Management of Dental Demineralization in a Patient with Complex Medical Conditions: A Case Report and Clinical Outcomes
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Phase 1: Patient Education and Introduction of Remineralizing Agents
2.2. Phase 2: Follow-Up and Clinical Outcomes
- •
- Day 14: The restoration of carious lesions in the first quadrant. Cavitated lesions were restored using Enamel plus HRi biofunction composite (Micerium S.p.A, Avegno, Italy). The adhesive technique involved 37% phosphoric acid etching, followed by the application of the Ena Bond Seal adhesive system (Micerium S.p.A, Avegno, Italy) and incremental layering of the composite resin.
- •
- Day 35: The restoration of the second quadrant. The patient’s adherence to oral hygiene recommendations was assessed. Professional supragingival and subgingival debridement was performed. The reinforcement of oral hygiene instructions was provided, with additional guidance on optimizing brushing technique.
- •
- Day 40: The restoration of the third and fourth quadrants. The patient’s adherence to oral hygiene recommendations was assessed. The reinforcement of oral hygiene instructions was provided.
- •
- Day 90: The reinforcement of oral hygiene instructions. A clinical examination was performed to assess the integrity and adaptation of the previously placed restorations, ensuring their proper function and stability.
- •
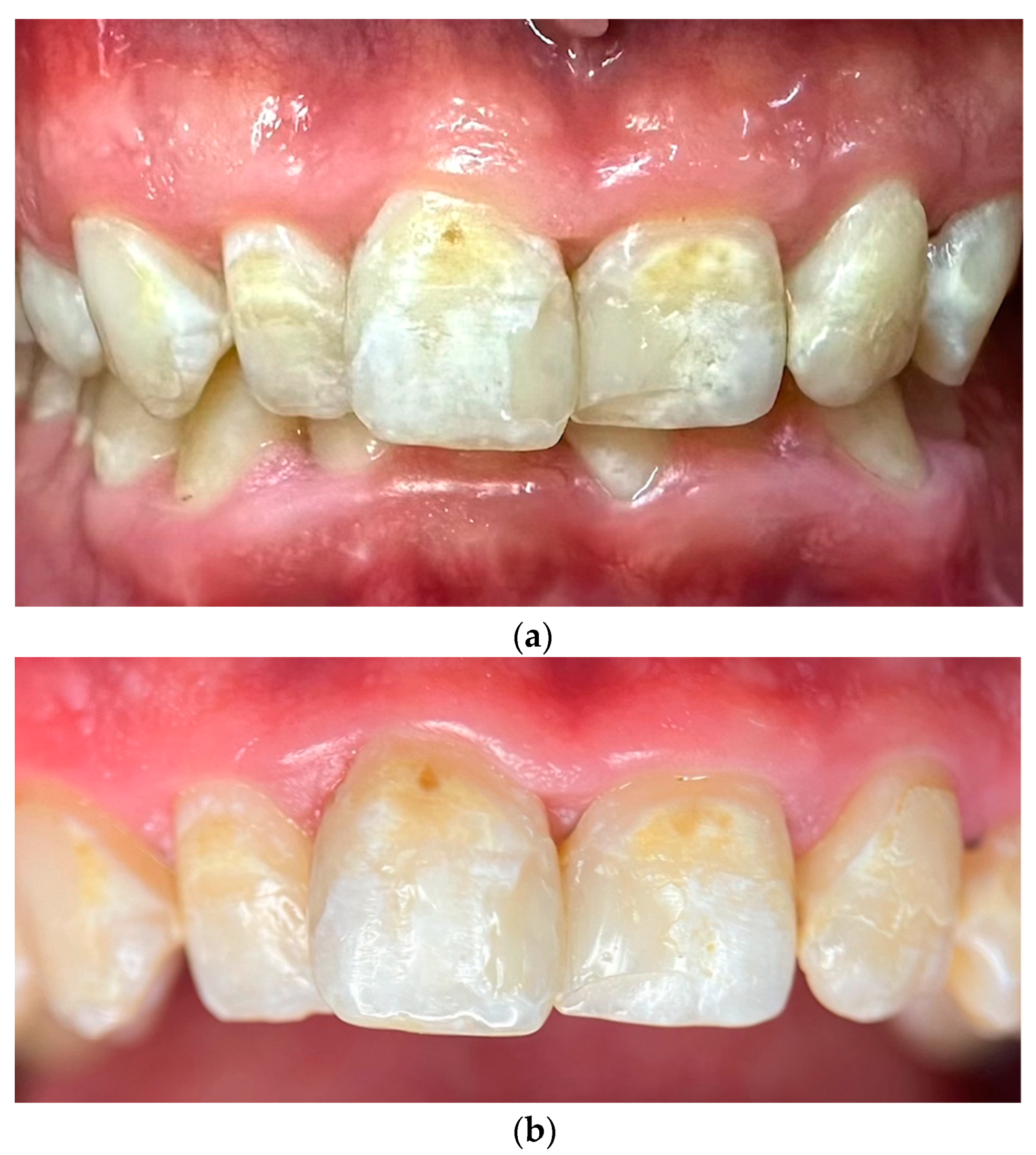
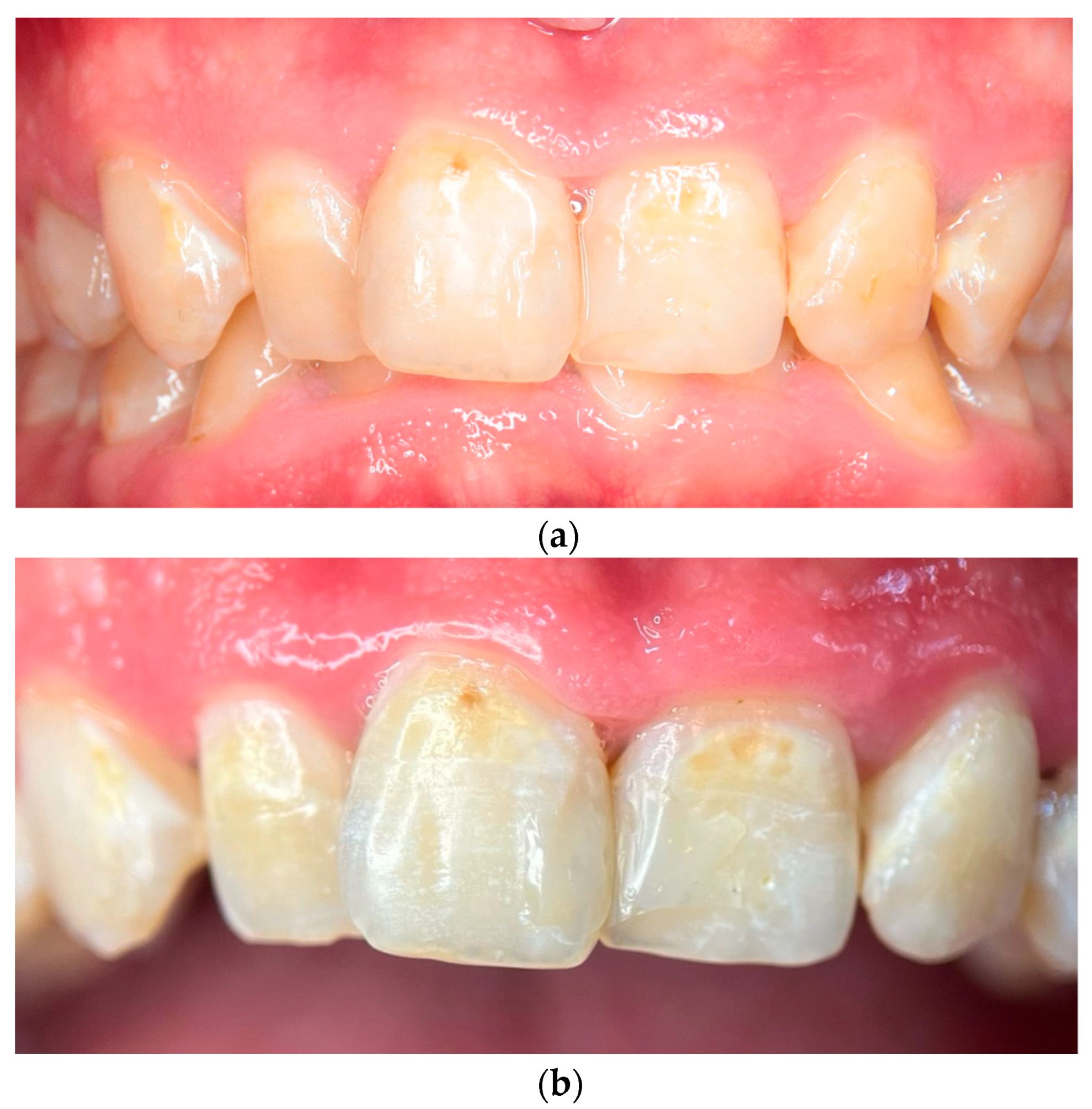
- Day 120: A comprehensive clinical examination was conducted to assess the patient’s progress. A significant remineralization of lesions was observed on the upper anterior teeth, indicating the effectiveness of the remineralization protocol. Gingival health showed marked improvement, with a complete resolution of inflammation. Objective periodontal assessments confirmed a reduction in Bleeding on Probing (BOP) from 38% to 12%, a Gingivitis Index improvement from 2.1 to 1.3, and a Plaque Index (PI) decrease from 50% to 25% (Table 1). The patient was scheduled for regular maintenance visits every six months to ensure long-term stability and prevent the recurrence of oral disease.
