Percutaneous Peripheral Nerve Stimulation in Chemotherapy-Induced Neuropathy: A Case Report
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Patient Information
2.2. Clinical Findings
2.3. Patient Evolution
2.4. Diagnostic Assessment
2.5. Therapeutic Intervention
2.6. Study Variables and Follow-Up
2.7. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIPN | Chemotherapy-induced peripheral neuropathy |
| FDA | Food and Drug Administration |
| PENS | Percutaneous peripheral nerve stimulation |
| EORTC QLQ-CIPN20 | European Organization for Research and Treatment of Cancer questionnaire for evaluating CIPN |
| QST | Quantitative sensory testing |
| MDT | Mechanical detection thresholds |
| VT | Vibration thresholds |
| TDT | Temperature detection thresholds |
| CDT | Cold detection test |
| WDT | Heat detection test |
References
- Hu, L.Y.; Mi, W.L.; Wu, G.-C.; Wang, Y.Q.; Mao-Ying, Q.L. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef]
- Hwang, M.S.; Lee, H.Y.; Lee, J.H.; Choi, T.Y.; Lee, J.H.; Ko, Y.S.; Choi, S.Y.; Park, T.Y. Protocol for a systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine 2019, 98, e15098. [Google Scholar] [CrossRef]
- Hershman, D.L.; Weimer, L.H.; Wang, A.; Kranwinkel, G.; Brafman, L.; Fuentes, D.; Awad, D.; Crew, K.D. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res. Treat. 2011, 125, 767–774. [Google Scholar] [CrossRef]
- Tofthagen, C. Surviving chemotherapy for colon cancer and living with the consequences. J. Palliat. Med. 2010, 13, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Buratti, T.; Golaszewski, S.; Bratti, A.; Caleri, F.; Tezzon, F.; Ladurner, G.; Mitterer, M. Delayed oxaliplatin-induced sensorimotor polyneuropathy. Onkologie 2009, 32, 10. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; Alam, U. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Saini, C.; Hussain, N.; Javed, S.; Prokop, L.; Her, Y.F. Global estimates of prevalence of chronic painful neuropathy among patients with chemotherapy-induced peripheral neuropathy: Systematic review and meta-analysis of data from 28 countries, 2000–2024. Reg. Anesth. Pain Med. 2025. [Google Scholar] [CrossRef]
