Adult Pleomorphic Rhabdomyosarcoma: Case Report
Abstract
1. Introduction and Clinical Significance
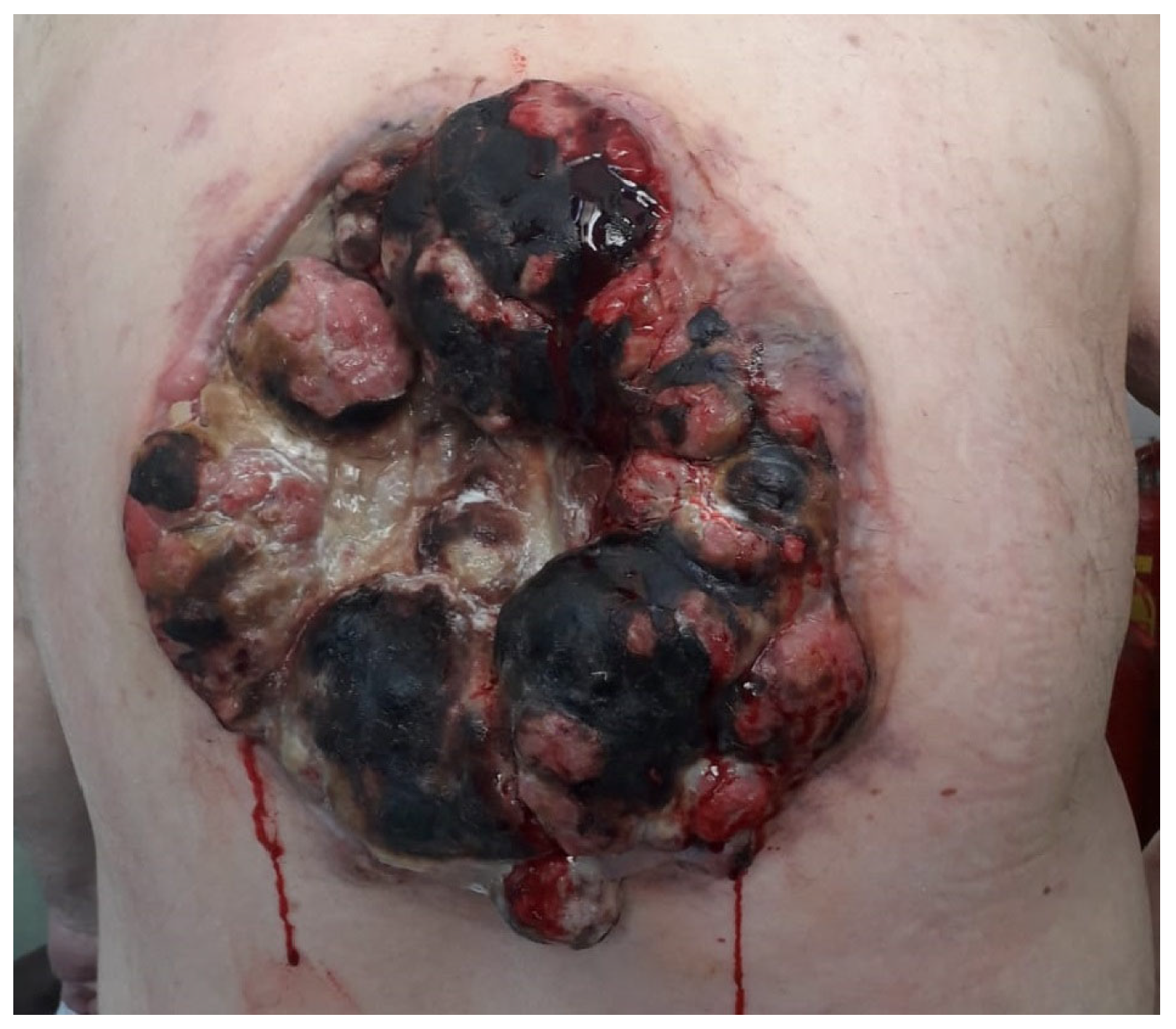
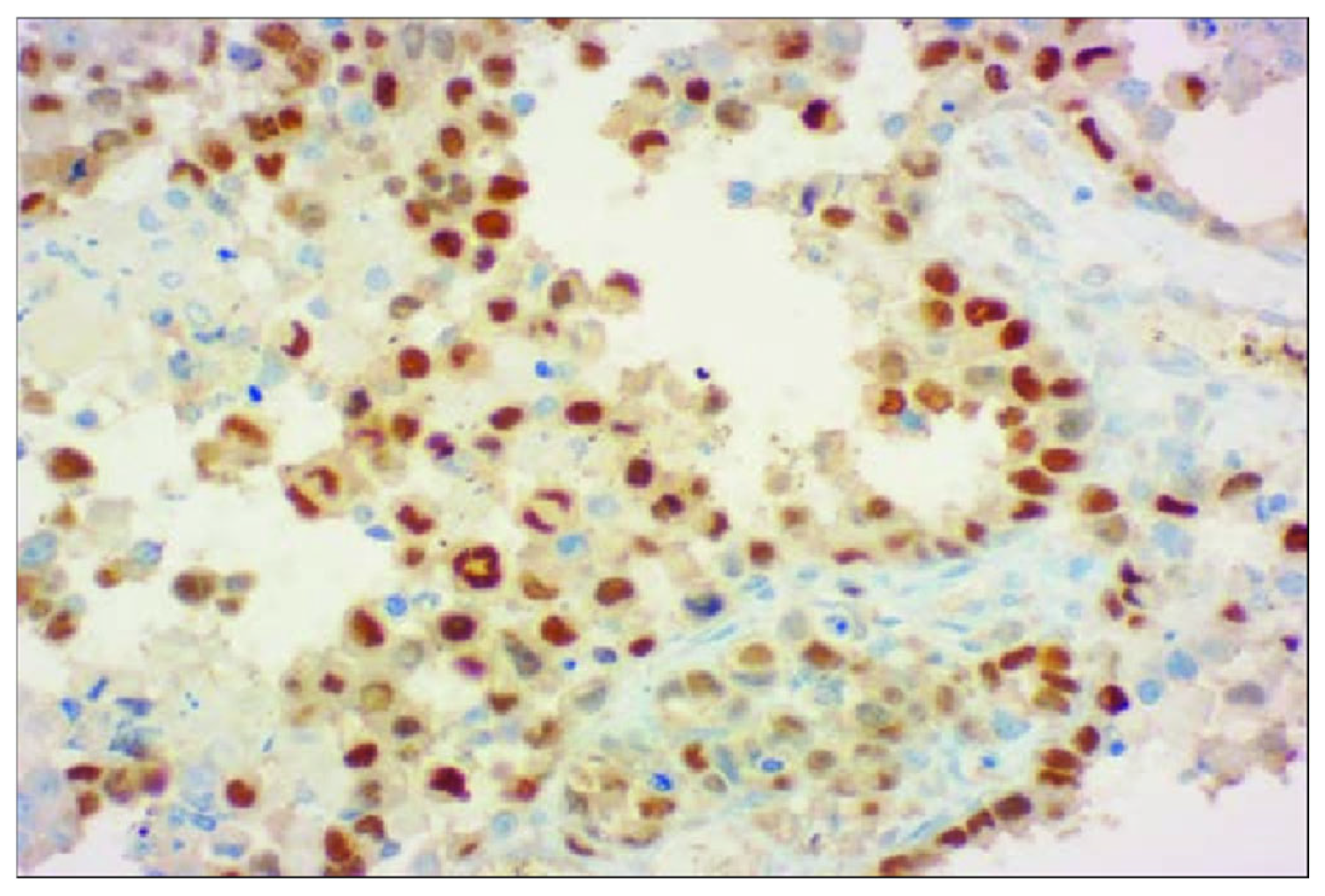