2.3. Photographic and Photometric Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Listl, S.; Galloway, J.; Mossey, P.A.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O. Concepts of dental caries and their consequences for understanding the disease. Community Dent. Oral Epidemiol. 1997, 25, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef]
- Yang, S.Y.; Piao, Y.Z.; Kim, S.M.; Lee, Y.K.; Kim, K.N.; Kim, K.M. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45s5 bioactive glass. Dent. Mater. 2013, 29, 1228–1235. [Google Scholar] [CrossRef]
- Dawes, C.; Wong, D.T.W. Role of Saliva and Salivary Diagnostics in the Advancement of Oral Health. J. Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, X.; Li, W.; Zhang, L. Human salivary proteins and their peptidomimetics: Values of function, early diagnosis, and therapeutic potential in combating dental caries. Arch. Oral Biol. 2019, 99, 31–42. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstro€m, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef]
- Yanushevich, O.O.; Maev, I.V.; Krikheli, N.I.; Andreev, D.N.; Lyamina, S.V.; Sokolov, F.S.; Bychkova, M.N.; Beliy, P.A.; Zaslavskaya, K.Y. Prevalence and Risk of Dental Erosion in Patients with Gastroesophageal Reflux Disease: A Meta-Analysis. Dent. J. 2022, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, K.; Papaioannou, W.; Seremidi, K.; Bougioukas, K.; Haidich, A.B. Prevalence and association of gastroesophageal reflux disease and dental erosion: An overview of reviews. J. Dent. 2023, 133, 104520. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, L.; Frigerio, F.; Piciocchi, C.; Piana, G.; Montevecchi, M.; Donini, L.M.; Mocini, E. Oro-dental manifestations of eating disorders: A systematic review. J. Eat. Disord. 2024, 24, 12–87. [Google Scholar] [CrossRef]
- Novotna, M.; Podzimek, S.; Broukal, Z.; Lencova, E.; Duskova, J. Periodontal diseases and dental caries in children with type 1 diabetes mellitus. Mediators Inflamm. 2015, 2015, 379626. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 88–97. [Google Scholar] [CrossRef]
- Southward, K. A hypothetical role for vitamin K2 in the endocrine and exocrine aspects of dental caries. Med. Hypotheses 2015, 84, 276–280. [Google Scholar] [CrossRef]
