1. Introduction and Clinical Significance
Drug-induced liver injury (DILI) is a complex hepatic condition marked by abrupt hepatic damage caused due to exposure to hepatotoxic substances or their metabolites [
1,
2]. DILI can be categorized into two types: dose-dependent or intrinsic and dose-independent or idiosyncratic. Dose-dependent injury, such as seen in Acetaminophen overdose, can develop when the drug dose exceeds the limit for toxicity, with the extent of damage being proportional to the amount administered. On the other hand, dose-independent injury is not influenced by dose or treatment duration, frequently arising even at therapeutic doses [
3].
A broad range of drugs may induce liver injury, from simple anti-pyretic drugs like Acetaminophen to multiple dietary herb supplements.
Acetaminophen is one of the most used analgesics, anti-pyretic, and anti-inflammatory drugs. While it is considered safe at therapeutic doses, overdoses of Acetaminophen can induce severe liver injury. After ingestion, 90% of the Acetaminophen is metabolized in nontoxic compounds (sulfate and glucuronide) in the liver and then excreted into the urine. Approximately 5–9% of Acetaminophen is metabolized by cytochrome p450 into a toxic form (
N-acetyl-
p benzoquinone imine—NAPQI) which can induce oxidative injury and hepatocellular necrosis. Only 2% of Acetaminophen is eliminated unchanged in the urine [
4,
5].
Dietary herb supplements become a major concern throughout the globe for liver injury [
6]. Whitania Somnifera (or Indian Ginseng), popularly known as Ashwagandha, widely used in Indian Ayurvedic medicine [
7], is a herb commonly used in supplements to improve stress and anxiety, enhance sleep and memory, support sexual function in both males and females, increase muscle mass and strength, and acts as “immune booster”. It can lead to liver injury, with the acute decompensation of cirrhosis, and acute-on-chronic liver failure. The primary pattern of liver injury is cholestatic, which may occur with or without inflammation and sometimes necrosis. Clinical presentation typically includes jaundice and pruritus [
8].
In multiple sclerosis (MS), there are many therapeutic options, and most of the available disease-modifying therapies (DMTs) cause DILI.
DMTs, used to reduce the rate of relapses and accumulation of disability, can induce a group of immunological mechanisms, not being free of potential hepatic toxic effects [
9,
10]. The mechanism of liver injury during DMT’s treatment can be autoimmune, with viral reactivation, and in rare cases, with idiosyncratic reactions with unpredictable acute liver failure [
2,
9].
DMTs can be classified into moderate efficiency therapies like beta interferon, glatiramer acetate, teriflunomide, and dimethyl fumarate and highly efficient treatments like natalizumab, fingolimod, alemtuzumab, cladribine, and ocrelizumab, every therapy being individualized for every patient due to the risk–benefit profile, with induction and escalation strategies [
11].
Natalizumab is a humanized monoclonal antibody anti-integrin that blocks the adhesion of leukocytes to vascular cell receptors on endothelial cells, thus preventing their migration into the central nervous system. Natalizumab was introduced for MS treatment for the first time in 2004, being administered in infusions at a dose of 300 mg and recently subcutaneously every 4 weeks [
9].
Several of the adverse effects of natalizumab reported are rash, abdominal discomfort, nausea, depression, fatigue, urinary tract, and lower and upper respiratory tract infections. One important complication is progressive multifocal leukoencephalopathy, a neurological condition associated with John Cunningham virus reactivation in the central nervous system [
9].
Liver injury induced by natalizumab can appear at any time during treatment. It has been reported after the first administration, or after multiple doses. There is an idiosyncratic autoimmune-like pattern, with hepatocellular involvement and sometimes with positive autoantibodies (anti-nuclear and anti-smooth muscle antibodies) [
9,
12].
Cases of liver injury or autoimmune hepatitis induced by natalizumab have been published in the literature, often presenting overlapping conditions, with autoantibodies and histological patterns of plasma cell infiltration. Notably, there had been no recurrence following the discontinuation of cortisone treatment [
9].
2. Case Presentation
We present the case of a female patient diagnosed with MS, with natalizumab as immunomodulatory treatment initialized 7 years ago, who suddenly developed acute liver injury.
A 39-year-old female patient was diagnosed with MS at the age of 27 years old, with brainstem syndrome as disease onset. She experienced multiple relapses over the course of her condition and had undergone multiple therapies: (1) Glatiramer Acetate for one year and a half and (2) interferon-beta for two years. Both therapies were discontinued, due to treatment failure, with continued clinical and imaging activity. She was then escalated to current immunomodulatory treatment to (3) natalizumab (infusion no. 81), with normal liver function monitored monthly since the beginning of the treatment.
