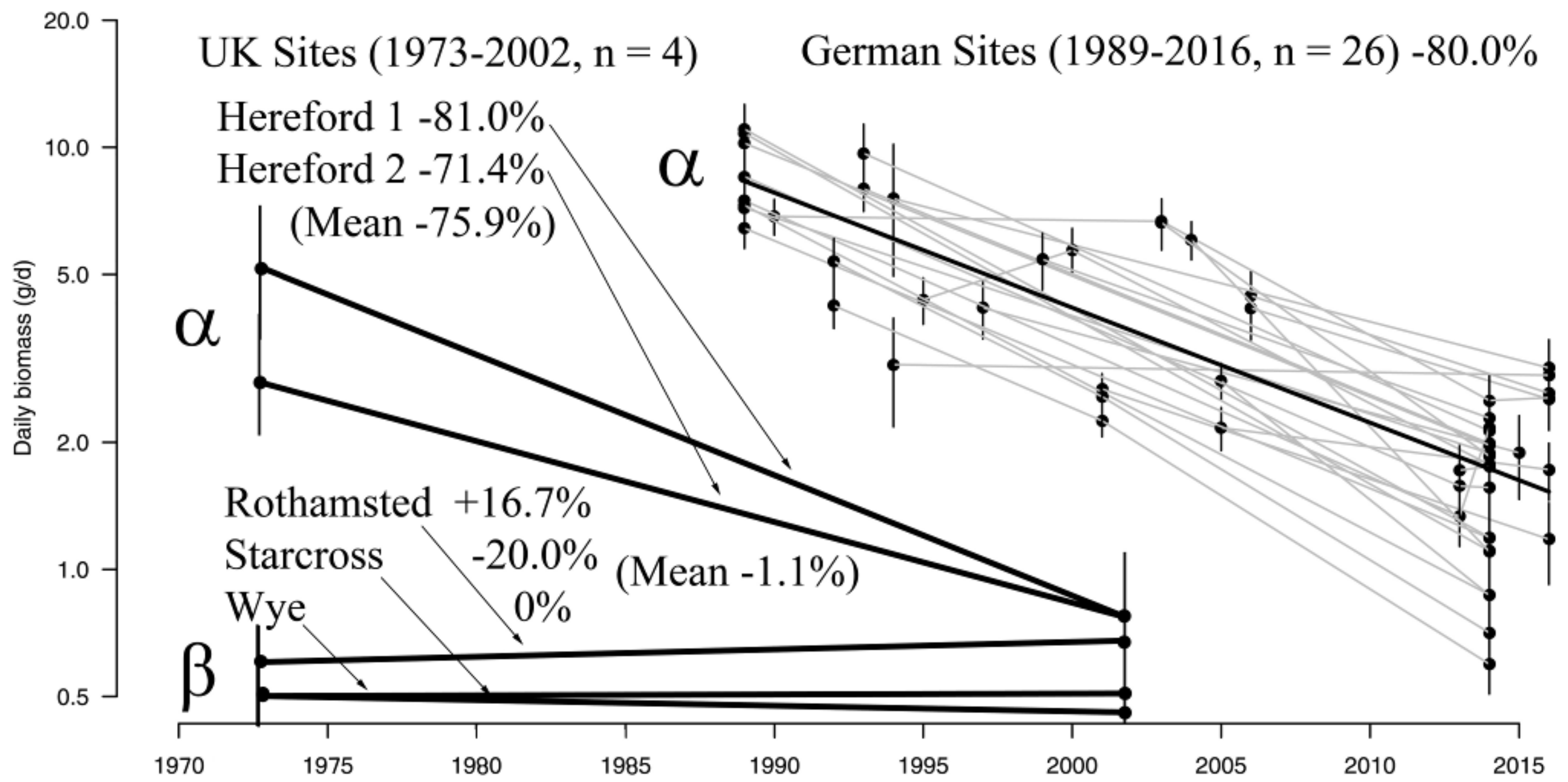
3.1. Insect Data
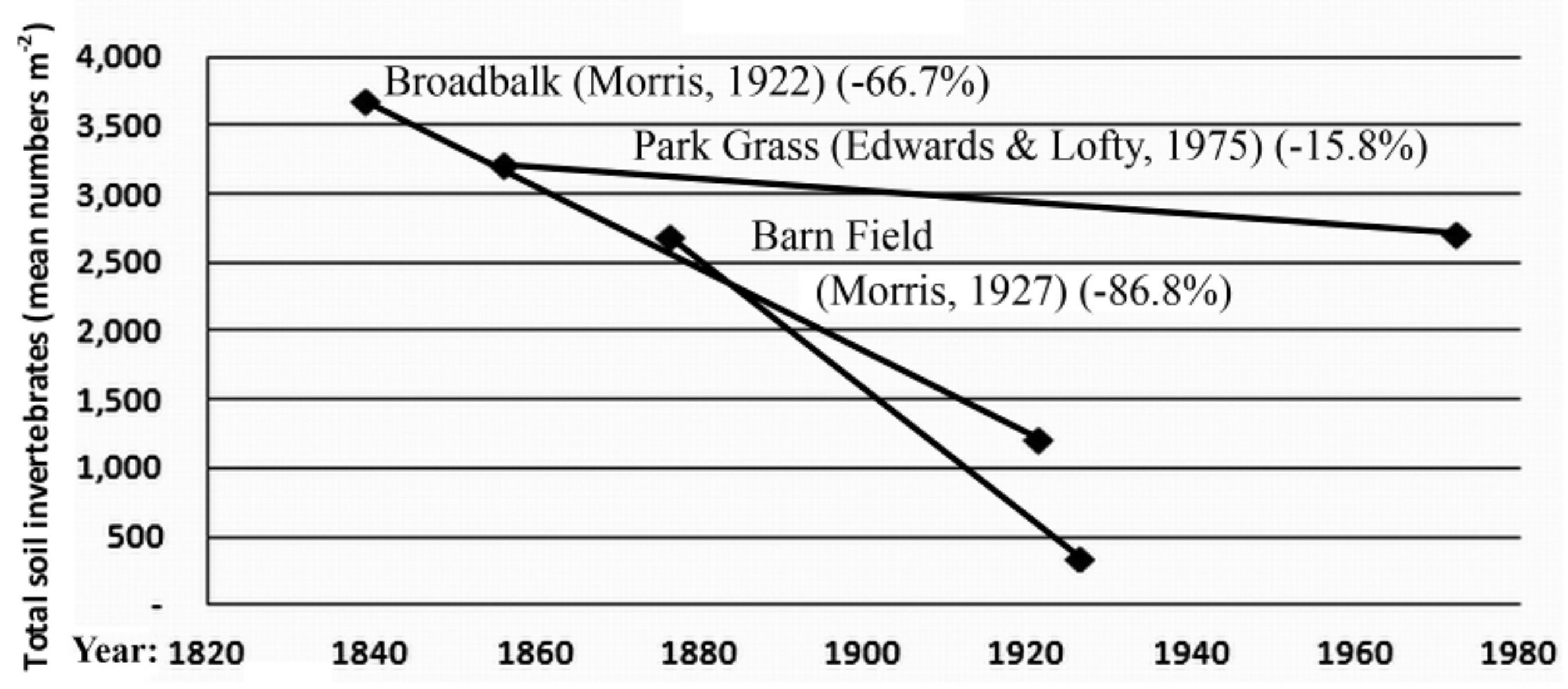
3.2. Invertebrate Data
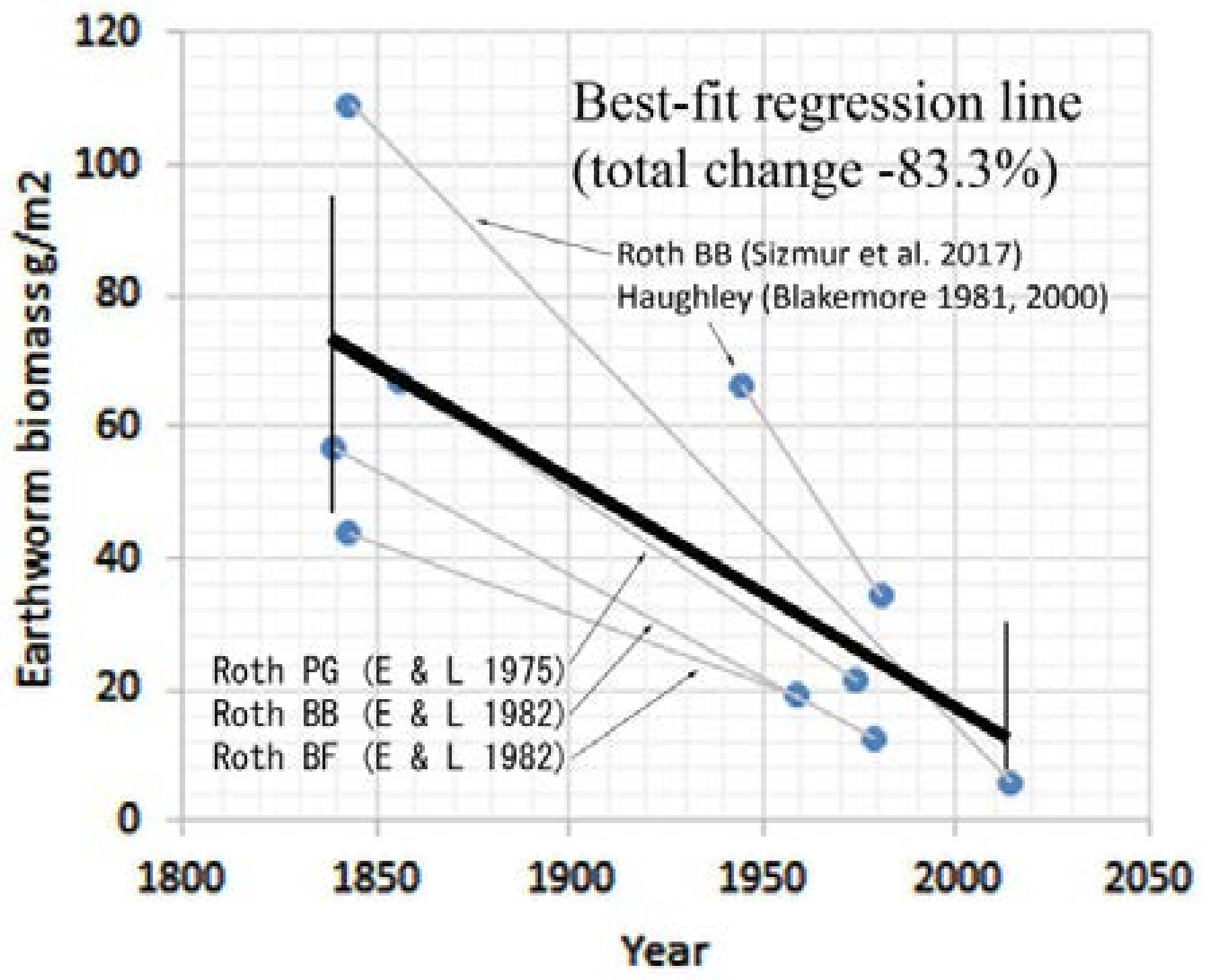
3.3. Earthworm Data
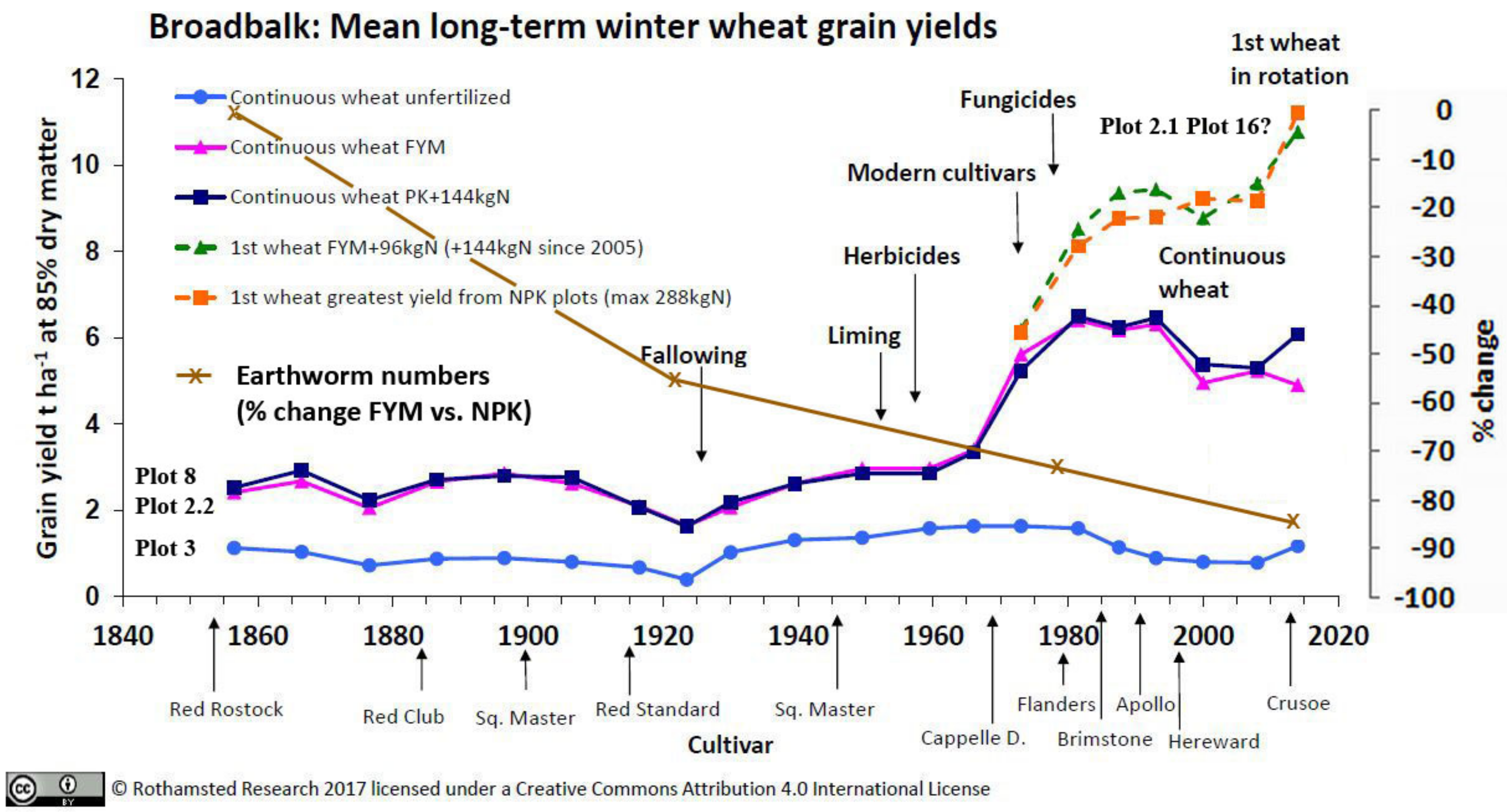
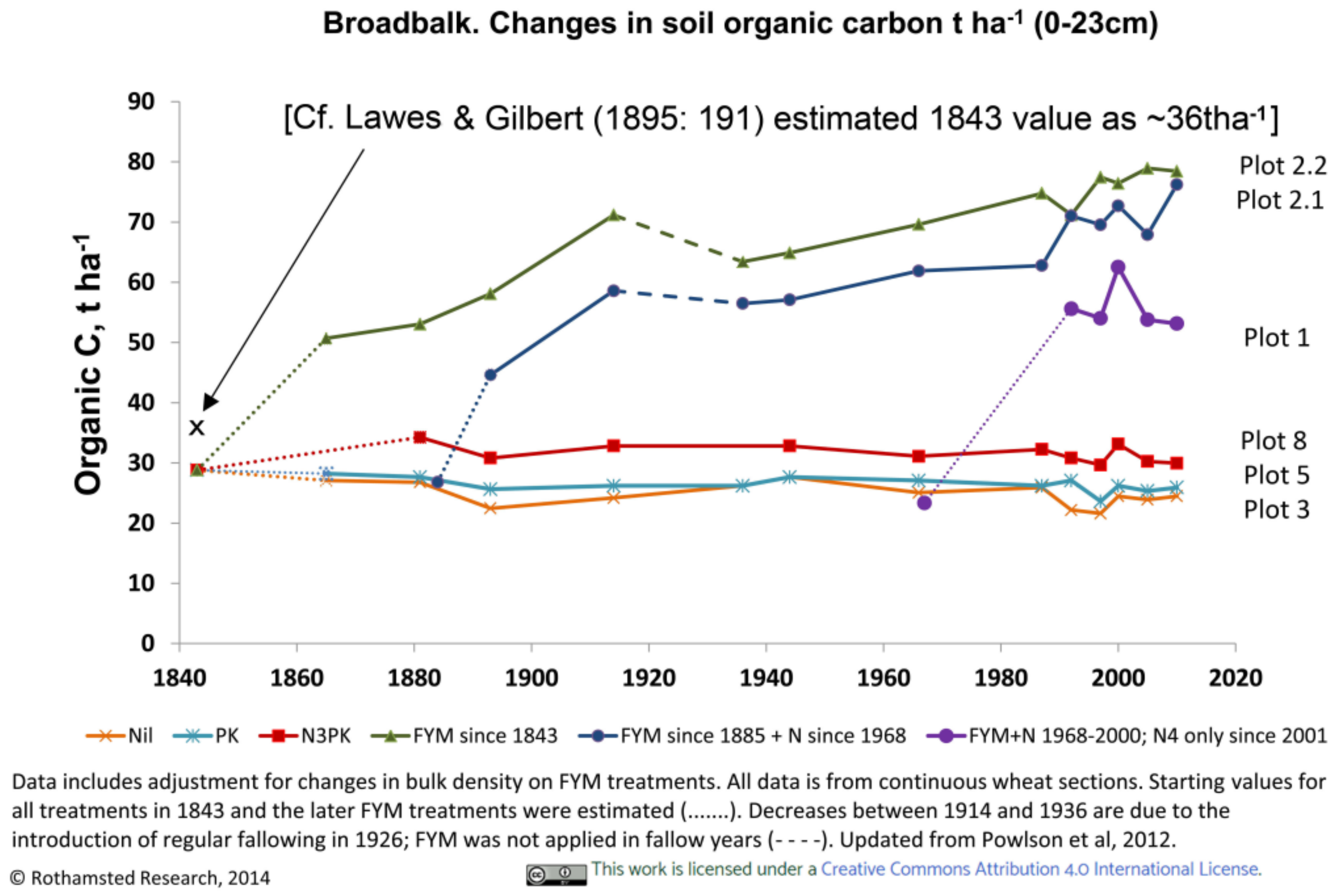
3.3.1. Rothamsted, UK
3.3.2. Haughley, UK
3.3.3. Swiss DOK Field Trials
3.3.4. Philippines Organic Farm Surveys
3.3.5. Summary of Earthworm Surveys
3.4. Soil Characteristics Relating to Earthworm Activity
3.5. Crop Yields and Economic Return
4. Discussion
4.1. Soils and Earthworms
4.2. Do Long-Term Controls Reasonably Represent Original States?
4.3. Comparison with Other Earthworm Monitoring Studies
4.4. Agriculture—The Primary Causal Factor of Biotic Declines
4.5. The Growing Failures of Synthetic Fertilizers
4.6. Significance of Decreasing Biodiversity
4.7. Practicability of Organic Restoration
4.8. Does Organic Farming Truly Require More Land?
4.9. Earthworm Benefits
4.10. Economic Considerations
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Shortall, C.R.; Moore, A.; Smith, E.; Hall, M.J.; Woiwod, I.P.; Harrington, R. Long-term changes in the abundance of flying insects. Insect Conserv. Divers. 2009, 2, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed]
- WWF International. Living Planet Report 2016: Risk and Resilience in a New Era. Gland, Switzerland. 2016. Available online: http://wwf.panda.org/about_our_earth/all_publications/lpr_2016/ (accessed on 10 May 2018).
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C.R. The Formation of Vegetable Mould through the Action of Worms, with Observation on Their Habits; Murray: London, UK, 1881. [Google Scholar]
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press: Sydney, Australia, 1985. [Google Scholar]
- Blakemore, R.J. Cosmopolitan Earthworms—An Eco-Taxonomic Guide to the Peregrine Species of the World, 6th ed.; VermEcology: Yokohama, Japan, 2016. [Google Scholar]
- Tuck, S.L.; Winqvist, C.; Mota, F.; Ahnström, J.; Turnbull, L.A.; Bengtsson, J. Land-use intensity and the effects of organic farming on biodiversity: A hierarchical meta-analysis. J. Appl. Ecol. 2014, 51, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.M. The insect and other invertebrate fauna of arable land at Rothamsted. Ann. Appl. Biol. 1922, 9, 282–305. [Google Scholar] [CrossRef]
- Morris, H.M. The insect and other invertebrate fauna of arable land at Rothamsted. Part II. Ann. Appl. Biol. 1927, 14, 442–464. [Google Scholar]
- Edwards, C.A.; Lofty, J.R. The invertebrate fauna of the Park Grass Plots. I. Soil fauna. Rothamsted Exp. Stn. Rep. 1974, 133–154. [Google Scholar]
