An Unexpected Finding of a Papillary Fibroelastoma in the Left Ventricle of an Asymptomatic Patient—A Case Report
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
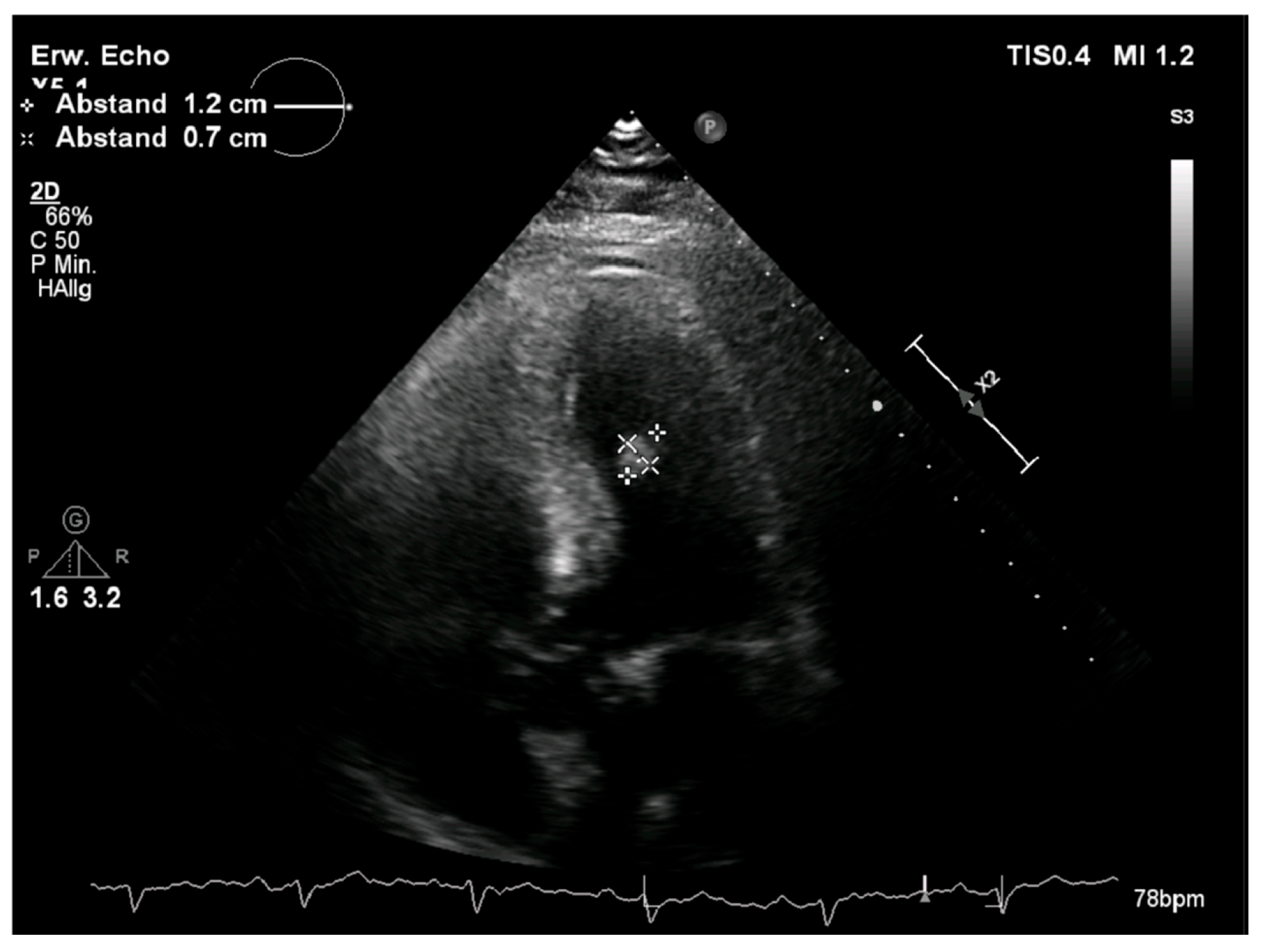
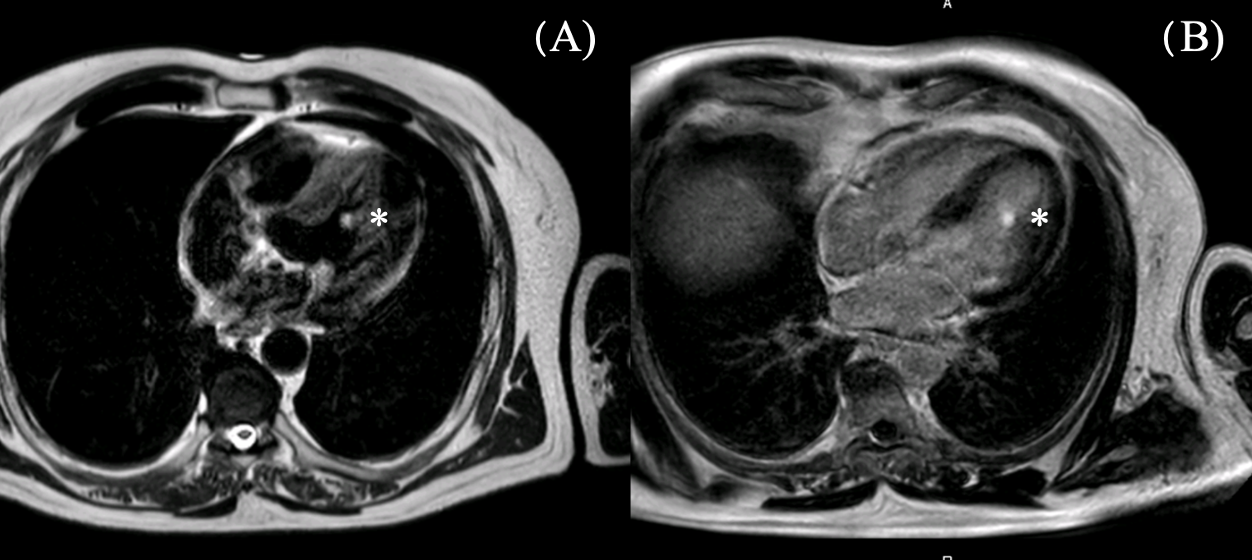
2.1. Diagnostic Assessment
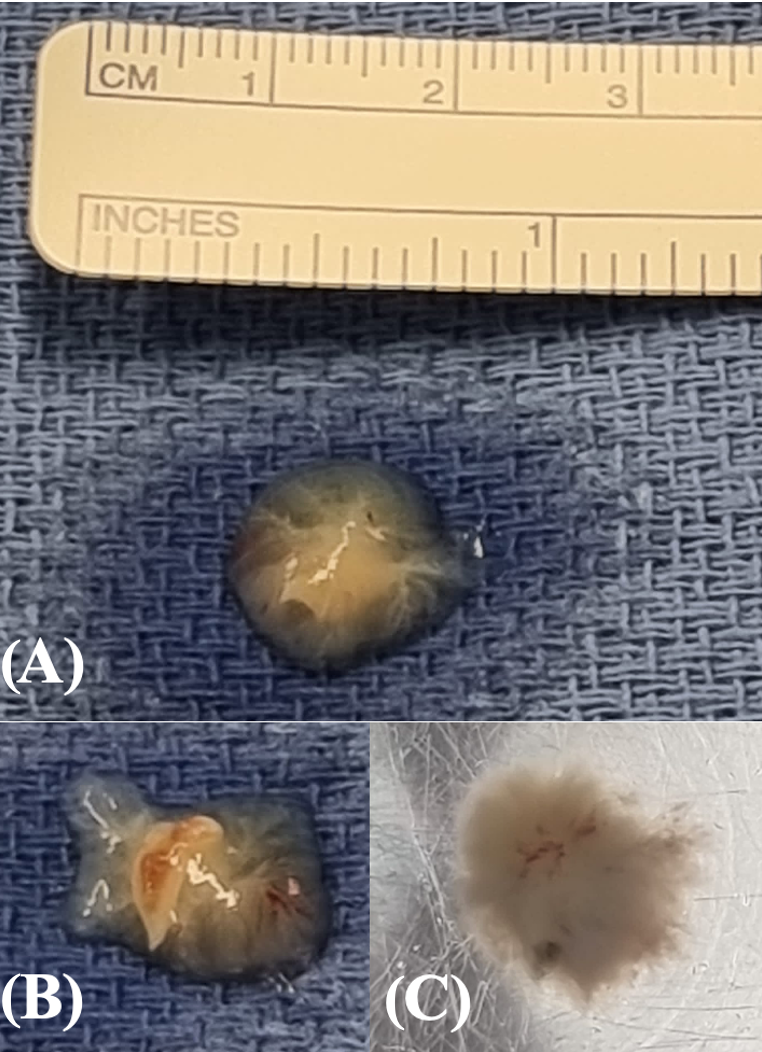
2.2. Therapeutic Intervention
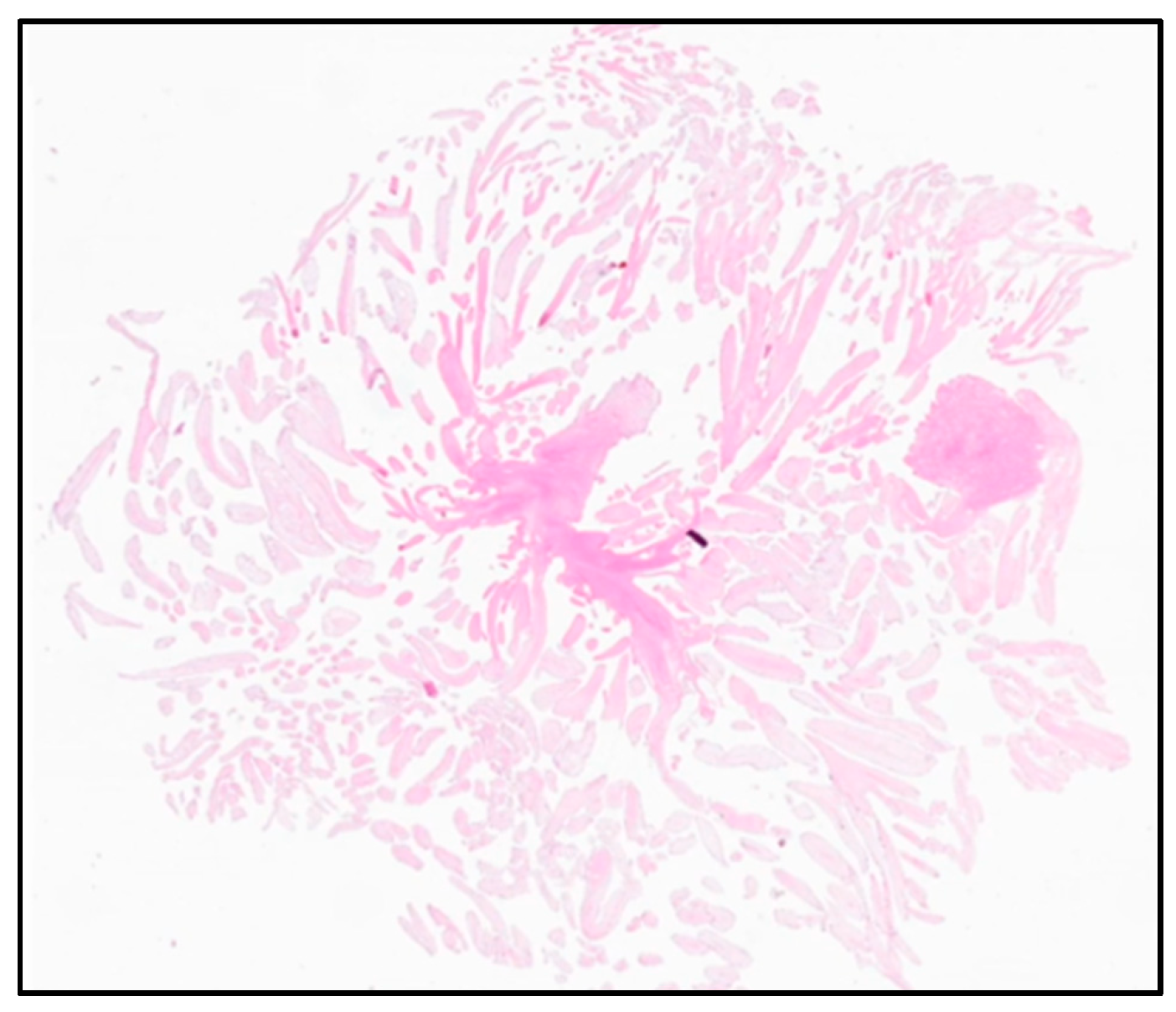
2.3. Outcomes
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFE | papillary fibroelastoma |
| CLL | chronic lymphatic leukemia |
| NYHA | New York Heart Association |
| CMR | cardiac magnetic resonance |
| LIMA | left internal mammary artery |
| PFO | persistent foramen ovale |
References
- Lam, K.Y.; Dickens, P.; Chan, A.C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch. Pathol. Lab. Med. 1993, 117, 1027–1031. [Google Scholar] [PubMed]
- Habertheuer, A.; Laufer, G.; Wiedemann, D.; Andreas, M.; Ehrlich, M.; Rath, C.; Kocher, A. Primary cardiac tumors on the verge of oblivion: A European experience over 15 years. J. Cardiothorac. Surg. 2015, 10, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, M.F.; Sagar, K.; Wynsen, J.C.; Kenny, D. Evolution of a papillary fibroelastoma. J. Am. Soc. Echocardiogr. 