A 91-Year-Old Female with Recurring Coma Due to Atypical Hyperammonemia
Abstract
1. Introduction
2. Case Presentation
Diagnostic Workup
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| CT | Computertomography |
| EEG | Electroencephalogram |
| Emergency department | ED |
References
- Skipina, T.; Macbeth, S.; Cummer, E.; Wells, O.; Kalathoor, S. Recurrent noncirrhotic hyperammonemia causing acute metabolic encephalopathy in a patient with a continent ileocecal pouch: A case report. J. Med. Case Rep. 2021, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, S.; Hojer, J.; Enander, C.; Ludwigs, U. Coma and impaired consciousness in the emergency room: Characteristics of poisoning versus other causes. Emerg. Med. J. 2009, 26, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E. The comatose patient. N. Engl. J. Med. 2016, 375, 2560–2570. [Google Scholar] [CrossRef]
- Thill, C.; Huynh-Moynot, S.; Giacardi, C.; Zandotti, C. Diagnosis of reversible causes of coma. Lancet 2015, 385, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J. Gastroenterol. 2013, 19, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Bleck, T.; Busl, K. Hyperammonemia: What urea-lly need to know: Case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Rep. Med. 2016, 2016, 8512721. [Google Scholar] [CrossRef] [PubMed]
- Flamm, S. Rifaximin treatment for reduction of risk of overt hepatic encephalopathy recurrence. Ther. Adv. Gastroenterol. 2011, 4, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Fiati Kenston, S.; Song, X.; Li, Z.; Zhao, J. Mechanistic insight, diagnosis, and treatment of ammonia-induced hepatic encephalopathy. J. Gastroenterol. Hepatol. 2019, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, J.; Jung, C.; Park, J.; Seo, J.; Kwon, D. Etiologies of altered level of consciousness in the emergency room. Sci. Rep. 2022, 12, 4972. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.; Rabinstein, A.; Traub, S.; Wijdicks, E. Diagnosis of reversible causes of coma. Lancet 2014, 384, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
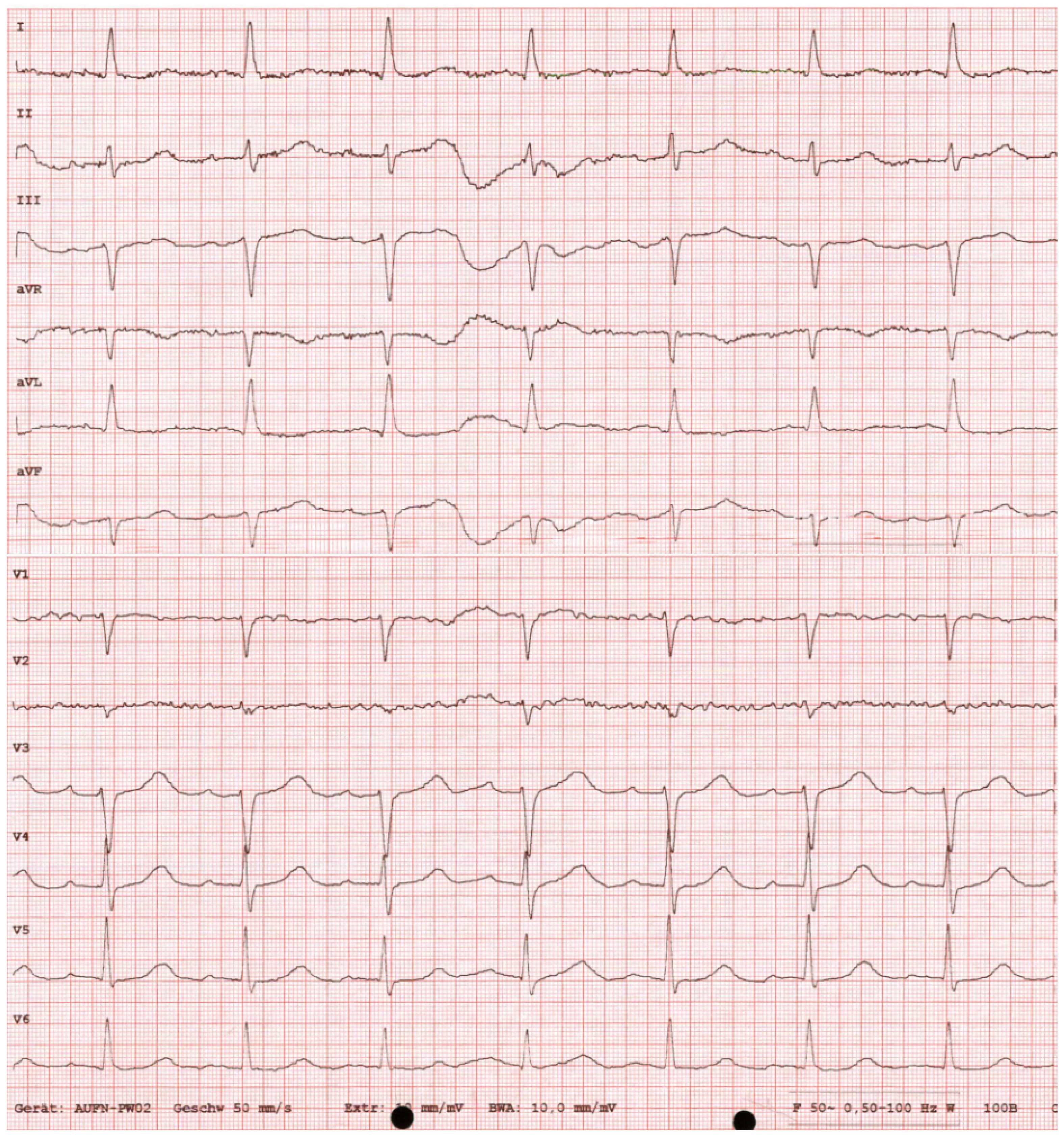
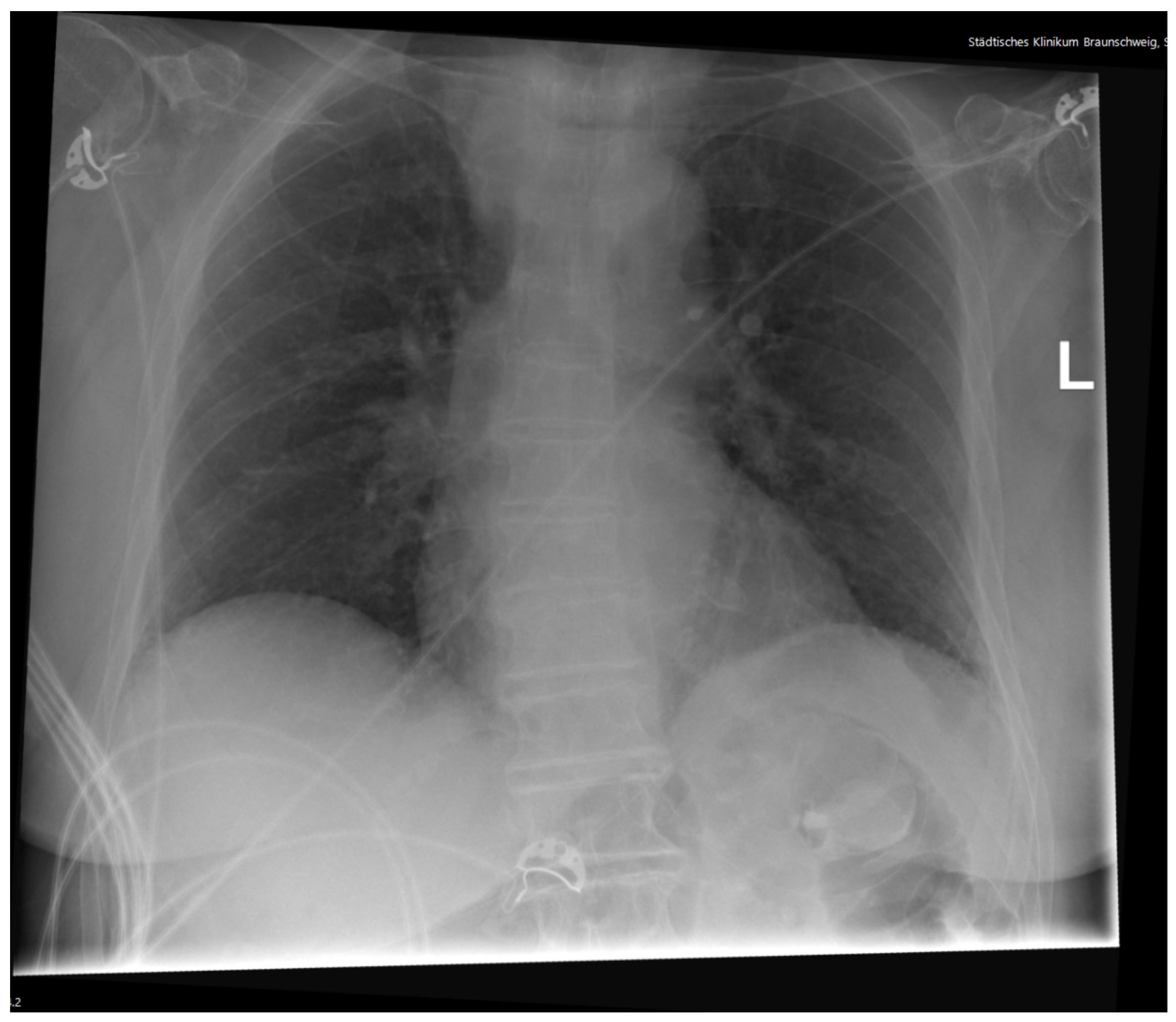
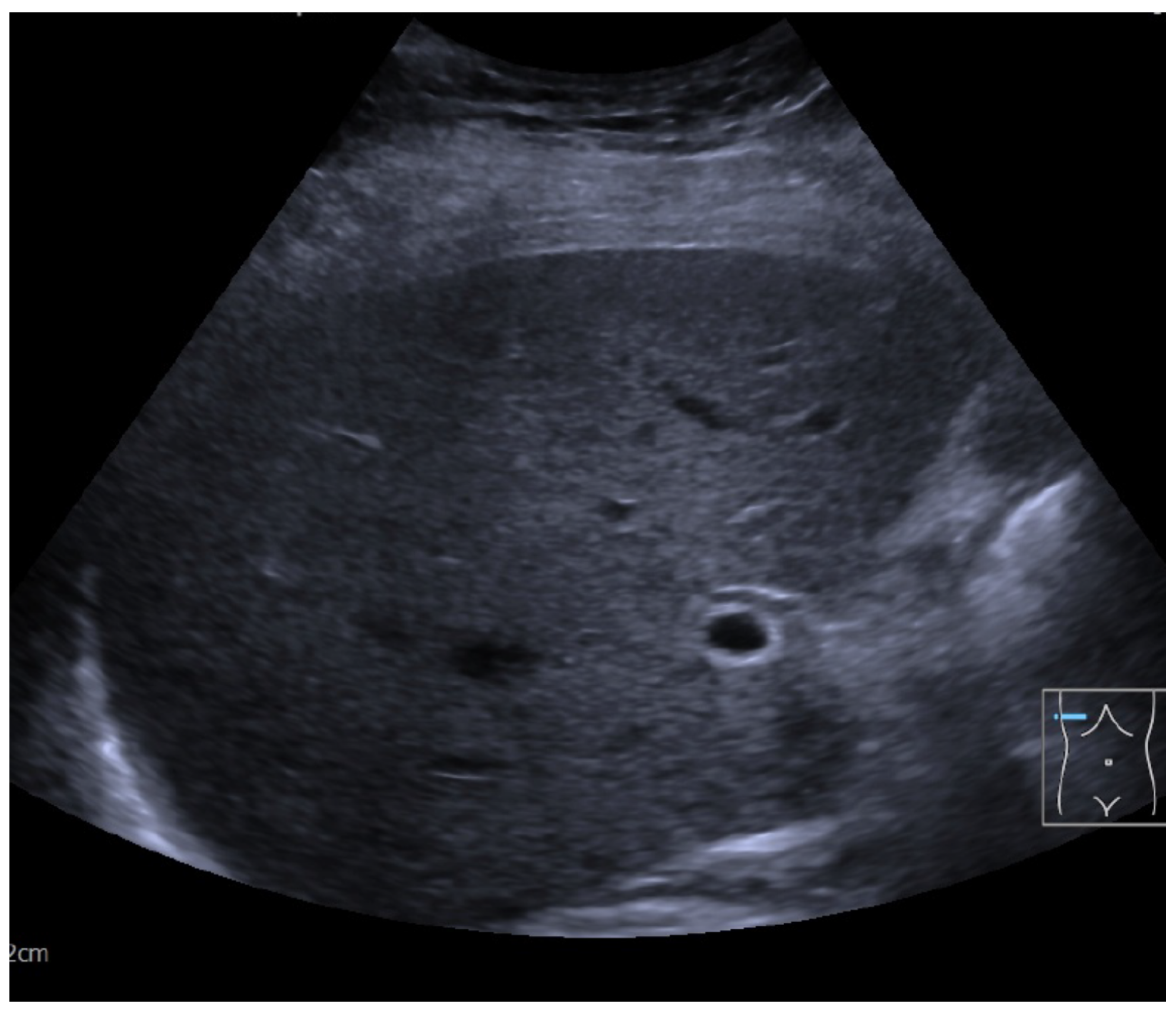
| Laboratory Test | Result | Normal Range | |
|---|---|---|---|
| Blood count | |||