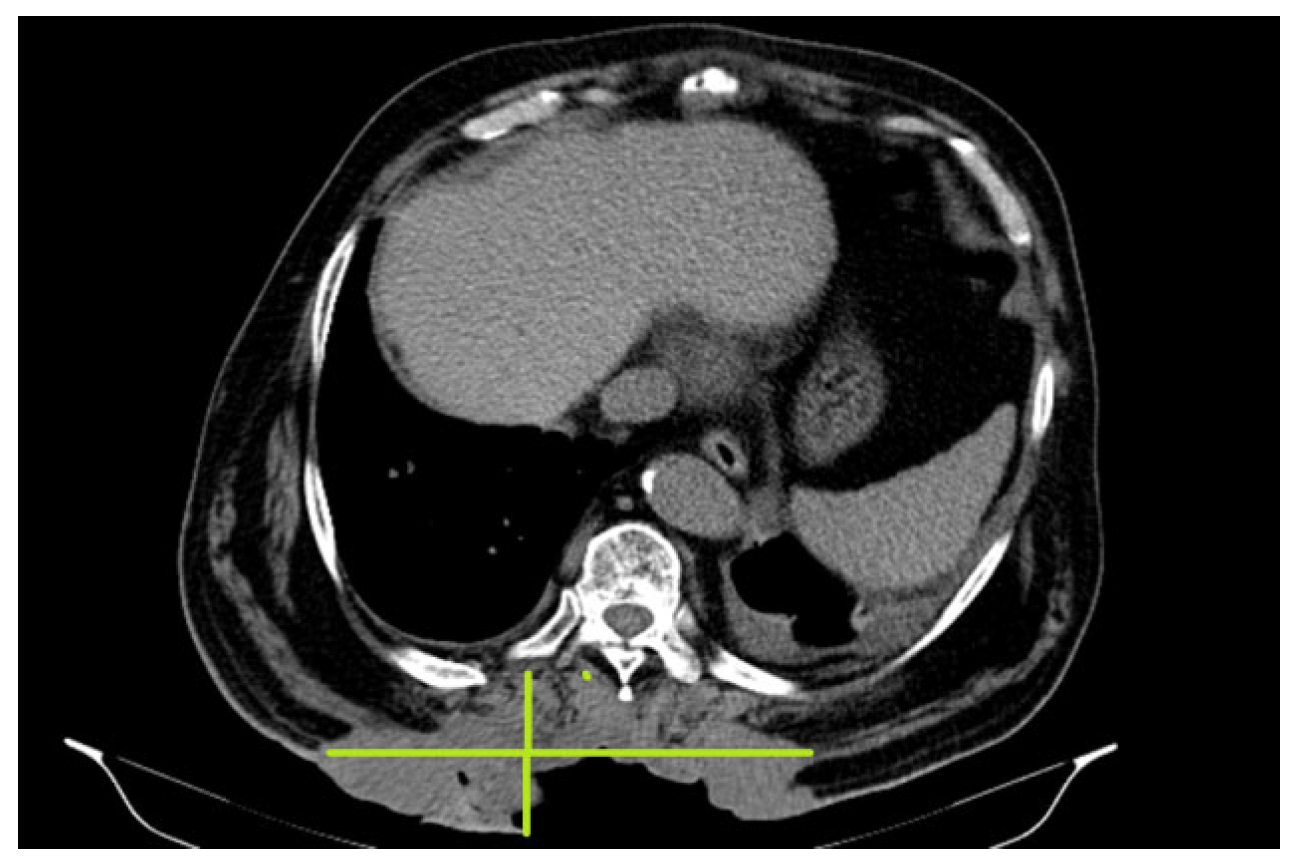
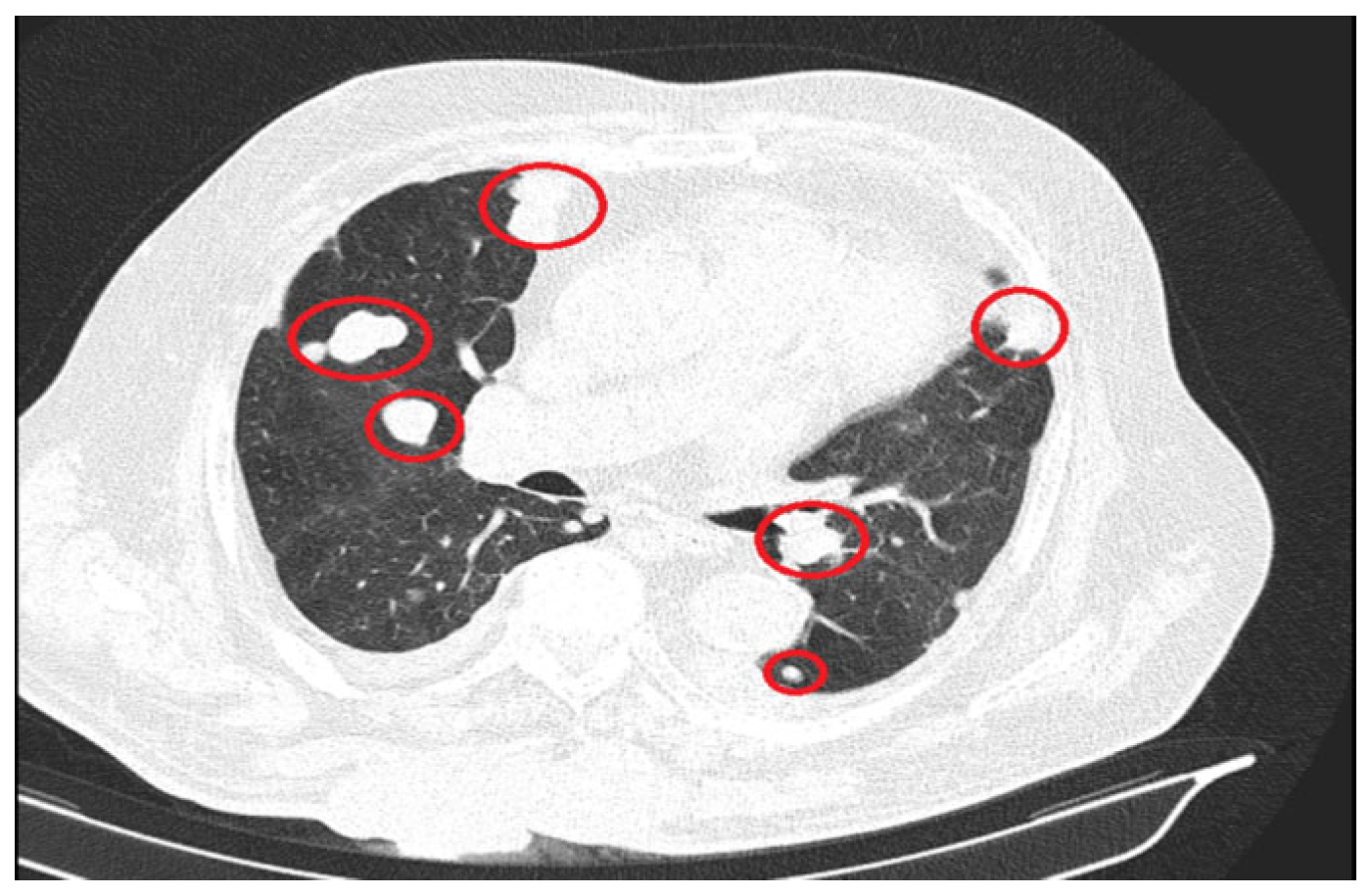
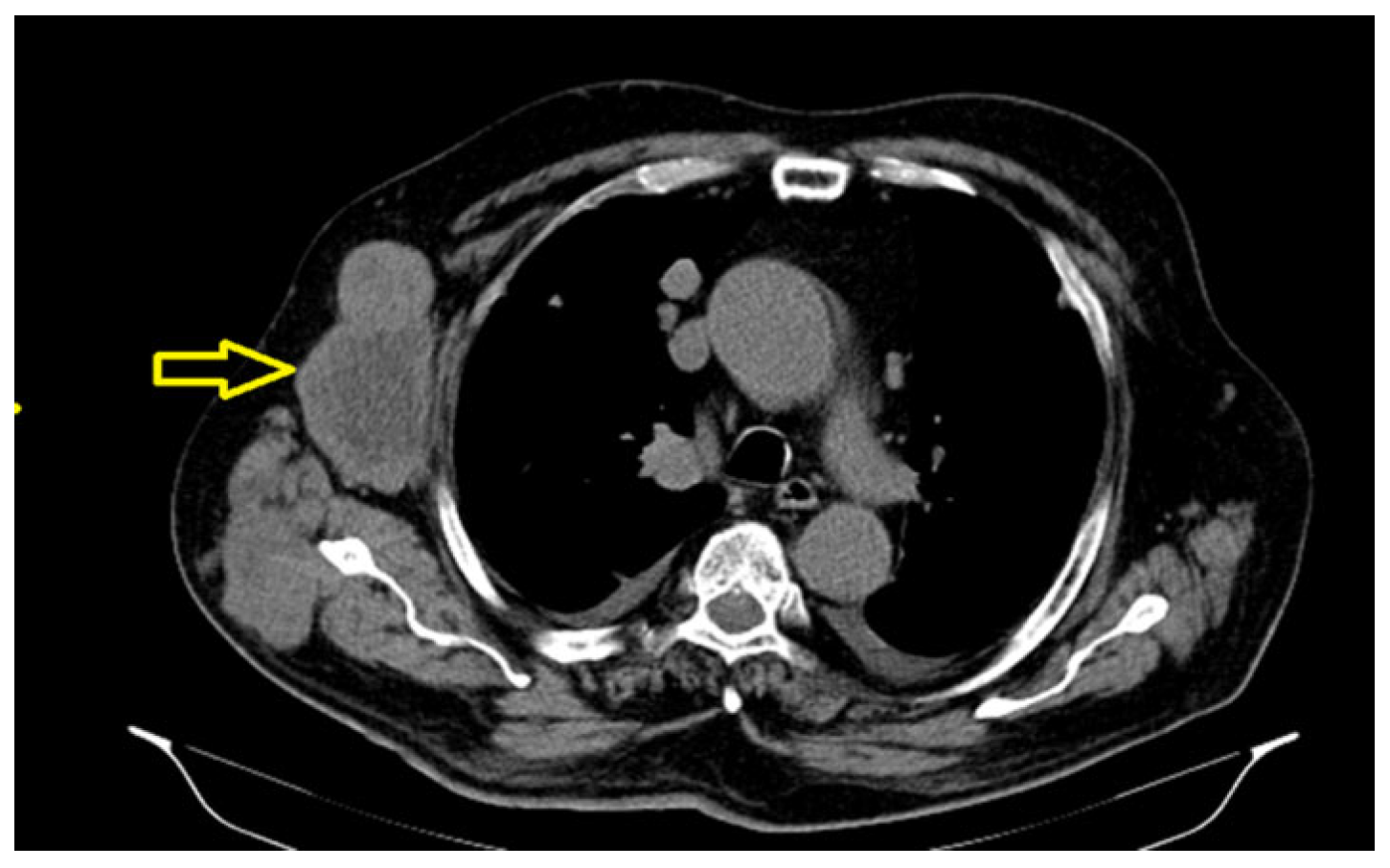
2. Case Presentation
3. Discussion
- Radiation exposure (PRMS being the most frequent histological type of radiation-induced soft-tissue sarcoma);
- Chemical exposure (vinyl chloride, arsenic, phenoxy herbicides, chlorinated dioxins) [16];
- Viruses (human herpesvirus 8 and Epstein–Barr) [17];
- Genetic factors—certain clinical syndromes are associated with a genetic predisposition for the development of sarcoma (e.g., Li–Fraumeni syndrome, Werner syndrome, neurofibromatosis type 1, Garner syndrome, Becwith–Wiedemann syndrome, Costello syndrome) [17].