- Hammond, E.A.; Pitz, M.; Steinfeld, K.; Lambert, P.; Shay, B. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabilit. Neural Repair. 2020, 34, 235–246. [Google Scholar] [CrossRef]
- Kim, J.H.; Dougherty, P.M.; Abdi, S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol. Oncol. 2015, 136, 453–459. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Bae, E.H.; Greenwald, M.K.; Schwartz, A.G. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics 2021, 18, 2384–2396. [Google Scholar] [CrossRef]
- Ristić, D.; Spangenberg, P.; Ellrich, J. Analgesic and antinociceptive effects of peripheral nerve neurostimulation in an advanced human experimental model. Eur. J. Pain 2008, 12, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Kupers, R.; Laere, K.V.; Calenbergh, F.V.; Gybels, J.; Dupont, P.; Baeck, A.; Plaghki, L. Multimodal therapeutic assessment of peripheral nerve stimulation in neuropathic pain: Five case reports with a 20-year follow-up. Eur. J. Pain 2011, 15, 161.e1–161.e9. [Google Scholar] [PubMed]
- D’souza, R.S.; Her, Y.F.; Jin, M.Y.; Morsi, M.; Abd-Elsayed, A. Neuromodulation Therapy for Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review. Biomedicines 2022, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Ghoname, E.-S.A.; White, P.F.; Ahmed, H.E.; Hamza, M.A.; Craig, W.F.; Noe, C.E. Percutaneous electrical nerve stimulation: An alternative to TENS in the management of sciatica. Pain 1999, 83, 193–199. [Google Scholar] [CrossRef]
- Hamza, M.A.; White, P.F.; Craig, W.F.; Ghoname, E.S.; Ahmed, H.E.; Proctor, T.J.; Noe, C.E.; Vakharia, A.S.; Gajraj, N. Percutaneous electrical nerve stimulation: A novel analgesic therapy for diabetic neuropathic pain. Diabetes Care 2000, 23, 365–370. [Google Scholar] [CrossRef]
- Raphael, J.H.; Raheem, T.A.; Southall, J.L.; Bennett, A.; Ashford, R.L.; Williams, S. Randomized double-blind sham-controlled crossover study of short-term effect of percutaneous electrical nerve stimulation in neuropathic pain. Pain Med. 2011, 12, 1515–1522. [Google Scholar] [CrossRef]