- Edwards, C.A.; Lofty, J.R. Nitrogenous fertilizers and earthworms populations in agricultural soils. Soil Biol. Biochem. 1982, 147, 515–521. [Google Scholar] [CrossRef]
- Sizmur, T.; Martin, E.; Wagner, K.; Parmenteir, E.; Watts, C.; Whitmore, A.P. Milled cereal straw accelerates earthworm (Lumbricus terrestris) growth more than selected organic amendments. Appl. Soil Ecol. 2017, 113, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.J. Ecology of Earthworms under Different Fertilizer Regimes in Agriculture. BSc (Hons.) Bachelor’s Dissertation, Westminster University, London, UK, 1981. [Google Scholar]
- Blakemore, R.J. Ecology of earthworms under the “Haughley Experiment” of organic and conventional management regimes. Biol. Agric. Hortic. 2000, 18, 141–159. Available online: http://orgprints.org/30000/ (accessed on 10 May 2018). [CrossRef]
- Blakemore, R.J. Veni, Vidi, Vermi—II. Earthworms in organic fields restore SOM & H2O and fix CO2. Verm Ecol. Occas. Pap. Veop 2016, 2, 1–26. [Google Scholar]
- Balfour, E.B. The Living Soil and the Haughley Experiment, 2nd ed.; Faber & Faber: London, UK, 1975. [Google Scholar]
- Balfour, E.B. Towards a Sustainable Agriculture The Living Soil. Address to an International Federation of Organic Agricultural Movements (IFOAM) Conference in Switzerland. 1977. Available online: http://www.soilandhealth.org/01aglibrary/010116Balfourspeech.html (accessed on 10 May 2018).
- Pfiffner, L.; Mäder, P. Effects of Biodynamic, Organic and Conventional Production Systems on Earthworm Populations. Biol. Agric. Hortic. 1997, 15, 3–10. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, T.M.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Setälä, H. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Rothamsted Research. Guide to the Classical and other Long-term Experiments, Datasets and Sample Archive. Lawes Agricultural Trust. 2012, p. 51. Available online: www.rothamsted.ac.uk/sites/default/files/LongTermExperiments_PDF.pdf (accessed on 10 May 2018).
- Franklin, J.F. Importance and justification of long-term studies in ecology. In Long-Term Studies in Ecology; Likens, G.E., Ed.; Springer-Verlag: New York, NY, USA, 1989; pp. 3–19. [Google Scholar]
- Poulson, P.R. The Rothamsted long-term experiments: Are they still relevant? Can. J. Plant Sci. 1996, 76, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Balfour, E.B. The Living Soil: Evidence of the Importance to Human Health of Soil Vitality, with Special Reference to Post-War Planning, 7th ed.; Faber and Faber Ltd.: London, UK, 1948. [Google Scholar]
