A Case Report of Tissue Mosaicism in 45,X0/46,XY: Diagnostic Complexity in a Newborn with Ambiguous Genitalia
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
- 1.1. Ten cells (50%): karyotype 45,X;
- 1.2. Six cells (30%): karyotype 46,XY with the presence of a translocation of a fragment of the short arm of the Y chromosome (Yp11.3) to another autosomal chromosome;
- 1.3. Four cells (20%): karyotype containing an unidentified chromosomal marker (mar), not included in the classical nomenclature.
- 2.1. A total of 46% of cells contained only a signal for the X centromere;
- 2.2. A total of 48% of cells contained signals for both X and Y, with the presence of the SRY gene;
- 2.3. A total of 6% of cells showed the presence of a signal for a marker chromosome with undetermined SRY status.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vining, B.; Ming, Z.; Bagheri-Fam, S.; Harley, V. Diverse regulation but conserved function: Sox9 in vertebrate sex determination. Genes 2021, 12, 486. [Google Scholar] [CrossRef]
- Lashkari, F.M.; Gilani, M.A.S.; Ghaheri, A.; Zamanian, M.R.; Boroujeni, P.B.; Meybodi, A.M.; Sabbaghian, M. Clinical aspects of 49 infertile males with 45,X/46,XY mosaicism karyotype: A case series. Andrologia 2018, 50, e13009. [Google Scholar] [CrossRef]
- Pan, L.; Su, Z.; Song, J.; Xu, W.; Liu, X.; Zhang, L.; Li, S. Growth data and tumour risk of 32 Chinese children and adolescents with 45,X/46,XY mosaicism. BMC Pediatr. 2019, 19, 143. [Google Scholar] [CrossRef]
- Becker, R.E.N.; Akhavan, A. Prophylactic Bilateral Gonadectomy for Ovotesticular Disorder of Sex Development in a Patient With Mosaic 45,X/46,X,idic(Y)q11.222 Karyotype. Urol. Case Rep. 2016, 5, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Fernández, J.; González-Peramato, P.; Estévez, A.R.; Villar, M.J.A.; Parera, L.A.; Julián, M.C.A.S.; Urquí, A.C.; González, L.A.C.; Tello, J.M.M.; Palma, C.M.; et al. Consensus guide on prophylactic gonadectomy in different sex development. Endocrinol. Diabetes Nutr. 2022, 69, 629–645. [Google Scholar] [CrossRef]
- Sandberg, D.E.; Gardner, M. Differences/Disorders of Sex Development: Medical Conditions at the Intersection of Sex and Gender. Annu. Rev. Clin. Psychol. 2022, 18, 201–231. [Google Scholar] [CrossRef]
- Ngun, T.C.; Ghahramani, N.; Sánchez, F.J.; Bocklandt, S.; Vilain, E. The genetics of sex differences in brain and behavior. Front. Neuroendocr. 2011, 32, 227–246. [Google Scholar] [CrossRef]
- Barbosa, L.G.; Siviero-Miachon, A.A.; Souza, M.A.; Spinola-Castro, A.M. Recognition of the Y chromosome in Turner syndrome using peripheral blood or oral mucosa tissue. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Canto, P.; Kofman-Alfaro, S.; Jiménez, A.L.; Söderlund, D.; Barrón, C.; Reyes, E.; Méndez, J.P.; Zenteno, J.C. Gonadoblastoma in Turner syndrome patients with nonmosaic 45,X karyotype and Y chromosome sequences. Cancer Genet. Cytogenet. 2004, 150, 70–72. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef]