1998, 11, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Zoltowska, D.M.; Sadic, E.; Becoats, K.; Ghetiya, S.; Ali, A.A.; Sattiraju, S.; Missov, E. Cardiac papillary fibroelastoma. J. Geriatr. Cardiol. 2021, 18, 346–351. [Google Scholar] [PubMed]
- Maleszewski, J.J.; Bois, M.C.; Bois, J.P.; Young, P.M.; Stulak, J.M.; Klarich, K.W. Neoplasia and the Heart: Pathological Review of Effects With Clinical and Radiological Correlation. J. Am. Coll. Cardiol. 2018, 72, 202–227. [Google Scholar] [CrossRef] [PubMed]
- Grolla, E.; Dalla Vestra, M.; Zoffoli, G.; D’Ascoli, R.; Critelli, A.; Quatrale, R.; Mangino, D.; Rigo, F. Papillary fibroelastoma, unusual cause of stroke in a young man: A case report. J. Cardiothorac. Surg. 2017, 12, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbasi, A.S.; Da Costa, M.; Hennessy, T.; Kiernan, T.J. Cardiac papillary fibroelastoma presenting as acute stroke. BMJ Case Rep. 2013, 2013, bcr2013010092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ljevak, J.; Mišmaš, A.; Bazina, A.; Matijević, V.; Alvir, D.; Supe, S.; Meaški, S.J.; Ozretić, D.; Poljaković, Z.; Habek, M. An infrequent type of stroke with an unusual cause and successful therapy: Basilar