- Johnston, A.E.; Garner, H.V. Rothamsted Experimental Station Report for 1968—Part 2. 1969, pp. 12–25. Available online: http://www.era.rothamsted.ac.uk/eradoc/OCR/ResReport1968p2-12-25 (accessed on 10 May 2018).
- Clark, I.M.; Buchkina, N.; Jhurreea, D.; Goulding, K.W.; Hirsch, P.R. Impacts of nitrogen application rates on the activity and diversity of denitrifying bacteria in the Broadbalk Wheat Experiment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1235–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martindale, W. The Rothamsted Long Term Agricultural Experiments. 2001. Available online: https://waynemartindale.files.wordpress.com/2017/04/oecdfellowship1004.pdf (accessed on 10 May 2018).
- Jones, K.C.; Johnston, A.E. Cadmium in cereal grain and herbage from long-term experimental plots at Rothamsted, UK. Environ. Pollut. 1989, 57, 199–216. [Google Scholar] [CrossRef]
- Addiscott, T.M. Nitrate, Agriculture, and the Environment; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Rothamsted Research. Open Access e-RA Report. December 2017. Available online: www.era.rothamsted.ac.uk/metadata/broad/Broadbalk_YieldsJune2017.xlsx (accessed on 10 May 2018).
- Widdowson, R.W. Towards Holistic Agriculture: A Scientific Approach; Pergammon: London, UK, 1987. [Google Scholar]
- Hogg, D. Technological Change in Agriculture: Locking in to Genetic Uniformity; St. Martin’s Press: New York, NY, USA, 2000; p. 296. [Google Scholar]
- Stanhill, G. The comparative productivity of organic agriculture. Agric. Ecosyst. Environ. 1990, 30, 1–26. [Google Scholar] [CrossRef]
- Mäder, P.; Fließbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Korcak, R.F. Early Roots of the Organic Movement: A Plant Nutrition Perspective. Hort. Technol. 1992, 2, 263–267. [Google Scholar]
- Loudon, J.C. An Encyclopædia of Agriculture; Longmans: London, UK, 1826. [Google Scholar]
- Rothamsted Research. Open Access e-RA Report. December 2017. Available online: http://www.rothamsted.ac.uk/sites/default/files/LongTermExperiments_PDF.pdf (accessed on 10 May 2018).
- Researchgate.net Article. December 2017. Available online: www.researchgate.net/post/What_are_the_typical_N_P_and_K_values_of_farmyard_manure (accessed on 10 May 2018).
- Blakemore, R.J. The diversity of exotic earthworms in Australia—A status report. In Proceedings of “The Other 99%”; Transactions of the Royal Zoological Society of NSW; Ponder, W., Lunney, D., Eds.; Royal Zoological Society of NSW: Sydney, NSW, Australia, 1999; pp. 182–187. Available online: http://www.annelida.net/earthworm/Australasian%20Earthworms/Australian%20Exotics.pdf (accessed on 10 May 2018).