Limitations and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, S.W.; Goldblum, J.R. (Eds.) Enzinger and Weiss’s Soft Tissue Tumors, 4th ed.; CV Mosby: St. Louis, MO, USA, 2001; pp. 785–835. [Google Scholar]
- Fletcher, C.D.; Gustafson, P.; Rydholm, A.; Willén, H.; Åkerman, M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: Prognostic relevance of subclassification. J. Clin. Oncol. 2001, 19, 3045. [Google Scholar] [CrossRef]
- World Health Organization Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3. [Google Scholar]
- Lawrence, W., Jr.; Donegan, W.L.; Natarajan, N.; Mettlin, C.; Beart, R.; Winchester, D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann. Surg. 1987, 205, 349–359. [Google Scholar] [CrossRef]
- Mariani, L.; Miceli, R.; Kattan, M.W.; Brennan, M.F.; Colecchia, M.; Fiore, M.; Casali, P.G.; Gronchi, A. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005, 103, 402. [Google Scholar] [CrossRef]
- Ferrari, A.; Dileo, P.; Casanova, M.; Bertulli, R.; Meazza, C.; Gandola, L.; Navarria, P.; Collini, P.; Gronchi, A.; Olmi, P.; et al. Rhabdomyosarcoma in adults: A retrospective analysis of 171 patients treated at a single institution. Cancer 2003, 98, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Rubin, B.P.; Baldini, E.H.; Vasudevan, N.; Demetri, G.D.; Fletcher, C.D.; Singer, S. Response to chemotherapy and predictors of survival in adult rhabdomyosarcoma. Ann. Surg. 2001, 234, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Qaddoumi, I.; Yaser, S.; Rodriguez-Galindo, C.; Ferrari, A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2600 patients. J. Clin. Oncol. 2009, 27, 3391. [Google Scholar] [CrossRef]
- Paulin, D.; Li, Z. Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 2004, 301, 1–7. [Google Scholar] [CrossRef]
- Muntoni, F.; Brown, S.; Sewry, C.; Patel, K. Muscle development genes: Their relevance in neuromuscular disorders. Neuromuscul. Disord. 2002, 12, 438–446. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Habeebu, S.; Farooqi, M.S.; Gamis, A.S.; Gonzalez, E.; Flatt, T.; Sherman, A.; Surrey, L.; Arnold, M.A.; Conces, M.; et al. MYOD1 as a prognostic indicator in rhabdomyosarcoma. Pediatr. Blood Cancer 2021, 68, e29085. [Google Scholar] [CrossRef]
- Dinakar, J.; Gowri, S.; Ann Tryphena, E.T. Alveolar type of rhabdomyosarcoma of maxilla—A case report. J. Oral Maxillofac. Pathol. 2023, 27, 406–410. [Google Scholar] [CrossRef]
- Shakya, S.; Banneyake, E.L.; Cholekho, S.; Singh, J.; Zhou, X. Soft tissue sarcoma: Clinical recognition and approach to the loneliest cancer. Explor. Musculoskelet. Dis. 2024, 2, 56–68. [Google Scholar] [CrossRef]
- Al Obaid, I.H. Soft tissue Sarcomas: Immunohistochemistry evaluation by Desmin, Myosin, Smooth muscle Actin and Vimentin. J. Fac. Med. Baghdad 2020, 62, 20–26. [Google Scholar] [CrossRef]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef]
- Kogevinas, M.; Becher, H.; Benn, T.; Bertazzi, P.A.; Boffetta, P.; Bueno-de-Mesqurta, H.B.; Coggon, D.; Colin, D.; Flesch-Janys, D.; Fingerhut, M.; et al. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. Am. J. Epidemiol. 1997, 145, 1061. [Google Scholar] [CrossRef]
- Elizabeth, H.B.; Jason, L.H. Pathogenic Factors in Soft Tissue and Bone Sarcomas. November 2024. Available online: https://www.uptodate.com/contents/pathogenetic-factors-in-soft-tissue-and-bone-sarcomas (accessed on 6 June 2025).
- Liau, J.Y.; Lee, J.C.; Tsai, J.H.; Yang, C.Y.; Liu, T.L.; Ke, Z.L.; Hsu, H.H.; Jeng, Y.M. Comprehensive screening of alternative lengthening of telomeres phenotype and loss of ATRX expression in sarcomas. Mod. Pathol. 2015, 28, 1545. [Google Scholar] [CrossRef]
- De Vita, A.; Vanni, S.; Fausti, V. Deciphering the Genomic Landscape and Pharmacological Profile of Uncommon Entities of Adult Rhabdomyosarcomas. Int. J. Mol. Sci. 2021, 22, 11564. [Google Scholar] [CrossRef]
- De Vita, A.; Ferrari, A.; Miserocchi, G. Identification of a novel RAB3IP-HMGA2 fusion transcript in an adult head and neck rhabdomyosarcoma. Oral. Dis. 2022, 28, 2052–2054. [Google Scholar] [CrossRef]
- Pappo, A.S.; Dirksen, U. Rhabdomyosarcoma, Ewing sarcoma, and other round cell sarcomas. J. Clin. Oncol. 2018, 36, 168. [Google Scholar] [CrossRef]
- Mentzel, T.; Katenkamp, D. Pleomorphic rhabdomyosarcoma in adults: Clinicopathologic and immunohistochemical study of 19 cases. Virchows Arch. 1999, 435, 534–539. [Google Scholar]
- Okcu, F.; Hicks, J.; Horowitz, M. Rhabdomyosarcoma in Childhood and Adolescence: Epidemiology, Pathology and Molecular Pathogenesis. November 2024. Available online: https://www.uptodate.com/contents/rhabdomyosarcoma-in-childhood-and-adolescence-epidemiology-pathology-and-molecular-pathogenesis (accessed on 6 June 2025).