artery occlusion caused by a cardiac papillary fibroelastoma recanalized 12 hours after onset. Intern. Med. 2013, 52, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Mkalaluh, S.; Szczechowicz, M.; Torabi, S.; Dib, B.; Sabashnikov, A.; Mashhour, A.; Karck, M.; Weymann, A. Surgery for Cardiac Papillary Fibroelastoma: A 12-Year Single Institution Experience. Med. Sci. Monit. Basic Res. 2017, 23, 258–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piestrzeniewicz, K.; Łuczak, K.; Jakubowski, P.; Kula, P.; Jaszewski, R.; Drożdż, J. Papillary fibroelastoma of the mitral valve as an unusual cause of myocardial infarction in a 20-year-old patient. Eur. Heart J. 2014, 35, 1970. [Google Scholar] [CrossRef] [PubMed]
- Maleszewski, J.J.; Anavekar, N.S.; Moynihan, T.J.; Klarich, K.W. Pathology, imaging, and treatment of cardiac tumours. Nat. Rev. Cardiol. 2017, 14, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Erlebach, M.; Lange, R.; Mazzitelli, D. Placement of Neochords in Mitral Valve Repair: Enhanced Exposure of the Papillary Muscles Using a Standard Valve Sizer. Ann. Thorac. Surg. 2016, 101, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.M.; Khan, I.A.; Nair, C.K.; Mehta, N.J.; Vasavada, B.C.; Sacchi, T.J. Cardiac