- Tapalaga, G.; Bumbu, B.A.; Reddy, S.R.; Vutukuru, S.D.; Nalla, A.; Bratosin, F.; Fericean, R.M.; Dumitru, C.; Crisan, D.C.; Nicolae, N.; et al. The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients 2023, 15, 3863. [Google Scholar] [CrossRef]
- Seow, W.K. Developmental defects of enamel and dentine: Challenges for basic science research and clinical management. Aust. Dent. J. 2014, 59, 143–154. [Google Scholar] [CrossRef]
- Moras, E.; Gandhi, K.; Narasimhan, B.; Brugada, R.; Brugada, J.; Brugada, P.; Krittanawong, C. Genetic and Molecular Mechanisms in Brugada Syndrome. Cells 2023, 12, 1791. [Google Scholar] [CrossRef]
- Veiga, N.; Figueiredo, R.; Correia, P.; Lopes, P.; Couto, P.; Fernandes, G.V.O. Methods of Primary Clinical Prevention of Dental Caries in the Adult Patient: An Integrative Review. Healthcare 2023, 11, 1635. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Gargani, A.; Parcianello, R.G.; Pezzato, L.; Bertolini, R.; Zuccon, A.; Stellini, E.; Ludovichetti, F.S. Protection against Dental Erosion and the Remineralization Capacity of Non-Fluoride Toothpaste, Fluoride Toothpaste and Fluoride Varnish. Appl. Sci. 2023, 13, 1849. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Limeback, H.; Amaechi, B.T.; Fabritius, H.-O.; Ganss, B.; O’hagan-Wong, K.; Wiesche, E.S.Z.; Meyer, F.; Enax, J. Hydroxyapatite as an active ingredient in oral care: An international symposium report. Bioinspired Biomim. Nanobiomater. 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Cocco, F.; Wierichs, R.J.; Wolf, T.G.; Salerno, C.; Arghittu, A. Efficacy of HAF toothpastes in primary and permanent dentitions. A 2-years triple-blind RCT. J. Dent. 2022, 121, 104049. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.; Manzoor, S.; Qazi, Z.; Ghaus, S.; Saleem, M.; Kashif, M. Remineralization Effect of Bioactive Glass With and Without Fluoride and Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) on Artificial Dentine Caries: An In Vitro Study. Cureus 2024, 16, e70801. [Google Scholar] [CrossRef] [PubMed]