- Leng, X.F.; Lei, K.; Li, Y.; Tian, F.; Yao, Q.; Zheng, Q.-M.; Chen, Z.-H. Gonadal dysgenesis in Turner syndrome with Y-chromosome mosaicism: Two case reports. World J. Clin. Cases 2020, 8, 5737–5743. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.A.; Shetty, D.P.; Kulshreshtha, P.S.; Kadandale, J.S. Mixed Gonadal Dysgenesis with 45,X/46,X,idic(Y)/46,XY Karyotype: A Case Report. J. Reprod. Infertil. 2024, 25, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Shinawi, M.; Cain, M.P.; VanderBrink, B.A.; Grignon, D.J.; Mensing, D.; Cooper, M.L.; Bader, P.; Cheung, S.W. Mixed gonadal dysgenesis in a child with isodicentric Y chromosome: Does the relative proportion of the 45,X line really matter? Am. J. Med. Genet. A 2010, 152, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Makroo, R.N.; Chowdhry, M.; Mishra, M.; Fauzdar, A. Mixed gonadal dysgenesis with 45,X/46,X,idic(Y) karyotype: A case report. Indian J. Pathol. Microbiol. 2011, 54, 655–657. [Google Scholar] [CrossRef]
- Josso, N.; Rey, R.A.; Picard, J.Y. Anti-Müllerian hormone: A valuable addition to the toolbox of the pediatric endocrinologist. Int. J. Endocrinol. 2013, 2013, 674105. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, M.; Lau, Y.F.C. The sex-determining factors SRY and SOX9 regulate similar target genes and promote testis cord formation during testicular differentiation. Cell Rep. 2014, 8, 723–733. [Google Scholar] [CrossRef]
- Lamanna, B.; Vinciguerra, M.; Dellino, M.; Cascella, G.; Cazzato, G.; Macorano, E.; Malvasi, A.; Scacco, S.; Cicinelli, E.; Loizzi, V.; et al. Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case. Int. J. Mol. Sci. 2022, 23, 14408. [Google Scholar] [CrossRef]
- Weidler, E.M.; Pearson, M.; van Leeuwen, K.; Garvey, E. Clinical management in mixed gonadal dysgenesis with chromosomal mosaicism: Considerations in newborns and adolescents. Semin. Pediatr. Surg. 2019, 28, 150841. [Google Scholar] [CrossRef]
- Fraccascia, B.; Sodero, G.; Pane, L.C.; Malavolta, E.; Gola, C.; Pane, L.; Paradiso, V.F.; Nanni, L.; Rigante, D.; Cipolla, C. Complete Androgen Insensitivity Syndrome in a Young Girl with Primary Amenorrhea and Suspected Delayed Puberty: A Case-Based Review of Clinical Management, Surgical Follow-Up, and Oncological Risk. Diseases. 2024, 12, 235. [Google Scholar] [CrossRef]
- Guzewicz, L.; Howell, S.; Crerand, C.E.; Umbaugh, H.; Nokoff, N.J.; Barker, J.; Davis, S.M. Clinical phenotype and management of individuals with mosaic monosomy X with Y chromosome material stratified by genital phenotype. Am. J. Med. Genet. A 2021, 185, 1437–1447. [Google Scholar] [CrossRef]
- Forger, N.G. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150114. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Is sexual differentiation of brain and behavior epigenetic? Curr. Opin. Behav. Sci. 2019, 25, 83–88. [Google Scholar] [CrossRef]
- Kundakovic, M.; Tickerhoof, M. Epigenetic mechanisms underlying sex differences in the brain and behavior. Trends Neurosci. 2024, 47, 18–35. [Google Scholar] [CrossRef]
- Swaab, D.F. Sexual differentiation of the human brain: Relevance for gender identity, transsexualism and sexual orientation. Gynecol. Endocrinol. 2004, 19, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, F.J.; Burgos, M.; Scherer, G.; Jiménez, R. Genes promoting and disturbing testis development. Cell. Mol. Biol. 2012, 27, 1361–1383. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Brain sexual differentiation and requirement of SRY: Why or Why Not? Front. Neurosci. 2017, 11, 632. [Google Scholar] [CrossRef]