| Red blood count [M/mcL] | 4.44 | 3.93–5.22 | |
| White blood count [K/mcL] | 5.8 | 3.98–10.04 | |
| Hemoglobin [g/dL] | 13.2 | 11.2–15.7 | |
| Hematocrit % | 38.1 | 34.1–44.9 | |
| Platelets [K/mcL] | 295 | 160–370 | |
| Chemistry | |||
| CRP [mg/L] | 1.5 | <5 | |
| Sodium [mmol/L] | 144 | 136–145 | |
| Potassium [mmol/L] | 3.95 | 3.4–4.5 | |
| Calcium [mmol/L] | 2.31 | 2.20–2.55 | |
| Magnesium [mmol/L] | 0.79 | 0.66–0.99 | |
| Creatinine [mg/dL] | 0.68 | 0.51–0.95 | |
| Bilirubin total [mg/dL] | 0.5 | <1.20 | |
| Alanine transaminase [U/L] | 26 | <33 | |
| Aspartate transaminase [U/L] | 31 | 10–35 | |
| Glucose [mg/dL] | 124 | 75–128 | |
| Cardiac troponin T [pg/mL] | 28 | <14 | |
| Arterial blood gas | |||
| Arterial carbon dioxide [mmHg] | 22.3 | 35–45 | |
| Arterial oxygen [mmHg] | 54.3 | 75–97 | |
| pH | 7.379 | 7.35–7.45 | |
| Base excess [mmol/L] | −9.8 | −3–2 | |
| HCO3− [mmol/L] | 13.2 | 20–26 |
| Differential Diagnosis | Consideration | Tests Performed | Results |
|---|---|---|---|
| Infection | Most frequent cause in the elderly | Leukocytes, CRP, vitals | No evidence of infection |