- Gilmore, C.; Ilfeld, B.; Rosenow, J.; Li, S.; Desai, M.; Hunter, C.; Rauck, R.; Kapural, L.; Nader, A.; Mak, J.; et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 44, 637–645. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Lu, C.; Bao, W.; Deng, D.; Li, R.; Li, G.; Zou, S.; Wang, Y. Efficacy of electroacupuncture with different frequencies in the treatment of chemotherapy-induced peripheral neuropathy: A study protocol for a randomized controlled trial. Front. Neurol. 2022, 13, 843886. [Google Scholar] [CrossRef]
- Li, R.; Lu, J.; Wang, M.; Zhang, P.; Fang, H.; Yang, K.; Wang, L.; Zhuang, J.; Tian, Z.; Yang, J.; et al. Ultrasound-Guided Median Nerve Electrical Stimulation to Promote Upper Limb Function Recovery after Stroke. Evid. Based Complement. Altern. Med. 2022, 2022, 3590057. [Google Scholar] [CrossRef]
- Chu, X.L.; Song, X.Z.; Li, Y.R.; Wu, Z.R.; Li, Q.; Li, Q.W.; Gu, X.S.; Ming, D. An ultrasound-guided percutaneous electrical nerve stimulation regimen devised using finite element modeling promotes functional recovery after median nerve transection. Neural Regen. Res. 2022, 18, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Huh, B.; Kim, H.K.; Kim, K.-H.; Abdi, S. Treatment of Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Recommendations. Pain Physician 2018, 21, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Bruna, J. Chemotherapy-induced peripheral neuropathy: An unresolved issue. Neurologia 2010, 25, 116–131. [Google Scholar] [CrossRef]
- Weaver, K.R.; Griffioen, M.A.; Klinedinst, N.J.; Galik, E.; Duarte, A.C.; Colloca, L.; Resnick, B.; Dorsey, S.G.; Renn, C.L. Quantitative Sensory Testing Across Chronic Pain Conditions and Use in Special Populations. Front. Pain Res. 2022, 2, 779068. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77. [Google Scholar] [CrossRef]
- Zhi, W.I.; Baser, R.E.; Kwon, A.; Chen, C.; Li, S.Q.; Piulson, L.; Seluzicki, C.; Panageas, K.S.; Harte, S.E.; Mao, J.J.; et al. Characterization of chemotherapy-induced peripheral neuropathy using patient-reported outcomes and quantitative sensory testing. Breast Cancer Res. Treat. 2021, 186, 761–768. [Google Scholar] [CrossRef]