- Markham, J.A.; Jurgens, H.A.; Auger, C.J.; De Vries, G.J.; Arnold, A.P.; Juraska, J.M. Sex differences in mouse cortical thickness are independent of the complement of sex chromosomes. Neuroscience 2003, 116, 71–75. [Google Scholar] [CrossRef]
- Berenbaum, S.A.; Beltz, A.M. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Front. Neuroendocr. 2011, 32, 183–200. [Google Scholar] [CrossRef]
- McCarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2008, 88, 91–134. [Google Scholar] [CrossRef]
- Schwarz, J.M.; McCarthy, M.M. Cellular mechanisms of estradiol-mediated masculinization of the brain. J. Steroid Biochem. Mol. Biol. 2008, 109, 300–306. [Google Scholar] [CrossRef]
- Todd, B.J.; Schwarz, J.M.; McCarthy, M.M. Prostaglandin-E2: A point of divergence in estradiol-mediated sexual differentiation. Horm. Behav. 2005, 48, 512–521. [Google Scholar] [CrossRef]
- Liu, P.; Meng, X.-H.; Wang, H.; Ji, Y.-L.; Zhao, M.; Zhao, X.-F.; Xu, Z.-M.; Chen, Y.-H.; Zhang, C.; Xu, D.-X. Effects of pubertal fenvalerate exposure on testosterone and estradiol synthesis and the expression of androgen and estrogen receptors in the developing brain. Toxicol. Lett. 2011, 201, 181–189. [Google Scholar] [CrossRef]
- Hughes, I.A.; Houk, C.; Ahmed, S.F.; Lee, P.A. Consensus statement on management of intersex disorders. J. Pediatr. Urol. 2006, 2, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.A. Shifting syndromes: Sex chromosome variations and intersex classifications. Soc. Stud. Sci. 2018, 48, 125–148. [Google Scholar] [CrossRef]
- Ratnu, V.S.; Emami, M.R.; Bredy, T.W. Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J. Neurosci. Res. 2017, 95, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Délot, E.C.; Papp, J.C.; Sandberg, D.E.; Vilain, E.; Fox, M.; Grody, W.; Lee, H.; Keegan, C.; Ramsdell, L.; Green, J.; et al. Genetics of Disorders of Sex Development: The DSD-TRN Experience. Metab. Clin. N. Am. 2017, 46, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Shah, U. Gender identity disorder (GID) in adolescents and adults with differences of sex development (DSD): A systematic review and meta-analysis. J. Pediatr. Urol. 2021, 17, 39–47. [Google Scholar] [CrossRef]
- Baetens, L.; Dhondt, K. Psychosocial challenges and hormonal treatment in gender diverse children and adolescents. A narrative review. Int. J. Impot. Res. 2021, 33, 217–227. [Google Scholar] [CrossRef]
- Skordis, N.; Kyriakou, A.; Dror, S.; Mushailov, A.; Nicolaides, N.C. Gender dysphoria in children and adolescents: An overview. Hormones 2020, 19, 267–276. [Google Scholar] [CrossRef]

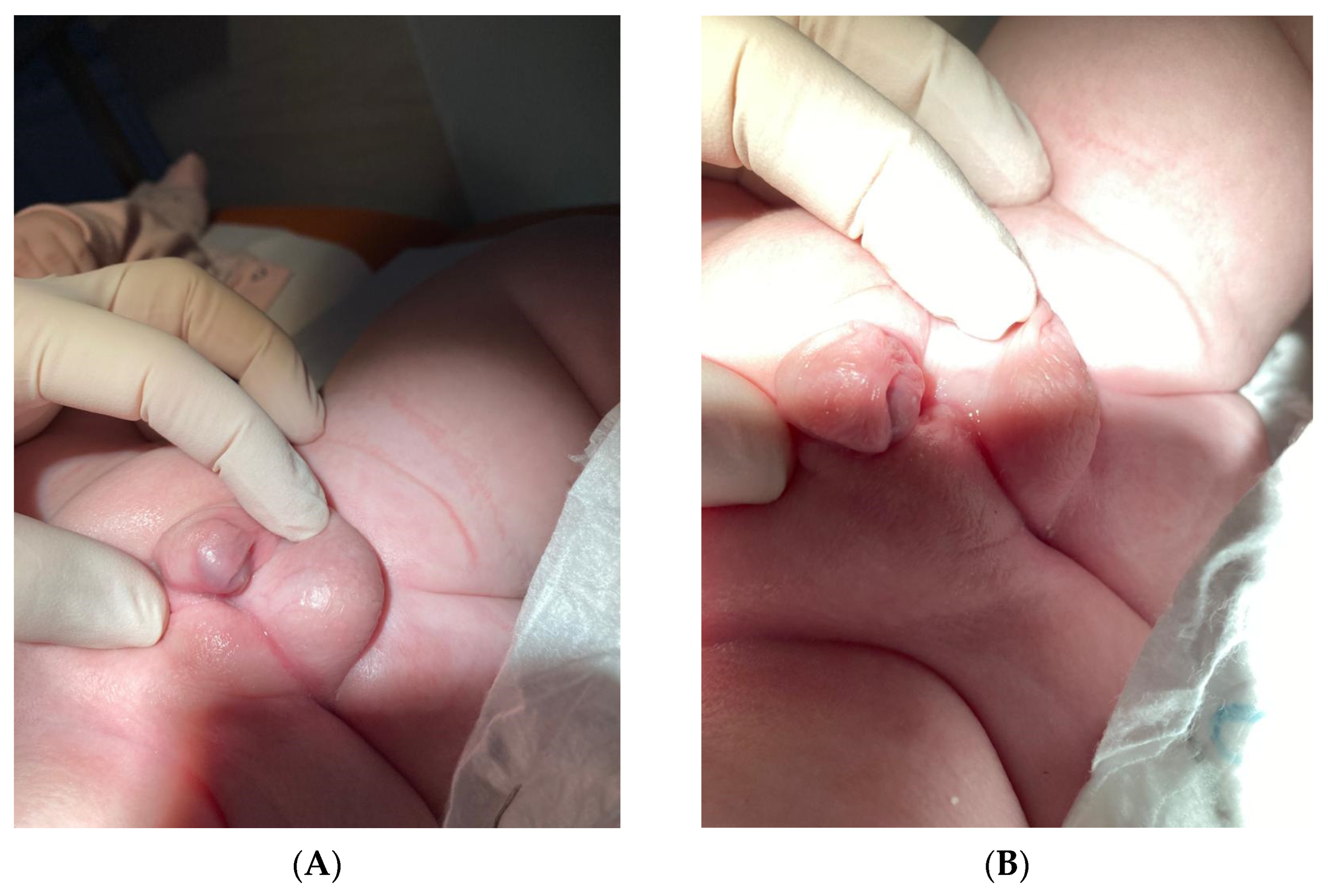

| (A) | |||
| Group | Subcategory | Examples | Key Features/Cause |
| Disorders of Gonadal Development | - Gonadal dysgenesis (complete/partial) - Ovotesticular DSD | - Swyer syndrome (46,XY complete gonadal dysgenesis) - 46,XX/XY ovotesticular DSD | Defects in genes regulating early gonad formation (e.g., SRY, NR5A1, DAX1) |