- Sathish, A.K.; Gopalkrishna, P.; Kumar, S. In vitro evaluation of remineralizing agents on dentinal tubule occlusion: A scanning electron microscopic study. J. Indian Soc. Periodontol. 2023, 27, 362–367. [Google Scholar] [CrossRef]
- Corrêa, M.C.; Lerco, M.M.; de Cunha, M.L.; Henry, M.A. Salivary parameters and teeth erosions in patients with gastroesophageal reflux disease. Arq Gastroenterol. 2012, 49, 214–218. [Google Scholar]
- Watanabe, M.; Nakatani, E.; Yoshikawa, H.; Kanno, T.; Nariai, Y.; Yoshino, A.; Vieth, M.; Kinoshita, Y.; Sekine, J. Oral soft tissue disorders are associated with gastroesophageal reflux disease: Retrospective study. BMC Gastroenterol. 2017, 17, 92. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Henriquez, C.C.; Mouawad, F.; Ristagno, C.; Barillari, M.R.; Schindler, A.; Nacci, A.; Bouland, C.; Laino, L.; et al. Laryngopharyngeal reflux, gastroesophageal reflux and dental disorders: A systematic review. PLoS ONE 2020, 15, e0237581. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Wierichs, R.J.; Schellwien, T.; Paris, S. Remineralizing efficacy of a CPP-ACP cream on enamel caries lesions in situ. Caries Res. 2015, 49, 56–62. [Google Scholar] [CrossRef]
- Souza, B.M.; Comar, L.P.; Vertuan, M.; Fernandes Neto, C.; Buzalaf, M.A.R.; Magalhães, A.C. Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2005, 49, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Covani, U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014, 45, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Ludovichetti, F.S.; Zambon, G.; Cimolai, M.; Gallo, M.; Signoriello, A.G.; Pezzato, L.; Bertolini, R.; Mazzoleni, S. Efficacy of Two Toothpaste in Preventing Tooth Erosive Lesions Associated with Gastroesophageal Reflux Disease. Appl. Sci. 2022, 12, 1023. [Google Scholar] [CrossRef]
- Fanfoni, L.; Costantinides, F.; Berton, F.; Marchesi, G.; Polo, L.; Di Lenarda, R.; Nicolin, V. From Erosion to Remineralization: The Possible Role of Two Topic Home Devices Used as Combined Treatment. Appl. Sci. 2020, 10, 4093. [Google Scholar] [CrossRef]

| Clinical Parameter | Baseline Value | 120-Day Follow-Up |
|---|---|---|
| Bleeding on Probing (BOP) (%) | 38% | 12% |