- Delate, K.; Cambardella, C.; Chase, C.; Turnbull, R. A review of long-term organic comparison trials in the U.S. Sustain. Agric. Res. 2015, 4, 5–14. [Google Scholar] [CrossRef]
- Rutgers, M.; Schouten, A.; Bloem, J.; Van Eekeren, N.; De Goede, R.; Jagersop Akkerhuis, G.; van der Wal, A.; Mulder, C.; Brussaard, L.; Breure, A.M. Biological measurements in a nationwide soil monitoring network. Eur. J. Soil Sci. 2009, 60, 820–832. [Google Scholar] [CrossRef]
- Jänsch, S.; Steffens, L.; Höfer, H.; Horak, F.; Roß-Nickoll, M.; Russell, D.; Nickoll, R.; Römbke, J. State of knowledge of earthworm communities in German soils as a basis for biological soil quality. Soil Org. 2013, 85, 215–233. [Google Scholar]
- Bauchhenß, J. Zeitliche veränderungen der regenwurm-taxozönosen auf grünland und auf ackerflachen. In 20 Jahre Bodendauerbeobachtung in Bayern. Zwischenbilanz der wichtigsten Ergebnisse 1985–2005; LfL-Schriftenreihe 8/2005; Bayerische Landesanstalt für Landwirtschaft: Freising, Germany, 2005; pp. 41–48. Available online: https://www.lfl.bayern.de/mam/cms07/publikationen/daten/schriftenreihe/p_19778.pdf (accessed on 10 May 2018).
- Van Leeuwen, J.P.; Saby, N.; Jones, A.; Louwagie, G.; Micheli E Rutgers, M. Gap assessment in current soil monitoring networks across Europe for measuring soil functions. Environ. Res. Lett. 2007, 12, 124007. [Google Scholar] [CrossRef]
- Rutgers, M.; Orgiazzi, A.; Gardi, C.; Römbke, J.; Jänsch, S.; Keith, A.M. Mapping earthworm communities in Europe. Appl. Soil Ecol. 2016, 97, 98. [Google Scholar] [CrossRef]
- Blakemore, R.J. Earthworms of South-East Queensland and Their Agronomic Potential in Brigalow Soils. Ph.D. Thesis, University of Queensland, St. Lucia, QLD, Australia, 1994; p. 605. [Google Scholar]
- Blakemore, R.J. Veni, Vidi, Vermi—I. On the contribution of Darwin’s ‘humble earthworm’ to soil health, pollution-free primary production, organic ‘waste’ management & atmospheric carbon capture for a safe and sustainable global climate. Verm Ecol. Occas. Pap. Veop. 2016, 2, 1–34. Available online: https://veop.files.wordpress.com/2016/09/vvv-i.pdf (accessed on 10 May 2018).
- Blakemore, R.J. Miscellaneous earthworm types in the Natural History Museum, London (Annelida: Oligochaeta: Megadrilacea: Eudrilidae, Lumbricidae, Megascolecidae, Moniligastridae, Octochaetidae). Opusc. Zool. Inst. Zoosyst. Oecol. Univ. Bp. 2014, 45, 119–155. [Google Scholar]
- Hutcheon, A.; Iles, D.R.; Kendall, D.A. Earthworm populations in conventional and integrated farming systems in the LIFE Project (SW England) in 1990–2000. Ann. Appl. Biol. 2001, 139, 361–372. [Google Scholar] [CrossRef]
- Sabrina, D.T.; Hanafi, M.M.; Nor Azwady, A.A.; Mahmud, T.M.M. Earthworm populations and cast properties in the soils of oil palm plantations. MJSS 2009, 13, 29–42. [Google Scholar]
- Lee, J.A.; McNeil, S.; Rorison, L.H. (Eds.) Nitrogen as an Ecological Parameter: Cereal Aphids; Blackwell Scientific Press: Oxford, UK, 1979; pp. 283–300. [Google Scholar]
- Heaton, S. Organic Farming, Food Quality and Human Health; Soil Association Report; Soil Association: Bristol, UK, 2001; p. 87. [Google Scholar]
- Soule, J.; Piper, J. Farming in Nature’s Image; Island Press: Washington, DC, USA, 1992. [Google Scholar]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Schmidt, O. Conventional tillage decreases the abundance and biomass of earthworms and alters their community structure in a global meta-analysis. Glob. Chang. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, S.J.; Eswaramurthy, T.; de-Goede, R.G.M.; Brussaard, L.; Pulleman, M.M. Effect of tillage on earthworms over short- and medium-term in conventional and organic farming. Appl. Soil Ecol. 2014, 83, 140–148. [Google Scholar] [CrossRef]
- Howard, A. An Agricultural Testament; Oxford University Press: London, UK, 1947. [Google Scholar]
- Gill, E. Lady Eve Balfour and the British Organic Food and Farming Movement. Ph.D. Thesis, Aberystwyth University, Aberystwyth, UK, 2010. [Google Scholar]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10. [Google Scholar] [CrossRef]
- NOAA. A Primer on pH. 2017. Available online: https://www.pmel.noaa.gov/co2/story/A+primer+on+pH (accessed on 10 May 2018).
- Gilbert, N. Acid soil threatens Chinese farms. Nature 1989. [Google Scholar] [CrossRef]
- UK Government Reports. UK Soil Degradation. July 2006 Number 265. Available online: http://www.parliament.uk/documents/post/postpn265.pdf (accessed on 10 May 2018).
- Research. Open Access e-RA Report. December 2017. Available online: https://www.rothamsted.ac.uk/sites/default/files/soil-health.pdf (accessed on 10 May 2018).
- Edmondson, J.L.; Davies, Z.G.; Gaston, K.J.; Leake, J.R. Urban cultivation in allotments maintains soil qualities adversely affected by conventional agriculture. J. Appl. Ecol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Withnall, A. Independent Newspaper Article. 2014. Available online: http://www.independent.co.uk/news/uk/home-news/britain-facing-agricultural-crisis-as-scientists-warn-there-are-only-100-harvests-left-in-our-farm-9806353.html (accessed on 10 May 2018).
- Crawford, J. What if the World’s Soil Runs Out? Time, 14 December 2012. Available online: http://world.time.com/2012/12/14/what-if-the-worlds-soil-runs-out/(accessed on 10 May 2018).
- Arsenault, C.; Only 60 Years of Farming Left if Soil Degradation Continues. Sci. Am. 2014. Available online: https://www.scientificamerican.com/article/only-60-years-of-farming-left-if-soil-degradation-continues/ (accessed on 10 May 2018).
- Bengtsson, J.; Ahnstrom, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2015, 42, 261–269. [Google Scholar] [CrossRef]
- Ewald, J.A.; Wheatley, C.J.; Aebischer, N.J.; Moreby, S.J.; Duffield, S.J.; Crick, H.Q. Influences of extreme weather, climate and pesticide use on invertebrates in cereal fields over 42 years. Glob. Chang. Biol. 2015, 21, 3931–3950. [Google Scholar] [CrossRef] [PubMed]
- Lawes, J.B.; Gilbert, J.H. The Rothamsted Memoirs on Agricultural Chemistry and Physiology. Vol. I: Reports of Field Experiments, &c, &c; Clowes & Sons: London, UK, 1893. [Google Scholar]
- Worldwatch Institute. Can Organic Farming Feed Us All? 2006. Available online: http://www.worldwatch.org/node/4060 (accessed on 10 May 2018).
- UN Report 2010. Available online: http://www2.ohchr.org/english/issues/food/docs/A-HRC-16-49.pdf (accessed on 10 May 2018).
- Muller, A.; Schader, C.; Scialabba, N.E.H.; Brüggemann, J.; Isensee, A.; Erb, K. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badgley, C.; Moghtader, J.; Quintero, E.; Zakem, E.; Chappell, M.J.; Aviles-Vazquez, K. Organic agriculture and the global food supply. Renew. Agric. Food Syst. 2007, 22, 86–108. [Google Scholar] [CrossRef]
- Mayall, S. An English Organic Farm; Soil Association: Haughley, UK, 1965. [Google Scholar]
- Stonehouse, B. Biological Husbandry: A Scientific Approach to Organic Farming; Butterworths: London, UK, 1981. [Google Scholar]
- Thomas, D. The mineral depletion of foods available to us as a nation (1940–2002)—A review of the 6th Edition of McCance & Widdowson. Nutr. Health 2007, 19, 21–55. [Google Scholar] [PubMed]
- Jorhem, L.; Sundström, B.; Engman, J. Trends in Cadmium and Certain. Other Metal in Swedish Household. Wheat and Rye Flours 1983–2009: National Food Agency, Sweden. Livsmedels. Verket. Rapport 16, 2013. Available online: https://www.livsmedelsverket.se/globalassets/rapporter/2013/2013-national-food-agency-16---trends-in-cadmium-and-certain-other-metal-in-swedish-household-wheat-and-rye-flours-1983-2009.pdf (accessed on 10 May 2018).