- Cata, J.P.; Cordella, J.V.; Burton, A.W.; Hassenbusch, S.J.; Weng, H.R.; Dougherty, P.M. Spinal cord stimulation relieves chemotherapy-induced pain: A clinical case report. J. Pain Symptom Manag. 2004, 27, 72–78. [Google Scholar] [CrossRef]
- Li, T.; Mizrahi, D.; Goldstein, D.; Kiernan, M.C.; Park, S.B. Chemotherapy and peripheral neuropathy. Neurol. Sci. 2021, 42, 4109–4121. [Google Scholar] [CrossRef]
- San-Emeterio-Iglesias, R.; Minaya-Muñoz, F.; Romero-Morales, C.; De-La-Cruz-Torres, B. Correct Sciatic Nerve Management to Apply Ultrasound-Guided Percutaneous Neuromodulation in Patients with Chronic Low Back Pain: A Pilot Study. Neuromodulation 2021, 24, 1067–1074. [Google Scholar] [CrossRef]
- Xin, Y.Y.; Wang, J.X.; Xu, A.J. Electroacupuncture ameliorates neuroinflammation in animal models. Acupunct. Med. 2022, 40, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ellrich, J.; Lamp, S. Peripheral nerve stimulation inhibits nociceptive processing: An electrophysiological study in healthy volunteers. Neuromodulation 2005, 8, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Pouratian, N. Brain imaging correlates of peripheral nerve stimulation. Surg. Neurol. Int. 2012, 3, 260–268. [Google Scholar] [CrossRef]
- Lai, M.-I.; Pan, L.-L.; Tsai, M.-W.; Shih, Y.-F.; Wei, S.-H.; Chou, L.-W. Investigating the Effects of Peripheral Electrical Stimulation on Corticomuscular Functional Connectivity Stroke Survivors. Top. Stroke Rehabil. 2016, 23, 154–162. [Google Scholar] [CrossRef]
- Chen, X.H.; Han, J.S. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: Another cross-tolerance study. Behav. Brain Res. 1992, 47, 143–149. [Google Scholar] [CrossRef]
- Ghoname, E.S.; Craig, W.F.; White, P.F.; Ahmed, H.E.; Hamza, M.A.; Gajraj, N.M.; Vakharia, A.S.; Noe, C.E. The effect of stimulus frequency on the analgesic response to percutaneous electrical nerve stimulation in patients with chronic low back pain. Anesth. Analg. 1999, 88, 841–846. [Google Scholar]