| Metabolic disturbances | Common cause of altered consciousness | Glucose, electrolytes, pH, BE, , EtOH, NH3 | NH3 ↑↑ |
| Neurological events | Very frequent cause in the elderly | Examination, CCT, spinal fluid, EEG | Suspected encephalopathy |
| Toxic ingestion | Possible, often overdose of sedatives | Clinical examination, antidotes administered | Not suspected |
| Endocrine disorders | Rare but possible | Electrolytes, ECG, lab testing, steroid administered | Not suspected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichert, M. A 91-Year-Old Female with Recurring Coma Due to Atypical Hyperammonemia. Reports 2025, 8, 107. https://doi.org/10.3390/reports8030107
Reichert M. A 91-Year-Old Female with Recurring Coma Due to Atypical Hyperammonemia. Reports. 2025; 8(3):107. https://doi.org/10.3390/reports8030107
Chicago/Turabian StyleReichert, Manuel. 2025. "A 91-Year-Old Female with Recurring Coma Due to Atypical Hyperammonemia" Reports 8, no. 3: 107. https://doi.org/10.3390/reports8030107
APA StyleReichert, M. (2025). A 91-Year-Old Female with Recurring Coma Due to Atypical Hyperammonemia. Reports, 8(3), 107. https://doi.org/10.3390/reports8030107