- Johnson, N. Do Industrial Agricultural Methods Actually Yield More Food Per Acre Than Organic Ones? grist.org Commentary, 14 October 2015. Available online: http://grist.org/food/do-industrial-agricultural-methods-actually-yield-more-food-per-acre-than-organic-ones/(accessed on 10 May 2018).
- Mollison, B. Permaculture: A Designers’ Manual; Tagari Publications: Sisters Creek, Australia, 1988. [Google Scholar]
- Russell, E.J. A Student’s Book on Soils & Manures, 3rd ed.; Cambridge University Press: Cambridge, UK, 1940. [Google Scholar]
- UN Report December 2017. Available online: https://documents-dds-ny.un.org/doc/UNDOC/GEN/G17/017/85/PDF/G1701785.pdf?OpenElement (accessed on 10 May 2018).
- Guardian Newspaper. 7 March 2017. UN Experts Denounce Myth that Pesticides are Necessary to Feed the World. Available online: https://www.theguardian.com/environment/2017/mar/07/un-experts-denounce-myth-pesticides-are-necessary-to-feed-the-world (accessed on 10 May 2018).
- Wong, E. One-Fifth of China’s Farmland is Polluted, State Study Finds. The New York Times, 17 April 2014. [Google Scholar]
- Zhou, C.; Zhang, Y.; China’s Tainted Soil Initiative Lacks Pay plan. Caixinglobal. 2016. Available online: https://www.caixinglobal.com/2016-06-08/101011627.html (accessed on 10 May 2018).
- USCC Report. 14 February 2017. Available online: https://www.uscc.gov/sites/default/files/Research/The%2013th%20Five-Year%20Plan_Final_2.14.17_Updated%20%28002%29.pdf (accessed on 10 May 2018).
- Bhattacharyya, R.; Ghosh, B.N.; Mishra, P.K.; Mandal, B.; Rao, C.S.; Sarkar, D. Soil degradation in India: Challenges and potential solutions. Sustainability 2015, 7, 3528–3570. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Wollny, E. Forschungen auf der Gebiet der Agrikultur-Physik; Carl Winter´s Universitätsbuchhandlung: Heidelberg, Germany, 1890. [Google Scholar]
- Kretzschmar, A. Description des galeries de vers de terre et variations saisonnières des réseaux (observations en conditions naturelles). Rev. Ecol. Biol. Sol. 1982, 19, 579–591. [Google Scholar]
- Costanza, R.; Groot RS de Sutton, P.; Ploeg S van der Anderson, S.J. Changes in the global value of ecosystem services. Global Environ. Chang. Hum. Policy Dimens. 2014, 26, 52–158. [Google Scholar] [CrossRef]
- Fitzpatrick, I. Valuing Nature and the Hidden Costs of Biodiversity Loss. 2017. Available online: http://sustainablefoodtrust.org/articles/valuing-nature-and-the-hidden-costs-of-biodiversity-loss/?utm_source=SFT+Newsletter&utm_campaign=5117bb6a15-Newsletter_26_10_2017&utm_medium=email&utm_term=0_bf20bccf24-5117bb6a15-105104433 (accessed on 10 May 2018).
- UN-FAO Report. 2015. Available online: www.fao.org/fileadmin/templates/ess/documents/GDP/IND_NewsRelease_EN__27Apr2015_.pdf (accessed on 10 May 2018).