- Ahmed, S.; Haddad, C.; Subramaniam, S.; Khattab, S.; Kumbhare, D. The Effect of Electric Stimulation Techniques on Pain and Tenderness at the Myofascial Trigger Point: A Systematic Review. Pain Med. 2019, 20, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
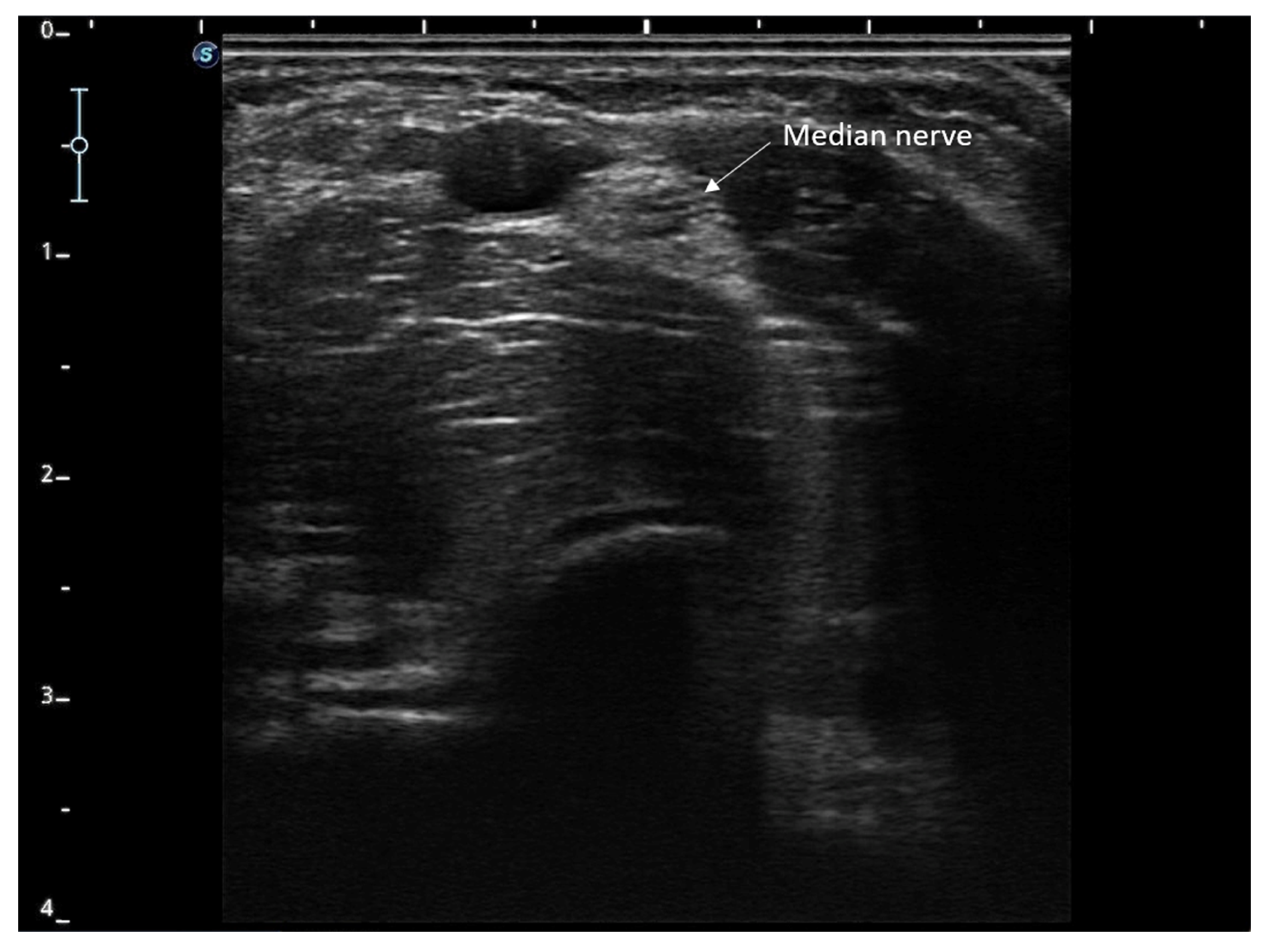
| Variable | Baseline | Week 4 | Week 12 | Change (Baseline–Week 12) | Percentage Change |
|---|---|---|---|---|---|
| Tingling (0–4) | 3/4 | 3/4 | 2/4 | −1 | −33% |
| Cramps (0–4) | 2/4 | 2/4 | 2/4 | 0 | 0% |
| Stabbing pain (0–4) | 3/4 | 0/4 | 0/4 | −3 | −100% |
| Functional difficulties (0–4) | 2/4 | 0/4 | 0/4 | −2 | −100% |
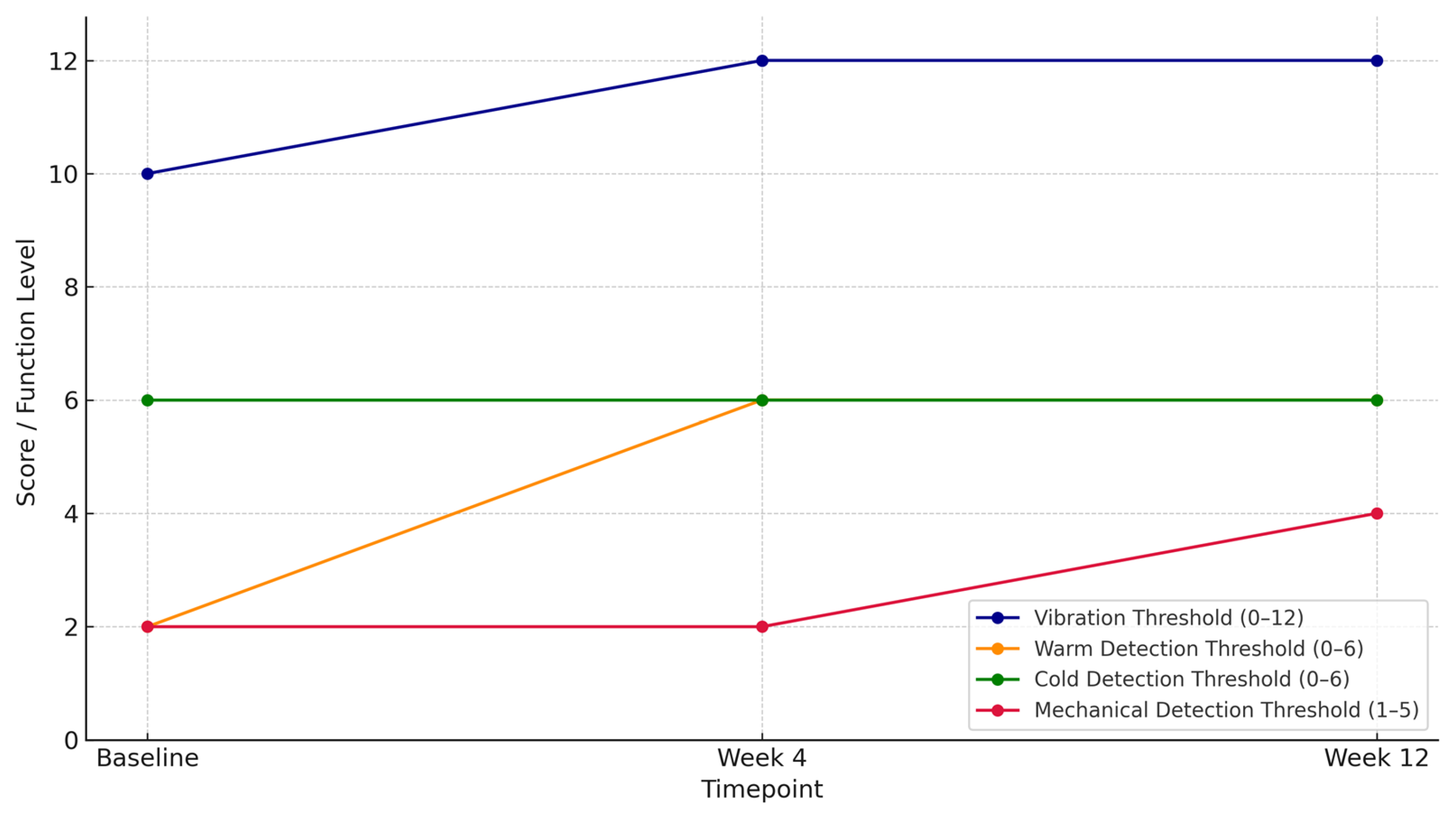
| Vibration Thresholds (0–12) | 10/12 | 12/12 | 12/12 | +2 | +20% |
| Warm Detection Thresholds (0–6) | 2/6 | 6/6 | 6/6 | +4 | +67% |
| Cold Detection Thresholds (0–6) | 6/6 | 6/6 | 6/6 | 0 | 0% |
| Mechanical Detection Thresholds | Decreased protective sensation | No change | Reduced light-touch sensitivity | Partial recovery | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogedano-Cruz, S.; Romero-Morales, C.; de la Cueva-Reguera, M.; Campbell, K.L.; Herrero, P. Percutaneous Peripheral Nerve Stimulation in Chemotherapy-Induced Neuropathy: A Case Report. Reports 2025, 8, 133. https://doi.org/10.3390/reports8030133
Mogedano-Cruz S, Romero-Morales C, de la Cueva-Reguera M, Campbell KL, Herrero P. Percutaneous Peripheral Nerve Stimulation in Chemotherapy-Induced Neuropathy: A Case Report. Reports. 2025; 8(3):133. https://doi.org/10.3390/reports8030133
Chicago/Turabian StyleMogedano-Cruz, Sara, Carlos Romero-Morales, Mónica de la Cueva-Reguera, Kristin L. Campbell, and Pablo Herrero. 2025. "Percutaneous Peripheral Nerve Stimulation in Chemotherapy-Induced Neuropathy: A Case Report" Reports 8, no. 3: 133. https://doi.org/10.3390/reports8030133
APA StyleMogedano-Cruz, S., Romero-Morales, C., de la Cueva-Reguera, M., Campbell, K. L., & Herrero, P. (2025). Percutaneous Peripheral Nerve Stimulation in Chemotherapy-Induced Neuropathy: A Case Report. Reports, 8(3), 133. https://doi.org/10.3390/reports8030133