papillary fibroelastoma: A comprehensive analysis of 725 cases. Am. Heart J. 2003, 146, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sovic, W.R.; Arunthamakun, J.; Lemieux, A.; Guileyardo, J.M. Papillary fibroelastoma in the left ventricle. Bayl. Univ. Med. Cent. Proc. 2021, 34, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.D.; Ferreira, J.; Almeida, J.; Campelo, M.; Maciel, M.J.; Pinho, P. Cardiac papillary fibroelastoma: Report of a surgical series. Rev. Port. Cardiol. 2018, 37, 981–986, (In English, Portuguese). [Google Scholar] [CrossRef] [PubMed]
- Galea, N.; Conia, L.; Ascione, A.; Bruno, N.; Miraldi, F. Fibroelastoma of the papillary muscle mimicking a left ventricular myxoma. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e298. [Google Scholar] [CrossRef] [PubMed]
- Stiru, O.; Geana, R.C.; Dragulescu, P.R.; Tulin, A.; Raducu, L.; Bacalbasa, N.; Balescu, I.; Cretoiu, D.; Diaconu, C.; Iliescu, L.; et al. Transapical Left Ventricular Approach for Cardiac Papillary Fibroelastomas: A Case Report. In Vivo 2020, 34, 3681–3685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiu, A.C.; Hussain, A.; Okonkwo, U.A.; O’Shea, J.P. A Case