- Van Grinsven, H.J.; Holland, M.; Jacobsen, B.H.; Klimont, Z.; Sutton, M.A.; Jaap Willems, W. Costs and benefits of nitrogen for Europe and implications for mitigation. Environ. Sci. Technol. 2013, 47, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, I.; Young, R.; Perry, M.; Rose, E. The Hidden Cost of UK Food: Summary Report November 2017, p. 20. Available online: http://sustainablefoodtrust.org/wp-content/uploads/2013/04/Summary-PDF-9-Nov-for-online.pdf (accessed on 10 May 2018).
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Cheeke, T.E.; Coleman, C.; Wall, D.H. Microbial Ecology in Sustainable Agroecosystems; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- UK National Trust “The State of Nature Report 2016”. Available online: https://www.nationaltrust.org.uk/features/the-state-of-nature-report-2016 (accessed on 10 May 2018).
- Storkey, J.; Macdonald, A.J.; Poulton, P.R.; Scott, T. Grassland biodiversity bounces back from long-term nitrogen addition. Nature 2015, 528, 401–404. [Google Scholar] [CrossRef] [PubMed]
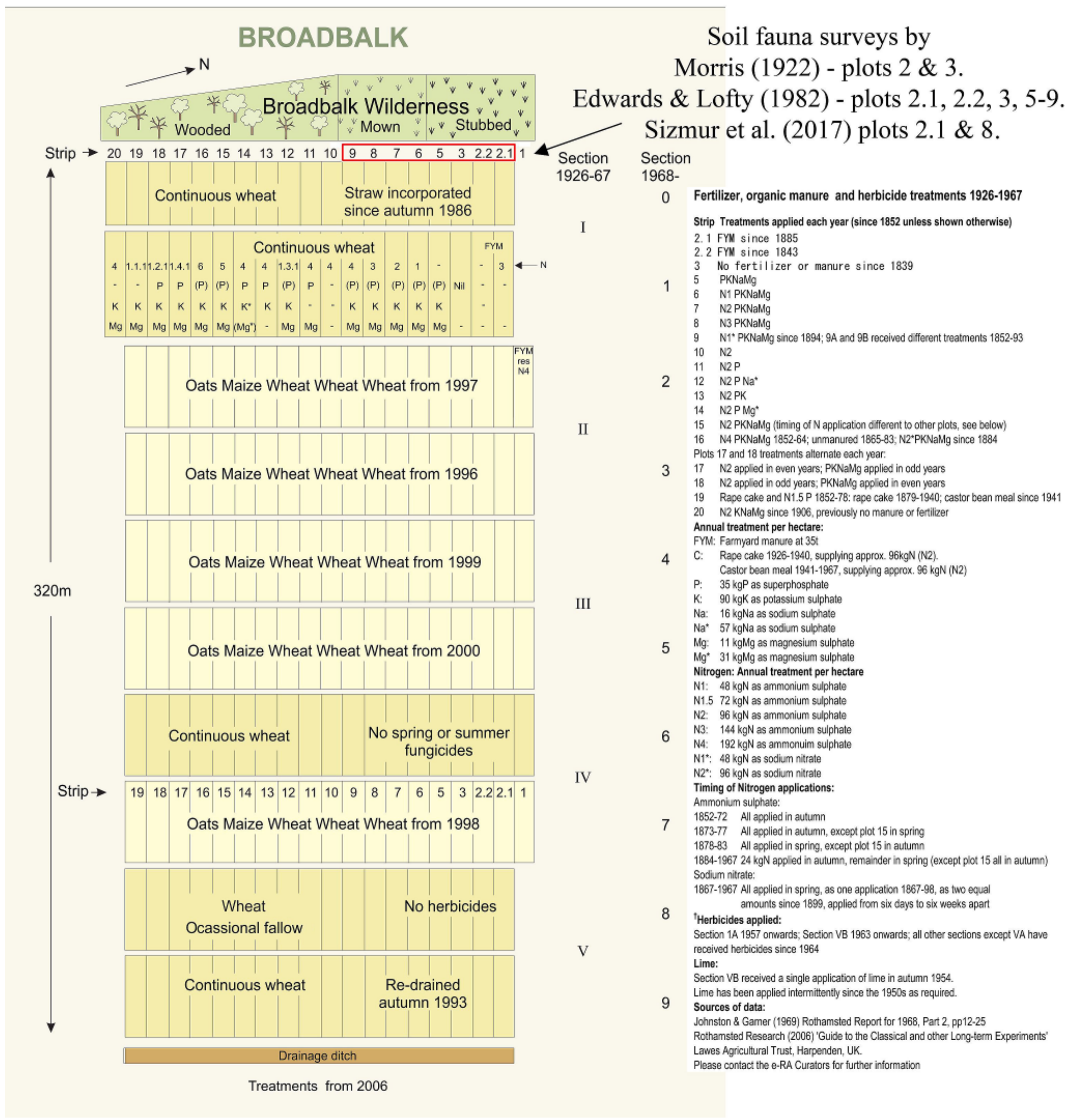
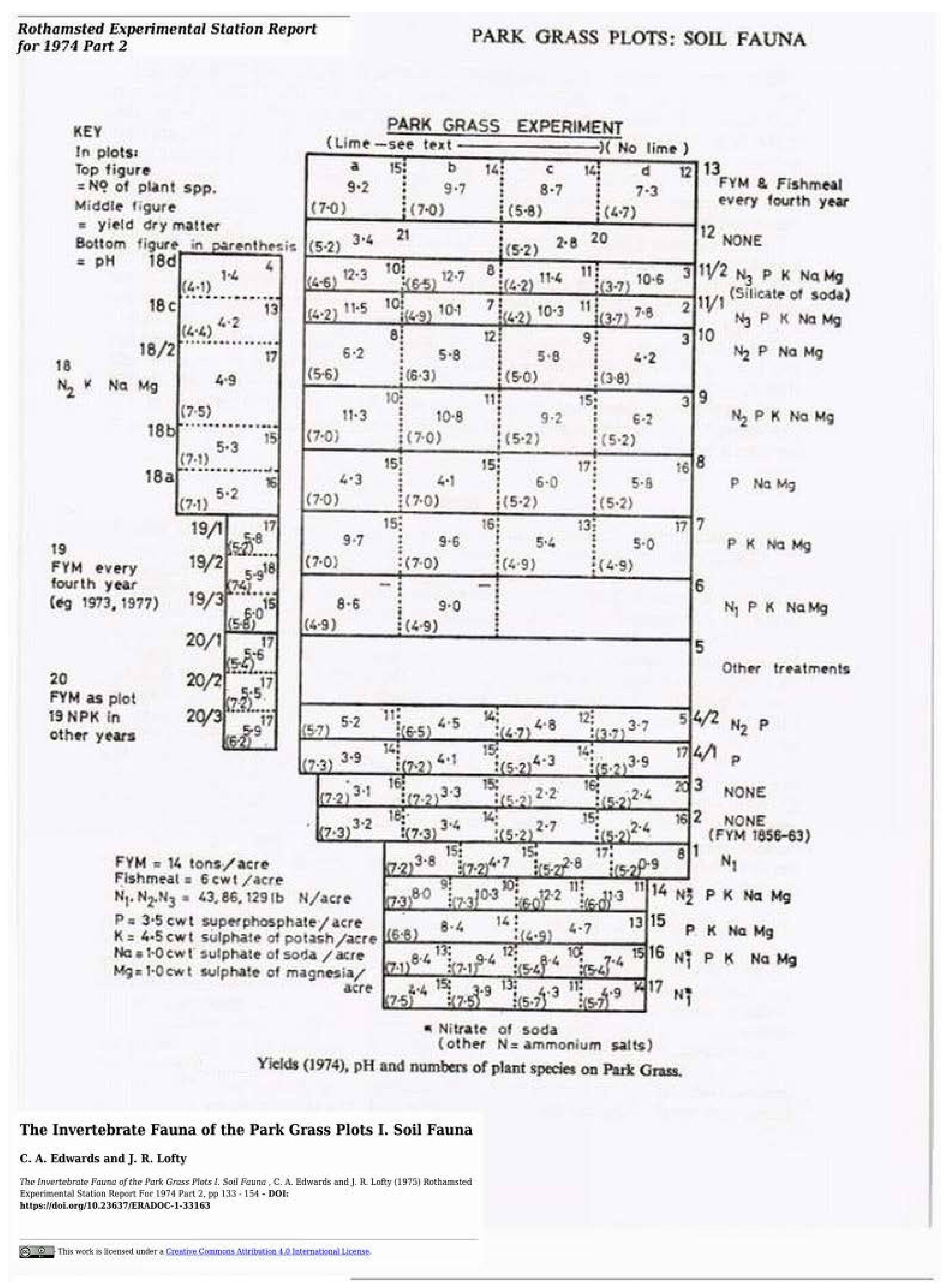
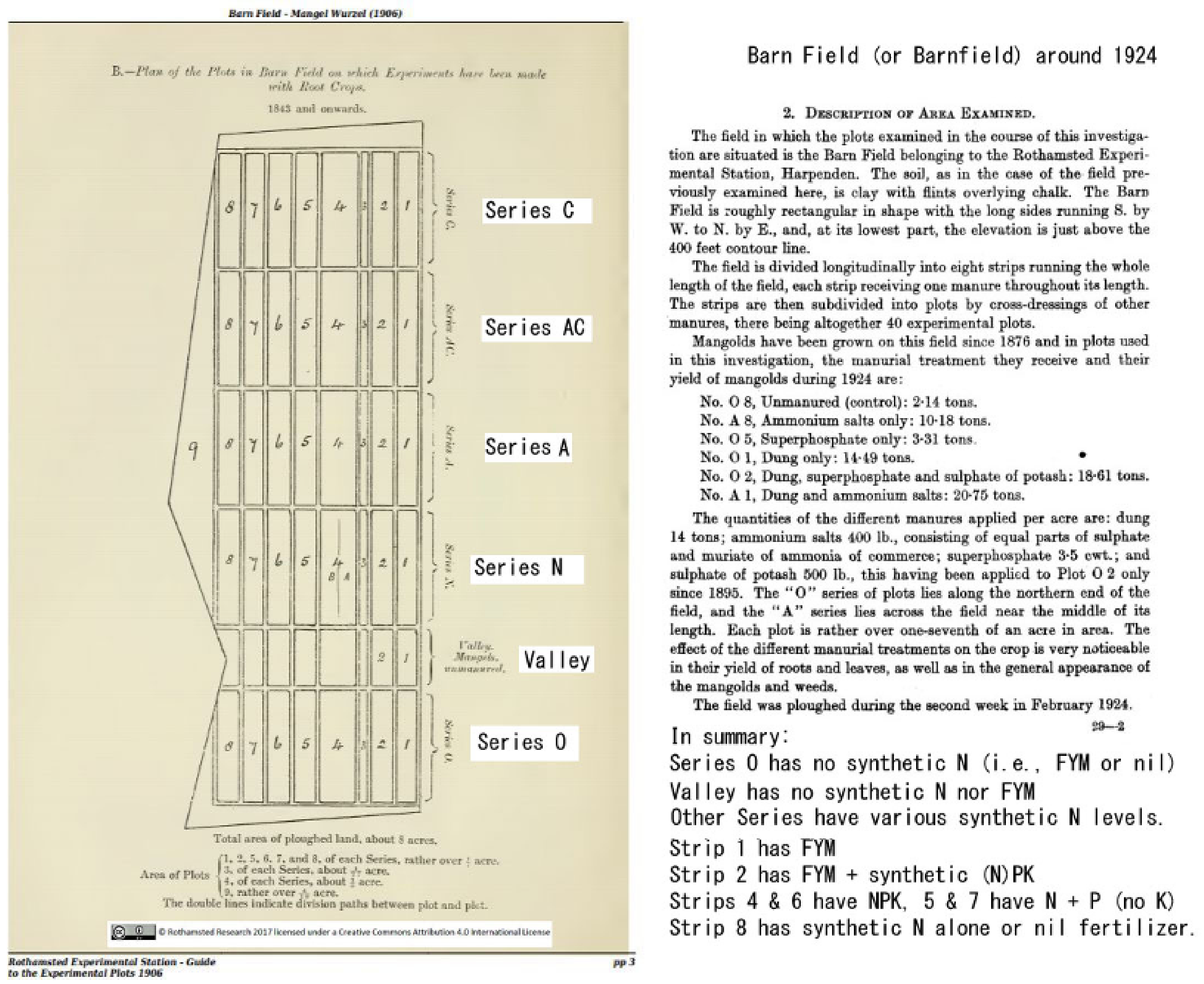
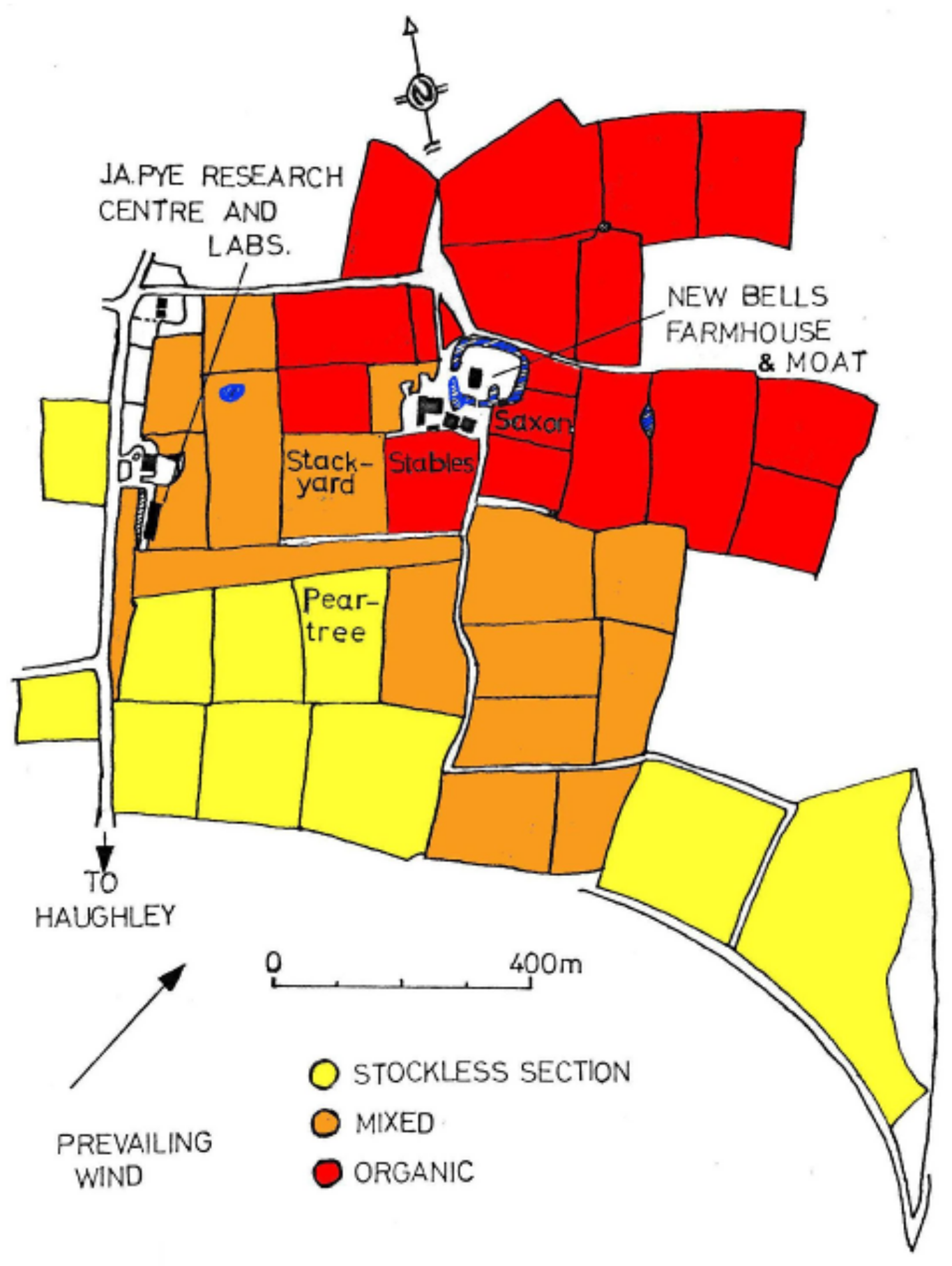
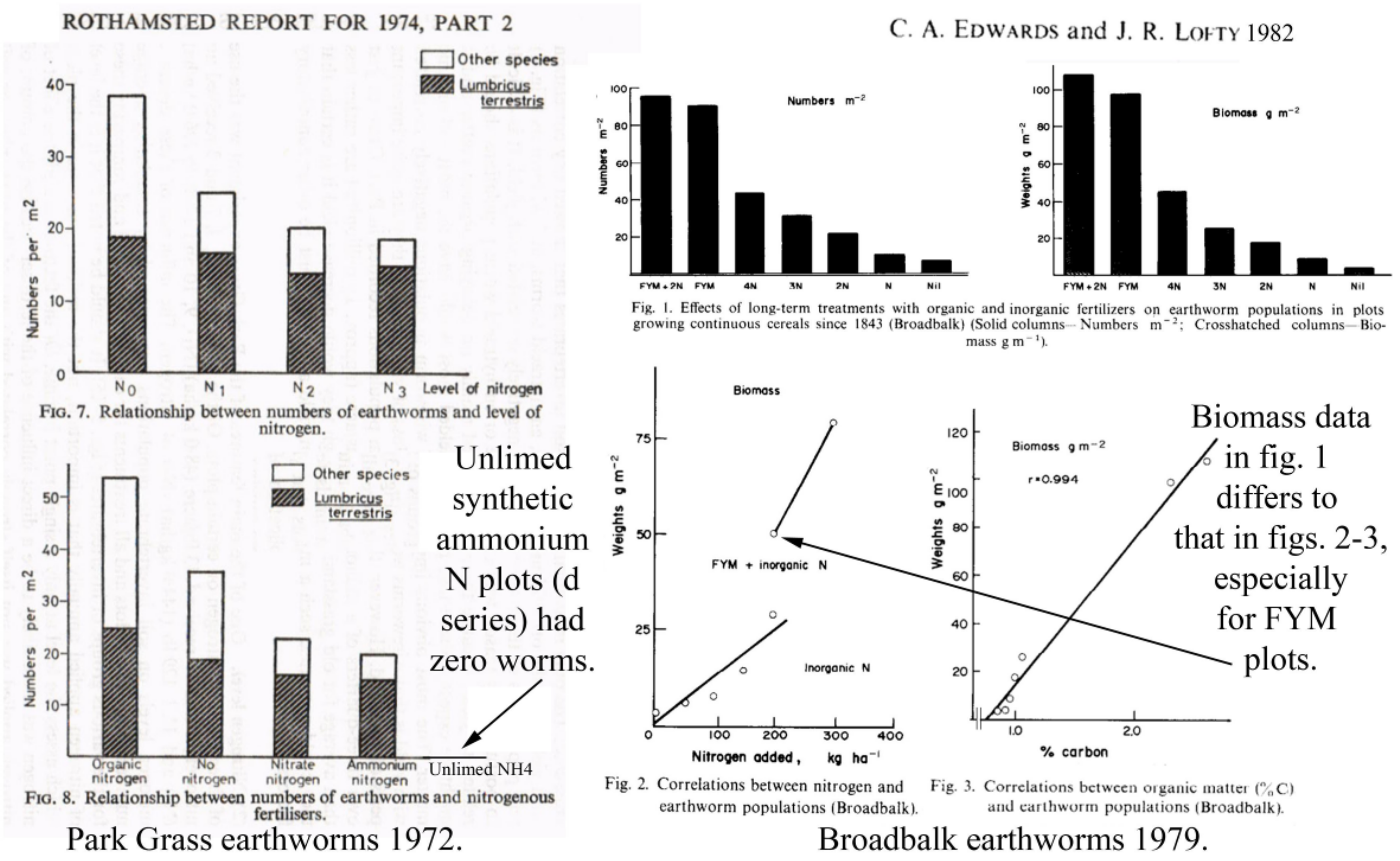
| Treatment − Plot Code | Totals m−2 (% Change) | Earthworms m−2 (%) |
|---|---|---|
| Farm-yard manure (FYM) − O1 | 3198 (0%) | 237 (0%) |
| Mix FYM + NH4 salts − A1 | 2597 (−19%) | 153 (−35%) |
| Mix FYM + PK − O2 | 2334 (−27%) | 137.9 (−42%) |
| Superphosphate − O5 | 428 (−87%) | 0 (−100%) |
| Ammonium NH4 salts − A8 | 348 (−89%) | 4 (−98%) |
| Nil fertilizer − O8 | 298 (−98%) | 6 (−98%) |
| Treatment (Plot) | Abundance m−2 (% Change) | Biomass gm−2 (% Change) |
|---|---|---|
| Mean FYM (plot 2.1; n = 2) | 400.00 (0%) a | 108.90 (0%) c |
| Mean NPK (plot 8; n = 2) | 70.35 (−82.4%) b** | 6.05 (−94.4%) d*** |
| Treatment (Plot) | Abundance m−2 (%) | Biomass gm−2 (%) |
|---|---|---|
| FYM (plots 2.1, 2.2; n = 2) # | (94.2 + 89.4)/2 = 91.8 (0%) | (71 + 44)/2 = 57.1 (0%) |
| 4N (plot 9, n = 4) | 42.9 (−53.3%) | 25.9 (−54.6%) |
| 3N (plot 8, n = 4) | 30.6 (−66.7%) | 13.7 (−76.0%) |
| 2N (plot 7, n = 4) | 20.9 (−77.2%) | 6.2 (−89.1%) |
| 1N (plot 6, n = 4) | 10.0 (−89.1%) | 4.7 (−91.8%) |
| Mean of all four N plots | 26.1 (−71.6%) | 12.6 (−77.9%) |
| Nil fertilizers (plots 5, 3; n = 2) | (7.5 + 5.6)/2 = 6.5 (−92.9%) | (8.9 + 2.7)/2 = 5.8 (−89.8%) |
| Treatment (Plot) | Abundance m−2 (% Change) | Biomass gm−2 (% Change) |
|---|---|---|
| FYM Plot 13 (n = 4) # | 55.0 (0%) | 66.9 (0%) |
| Nil fertilizer Plots 2, 3, 12 (n = 8) | 37.9 (−31.1%) | 43.1 (−43.16%) |
| NPK Plots 14, 16 (n = 4) | 17.3 (−68.6%) | 21.8 (−67.5%) |
| Treatment (and Plots?) # | Abundance m−2 (%) | Biomass gm−2 (%) |
|---|---|---|
| FYM (n = 1, O1) | 78.7 (0%) | 43.7 (0%) |
| FYM + 2N (NH4) (A1+?) | 76.8 (−2.4%) | 46.4 (6.2%) |
| FYM + 2N (NaNO3) (O2?) | 35.8 (−54.5%) | 17.1 (−60.9%) |
| 2N + superphosphate | 28.9 (−63.3%) | 19.2 (−56.1%) |
| Nil fertilizer (n = 1, O8?) | 10.6 (−86.5%) | 6.9 (−84.2%) |
| Plot Series (Treatment) | “Dung (14 t/yr)” | P-K-Na-Mg | “No Manure” |
|---|---|---|---|
| AC, (NaNO3 + rape) | 78.8 | 19.3 | 24.1 |
| N, ((HN4)2SO4) | 106.2 | 1.7 | 2.5 |
| A, (NaNO3) | 62.4 | 30.3 | 10.2 |