- Billingsley, K.G.; Burt, M.E.; Jara, E.; Ginsberg, R.J.; Woodruff, J.M.; Leung, D.H.; Brennan, M.F. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann. Surg. 1999, 229, 602. [Google Scholar] [CrossRef]
- Sinha, S.; Peach, A.H. Diagnosis and management of soft tissue sarcoma. BMJ 2010, 341, c7170. [Google Scholar] [CrossRef]
- Ryan, C.W.; Meyer, J.; Pollock, R.E. Clinical Presentation, Diagnostic Evaluation and Staging of Soft Tissue Sarcoma. Available online: https://www.uptodate.com/contents/clinical-presentation-diagnostic-evaluation-and-staging-of-soft-tissue-sarcoma (accessed on 6 June 2025).
- Weigel, B.J.; Lyden, E.; Anderson, J.R.; Meyer, W.H.; Parham, D.M.; Rodeberg, D.A.; Michalski, J.M.; Hawkins, D.S.; Arndt, C.A. Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients with High-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2016, 34, 117. [Google Scholar] [CrossRef]
- Noria, S.; Davis, A.; Kandel, R.; Levesque, J. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J. Bone Jt. Surg. Am. 1996, 78, 650. [Google Scholar] [CrossRef]
- Okcu, M.F.; Pappo, A.S.; Savarese, D.M.F. Rhabdomyosarcoma in Childhood and Adolescence and Adulthood: Treatment. Available online: https://www.uptodate.com/contents/rhabdomyosarcoma-in-childhood-adolescence-and-adulthood-treatment (accessed on 6 June 2025).
- Salerno, K.E.; Alektiar, K.M.; Baldini, E.H.; Bedi, M.; Bishop, A.J.; Bradfield, L.; Chung, P.; DeLaney, T.F.; Folpe, A.; Kane, J.M.; et al. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 339. [Google Scholar] [CrossRef]
- Maki, R. Adjuvant and Neoadjuvant Chemotherapy for Soft Tissue Sarcoma of the Extremities. Available online: https://www.uptodate.com/contents/adjuvant-and-neoadjuvant-systemic-therapy-for-soft-tissue-sarcoma-of-the-extremities (accessed on 6 June 2025).
- Mowery, Y.M.; Ballman, K.V.; Hong, A.M.; Schuetze, S.M.; Wagner, A.J.; Monga, V.; Heise, R.S.; Attia, S.; Choy, E.; Burgess, M.A.; et al. Safety and efficacy of pembrolizumab, radiation therapy, and surgery versus radiation therapy and surgery for stage III soft tissue sarcoma of the extremity (SU2C-SARC032): An open-label, randomised clinical trial. Lancet 2024, 404, 2053. [Google Scholar] [CrossRef]
- Drabbe, C.; Benson, C.; Younger, E.; Zaidi, S.; Jones, R.L.; Judson, I.; Chisholm, J.; Mandeville, H.; Fisher, C.; Thway, K.; et al. Embryonal and alveolar rhabdomyosarcoma in adults: Real-life data from a tertiary sarcoma centre. Clin. Oncol. 2020, 32, e27–e35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, B.; Mirică, R.E. Adult Pleomorphic Rhabdomyosarcoma: Case Report. Reports 2025, 8, 166. https://doi.org/10.3390/reports8030166
Oancea B, Mirică RE. Adult Pleomorphic Rhabdomyosarcoma: Case Report. Reports. 2025; 8(3):166. https://doi.org/10.3390/reports8030166
Chicago/Turabian StyleOancea, Beatrice, and Roxana Elena Mirică. 2025. "Adult Pleomorphic Rhabdomyosarcoma: Case Report" Reports 8, no. 3: 166. https://doi.org/10.3390/reports8030166
APA StyleOancea, B., & Mirică, R. E. (2025). Adult Pleomorphic Rhabdomyosarcoma: Case Report. Reports, 8(3), 166. https://doi.org/10.3390/reports8030166