of Cardiac Papillary Fibroelastoma—An Increasingly Described Cardiac Tumor with Fatal Consequences. Hawaii J. Health Soc. Welf. 2021, 80, 207–211. [Google Scholar] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piber, N.; Nöbauer, C.; Voss, B.; Krane, M.; Voss, S. An Unexpected Finding of a Papillary Fibroelastoma in the Left Ventricle of an Asymptomatic Patient—A Case Report. Reports 2025, 8, 90. https://doi.org/10.3390/reports8020090
Piber N, Nöbauer C, Voss B, Krane M, Voss S. An Unexpected Finding of a Papillary Fibroelastoma in the Left Ventricle of an Asymptomatic Patient—A Case Report. Reports. 2025; 8(2):90. https://doi.org/10.3390/reports8020090
Chicago/Turabian StylePiber, Nicole, Christian Nöbauer, Bernhard Voss, Markus Krane, and Stephanie Voss. 2025. "An Unexpected Finding of a Papillary Fibroelastoma in the Left Ventricle of an Asymptomatic Patient—A Case Report" Reports 8, no. 2: 90. https://doi.org/10.3390/reports8020090
APA StylePiber, N., Nöbauer, C., Voss, B., Krane, M., & Voss, S. (2025). An Unexpected Finding of a Papillary Fibroelastoma in the Left Ventricle of an Asymptomatic Patient—A Case Report. Reports, 8(2), 90. https://doi.org/10.3390/reports8020090