| Mean Gingivitis Index (Löe and Silness) | 2.1 (Moderate to severe gingivitis) | 1.3 (Improved gingival health) |
| Mean Plaque Index (Silness and Löe) | 50% | 25% |
| DMFT (Decayed/Missing/Filled Teeth) | 15 (D = 10, M = 0, F = 5) | No new lesions |
| Event | Details | Date |
|---|---|---|
| Visit 1 | Education on oral hygiene and dietary counselling. Initiated remineralizing mousse and toothpaste. Supragingival debridement performed. | Day 0 |
| Visit 2 | Restoration of carious lesions in the first quadrant using enamel plus HRi biofunction composite. Adhesive technique with 37% phosphoric acid etching and Ena Bond Seal adhesive system. | Day 14 |
| Visit 3 | Restoration of second quadrant. Patient’s adherence to oral hygiene recommendations assessed. Professional supragingival and subgingival debridement performed. | Day 35 |
| Visit 4 | Restoration of third and fourth quadrants. Patient’s adherence to oral hygiene recommendations assessed. Reinforcement of oral hygiene instructions. | Day 40 |
| Visit 5 | Reinforcement of oral hygiene instructions, along with an assessment of the integrity and adaptation of the previously placed restorations. | Day 90 |
| Visit 6 | Comprehensive clinical examination. | Day 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardellitti, L.; Filigheddu, E.; Milia, E. Management of Dental Demineralization in a Patient with Complex Medical Conditions: A Case Report and Clinical Outcomes. Reports 2025, 8, 39. https://doi.org/10.3390/reports8020039
Sardellitti L, Filigheddu E, Milia E. Management of Dental Demineralization in a Patient with Complex Medical Conditions: A Case Report and Clinical Outcomes. Reports. 2025; 8(2):39. https://doi.org/10.3390/reports8020039
Chicago/Turabian StyleSardellitti, Luigi, Enrica Filigheddu, and Egle Milia. 2025. "Management of Dental Demineralization in a Patient with Complex Medical Conditions: A Case Report and Clinical Outcomes" Reports 8, no. 2: 39. https://doi.org/10.3390/reports8020039
APA StyleSardellitti, L., Filigheddu, E., & Milia, E. (2025). Management of Dental Demineralization in a Patient with Complex Medical Conditions: A Case Report and Clinical Outcomes. Reports, 8(2), 39. https://doi.org/10.3390/reports8020039