| O, no Nitrogen | 49.5 | 0.6 | 3.9 |
| Mean of totals (gm−2) | 74.24 | 12.95 | 10.17 |
| Total means % change | 0.0% | −82.6% | −86.3 |
| Treatment − Plot | Abundance m−2 (%) | Root Crop Yield t (%) * |
|---|---|---|
| FYM − O1 | 237.2 (0%) | 14.7 (0%) |
| Mix FYM + NH4 salts − A1 | 153.2 (−35.4%) | 21.1 (43.2%) |
| Mix FYM + PK − O2 | 137.9 (−41.9%) | 18.9 (28.4%) |
| [Mean of both “mixed” plots] | 145.5 (−38.6%) | 20.0 (35.5%) |
| Superphosphate − O5 | 0 (−100%) | 3.4 (−77.2%) |
| Ammonium NH4 salts − A8 | 3.7 (−98.4%) | 10.3 (−29.7%) |
| [Mean of both synthetic plots] | 1.9 (−98.9%) | 6.9 (−53.4%) |
| Nil fertilizer − O8 | 5.7 (−97.6%) | 2.2 (−85.2%) |
| Abundance m−2 (% Change) | Biomass gm−2 (% Change) | |
|---|---|---|
| Organic Permanent Pasture | 424.0 a (+137.4) | 117.6 d (+77.6%) |
| Organic (wheat) | 178.6 b (0%) | 66.2 e (0%) |
| Mixed (wheat) | 97.5 c (−45.4%) | 35.4 f (−46.5%) |
| Stockless/Chemical (wheat) | 100.0 c (−44.0%) | 34.7 f (−47.6%) |
| Field (Section) | Stables (Organic) | Stackyard (Mixed) | Peartree (Stockless) |
|---|---|---|---|
| Size (ha) | 2.43 | 3.24 | 2.83 |
| Sown | 26 September 1980 | 26 September 1980 | 26 September 1980 |
| Fertilized | FYM & mulch | FYM, mulch & NPK | NPK |
| Ploughed | 8 August 1980 | 24 September 1980 | 25 July 1980 |
| Previous crop | 3 year ley | 3 year ley | 3 year ley |
| Previous (1976) | barley | barley | barley |
| Previous (1975) | beans | beans | winter wheat |
| General rotation | 4 yr arable/ 4 yr ley (pasture before 1945) | 4 yr arable/ 4 yr ley (pasture before 1945) | 4 year corn and root crops, reduced ley |
| Treatment | No. m−2 | % Change | gm−2 | % Change |
|---|---|---|---|---|
| Organic | 350.0 a | 0.0 | 300.0 a | 0.0 |
| Conventional (Mixed) | 120.0 b | −65.7 | 100.0 b | −66.7 |
| Mineral (Chemical) | 100.0 b | −71.4 | 98.0 b | −67.3 |
| Mean % changes | −68.6 | −67.0 |
| Years | No. m−2 (%) | Biomass gm−2 (%) | Comparative Crop Study |
|---|---|---|---|
| 5 | 36.0 (−36.1) | 13.8 (−97.1) | Filipino PI paddy rice (Blakemore 2016a) [17] |
| 10 | 44.3 (−54.9) | - | Filipino PI sugarcane (Blakemore 2016a) [17] |
| 13 | 350 (−65.7) | 300 (−66.7) | FiBL DOK wheat Mix (Pfiffner & Mäder 1997) [20] |
| 13 | 350 (−71.4) | 300 (−67.3) | FiBL DOK wheat Chem (Pfiffner & Mäder 1997) [20] |
| 27 | 148 (−59.5) | - | DOK (Birkhofer 2008) [21] |
| 35(40) | 179.6 (−45.4) | 66.2 (−46.5) | Haughley winter wheat Mix (Blakemore 2000) [16] |
| 35(40) | 179.6 (−44.0) | 66.2 (−47.6) | Haughley winter wheat Chem (Blakemore 2000) [16] |
| 50 (80) | 175.9 (−98.2) | - | Roth BF (Morris 1927) [11] |
| 80 | 250 (−54.8) | - | Roth BB (Morris 1922) [10] |
| 116 | 78.7 (−63.3) | 43.7 (−56.1) | Roth BF (Edwards & Lofty 1982) [13] |
| 135 | 91.8 (−71.6) | 57.1 (−77.8) | Roth BB (Edwards & Lofty 1982) [13] |
| 143 | 55.0 (−68.6) | 66.9 (−67.5) | Roth PG (Edwards & Lofty 1975) [12] |
| 170 | 400 (−82.4) | 108.9 (−94.4) | Roth BB (Sizmur et al. 2017) [14] |
| Mean | 163.3 (−62.7%) | 93.8 (−69.0%) | ← Corresponding to c. 1.6 million ha−1 and 0.94 tha−1 |