| Disorders of Androgen Synthesis or Action | - Defects in testosterone or DHT production - Androgen insensitivity syndromes | - 5α-reductase deficiency - Complete/partial AIS | Normal gonads (testes) but impaired masculinization of external genitalia |
| Disorders of Gonadal Hormone Excess | - Androgen excess in XX individuals - Aromatase deficiency | - Congenital adrenal hyperplasia (CAH) - Maternal virilization | Excessive androgen action during fetal development |
| Syndromic and Complex DSD | - Multisystem syndromes involving sex development | - Campomelic dysplasia (SOX9 mutation) - Denys–Drash syndrome (WT1) | DSD is part of broader genetic syndrome affecting multiple organs |
| Chromosomal Mosaicism or Chimerism | - Sex chromosome mosaicism or mixoploidy | 45,X/46,XY mosaicism 46,XX/46,XY chimerism | Discordant development due to mixed cell lines; variable phenotype |
| Unclassified/VUS (Variant of Uncertain Significance) | - Unknown or novel genetic cause | - Gene panel detects VUS - No clear correlation yet | Requires further research and individualized assessment |
| (B) | |||
| Karyotype | Category | Example Conditions | Key Features |
| 46,XX | XX DSD (ovarian) | - Congenital adrenal hyperplasia (CAH) | - Typical ovaries - Androgen excess may cause virilization |
| 46,XY | XY DSD (testicular) | - Androgen insensitivity syndrome (AIS) - 5α-reductase deficiency | - Gonads are testes - Variable external genitalia - Often undervirilization |
| Sex Chromosome DSD | Sex chromosome anomalies | - Turner syndrome (45,X) - Klinefelter syndrome (47,XXY) - Mixed gonadal dysgenesis | - Gonadal dysgenesis - Atypical development of genitalia or secondary sex traits |
| Parameters | Value |
|---|---|
| Testosterone | 1.21 ng/mL |
| FSH | 2.1 IU/L |
| LH | 4.3 IU/L |
| AMH | 14.6 ng/mL |
| Estradiol | <5 pg/mL |
| 17-OH-Progesterone | 36 ng/dL |
| Parameters | Result |
|---|---|
| TSH | 28–33 uIU/mL |
| Free T4 | 1.1 ng/dL |
| Free T3 | 3.0 pg/mL |
| Thyroid ultrasound | Hypoechogenic, heterogenous gland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyścin, M.; Brodowska, A.; Pietrzyk, D.; Zając, K.; Sowińska-Przepiera, E. A Case Report of Tissue Mosaicism in 45,X0/46,XY: Diagnostic Complexity in a Newborn with Ambiguous Genitalia. Reports 2025, 8, 146. https://doi.org/10.3390/reports8030146
Krzyścin M, Brodowska A, Pietrzyk D, Zając K, Sowińska-Przepiera E. A Case Report of Tissue Mosaicism in 45,X0/46,XY: Diagnostic Complexity in a Newborn with Ambiguous Genitalia. Reports. 2025; 8(3):146. https://doi.org/10.3390/reports8030146
Chicago/Turabian StyleKrzyścin, Mariola, Agnieszka Brodowska, Dominika Pietrzyk, Katarzyna Zając, and Elżbieta Sowińska-Przepiera. 2025. "A Case Report of Tissue Mosaicism in 45,X0/46,XY: Diagnostic Complexity in a Newborn with Ambiguous Genitalia" Reports 8, no. 3: 146. https://doi.org/10.3390/reports8030146
APA StyleKrzyścin, M., Brodowska, A., Pietrzyk, D., Zając, K., & Sowińska-Przepiera, E. (2025). A Case Report of Tissue Mosaicism in 45,X0/46,XY: Diagnostic Complexity in a Newborn with Ambiguous Genitalia. Reports, 8(3), 146. https://doi.org/10.3390/reports8030146