| Treatment | H2O % (%) | BD (gcm−3) | pH | SOC % (%) | Microbes | Cd (mg/kg) |
|---|---|---|---|---|---|---|
| Woodland | 41 (+28%) | 0.9 | 7.7 | 3.45 (+22%) | Highest | - |
| FYM (plot 2.2) | 32 (0%) | 1.1 | 7.8 | 2.83 (0%) | Higher | 40–46 |
| N288 (plot 16) | 25 (−22%) | 1.2 | 7.8 | 1.2 (−58%) | Reduced | - |
| N144 (plot 8) | 27 (−16%) | 1.2 | 7.3 | 1.13 (−60%) | Reduced | 71–83 |
| N0 (plot 5) | 25 (−22%) | 1.2 | 8.1 | 0.9 (−68%) | Reduced | - |
| Treatment | H2O % (%) | BD (gcm−3) (%) | pH | SOC % (%) | Temp. °C |
|---|---|---|---|---|---|
| Perm. Pasture | 42 (+36%) | 0.9 (+13%) | 7.5 | 9.9 (+148%) | - |
| Organic (wheat) | 31 (0%) | 0.8 (0%) | 7.2 | 4.0 (0%) | 2.5 |
| Mixed (wheat) | 24 (−23%) | 0.8 (0%) | 7.6 | 2.3 (−42%) | 1.5 |
| Stockless (wheat) | 22 (−29%) | 1.1 (+38%) | 7.5 | 1.8 (−56%) | 0.5 |
| Treatment (in 1843) | Grain (tha−1) | Straw (tha−1) | Total (% Change) |
|---|---|---|---|
| Nil (FYM in 1839) | 1.03 | 1.26 | 2.29 (−26.0) |
| FYM (35 tha−1) | 1.43 | 1.66 | 3.09 (0.0) |
| Ash from FYM | 0.99 | 1.24 | 2.24 (−27.6) |
| PK minerals (no N) | 1.13 | 1.29 | 2.42 (−21.6) |
| NPK (0.073 tha−1 N) | 1.43 | 1.60 | 3.03 (−2.0) |
| Plot 3/Nil | Plot 2.2/FYM | Plot 8/3NPK | |
|---|---|---|---|
| Cumulative yield | 21.38 a | 72.55 b | 74.63 b |
| % change | −70.5 | 0.0 | +2.9 |
| Crop | Org. | Org. | Chem. | Chem. | Yield (% Change) |
|---|---|---|---|---|---|
| Yield (t/ha) | Gross (£/ha) | Yield (t/ha) | Gross (£/ha) | Org. v. Chem. | |
| Winter wheat | 5.29 | 593 | 5.7 | 449 | —7.2 |
| Spring barley | 5.04 | 500 | 4.24 | 322.7 | +18.9 |
| Oats | 5.17 | 499 | 4.64 | 353.8 | +11.4 |
| Means | 5.2 | £530.6 | 4.9 | £375.2 | +7.7% |
| Earthworm Survey Site Crops [Reference] | Org. vs. Chem. Yield (% Change) |
|---|---|
| Rothamsted Broadbalk wheat [31] | −2.8 |
| Rothamsted Barnfield root crops [11] | +42.7 |
| Rothamsted Park Grass pasture [12] | −2.2 |
| Haughley grains [32,33] | +7.7 |
| Haughley wheat below-ground roots [16] | +10.8 |
| Haughley wheat above-ground shoots [16] | +13.9 |
| Haughley milk [18,19] | +15 |
| Swiss DOK crops (overall) [20,35] | −20 |
| Philippines PI paddy rice [17] | +33.3 |
| Philippines PI sugarcane [17] | +80.0 |
| Overall mean yield Org. vs. Chem. (n = 10) | +17.8 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blakemore, R.J. Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture. Soil Syst. 2018, 2, 33. https://doi.org/10.3390/soilsystems2020033
Blakemore RJ. Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture. Soil Systems. 2018; 2(2):33. https://doi.org/10.3390/soilsystems2020033
Chicago/Turabian StyleBlakemore, Robert J. 2018. "Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture" Soil Systems 2, no. 2: 33. https://doi.org/10.3390/soilsystems2020033
APA StyleBlakemore, R. J. (2018). Critical Decline of Earthworms from Organic Origins under Intensive, Humic SOM-Depleting Agriculture. Soil Systems, 2(2), 33. https://doi.org/10.3390/soilsystems2020033