The patient had a recent history of an airway infection treated with 4–5 tablets of Acetaminophen per day for 7 days. She had a history of consumption of dietary supplements with Ashwagandha herb in the last month, with the desire to lose weight.
She presented at the emergency room with jaundice, pruritus, and lower limb ecchymoses. She had no fever, myalgia, diarrhea, or abdominal pain. She denied alcohol intake and use of illicit drugs.
On physical examination, she was overweight and there was no flapping tremor or other clinical signs of chronic liver disease. She was medically stable with a blood pressure of 110/60 mmHg, a pulse of 75 beats per minute, a respiration rate of 20 breaths per minute, and a normal temperature.
Abdominal ultrasound showed normal morphology of the liver, with no cholelithiasis or cholecystitis, and no dilatation of the biliary tract. An abdominal CT scan was conducted, showing no hepatic abnormalities.
The patient’s complete blood count showed a white blood cell (WBC) count of 11.9 × 10
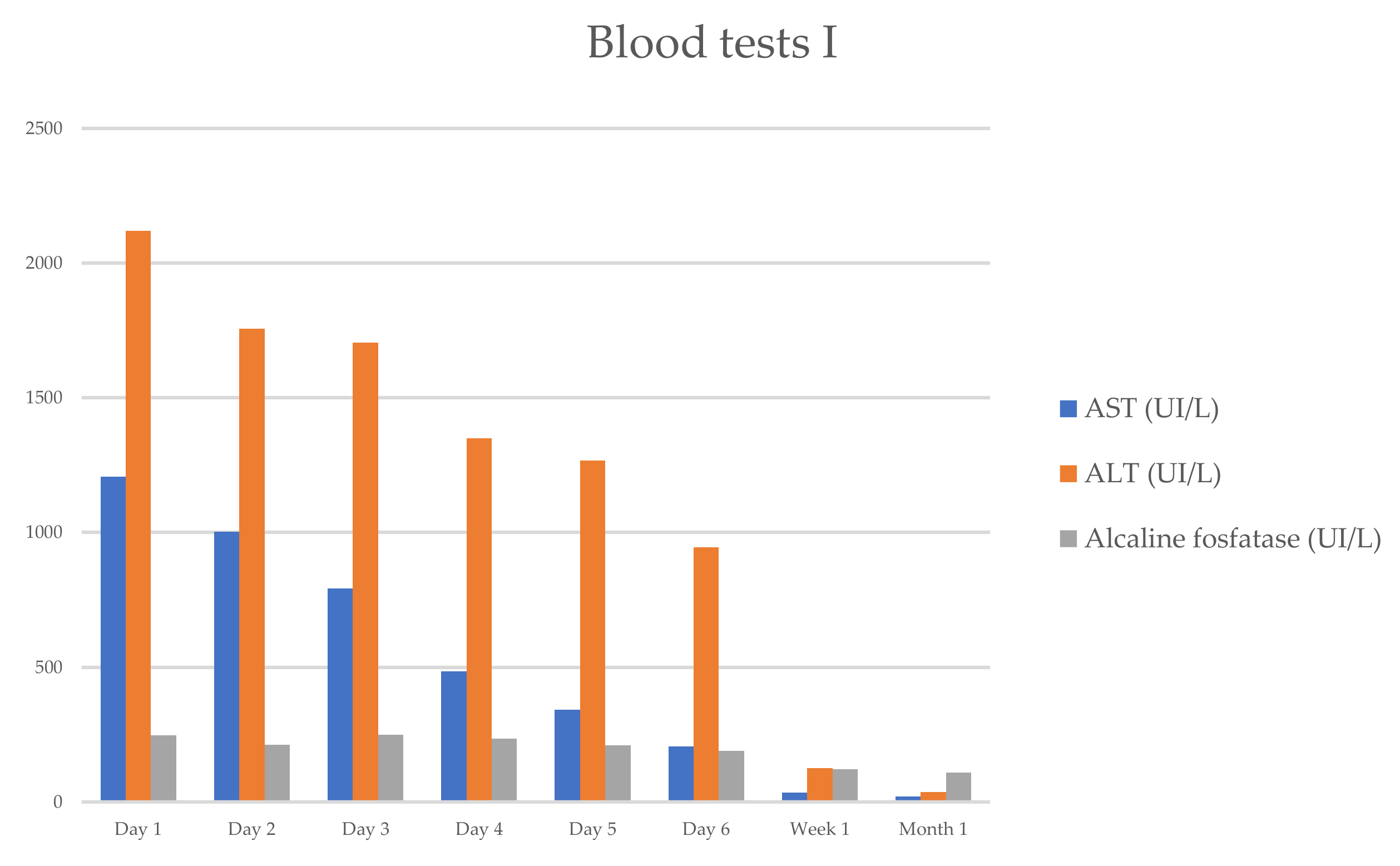
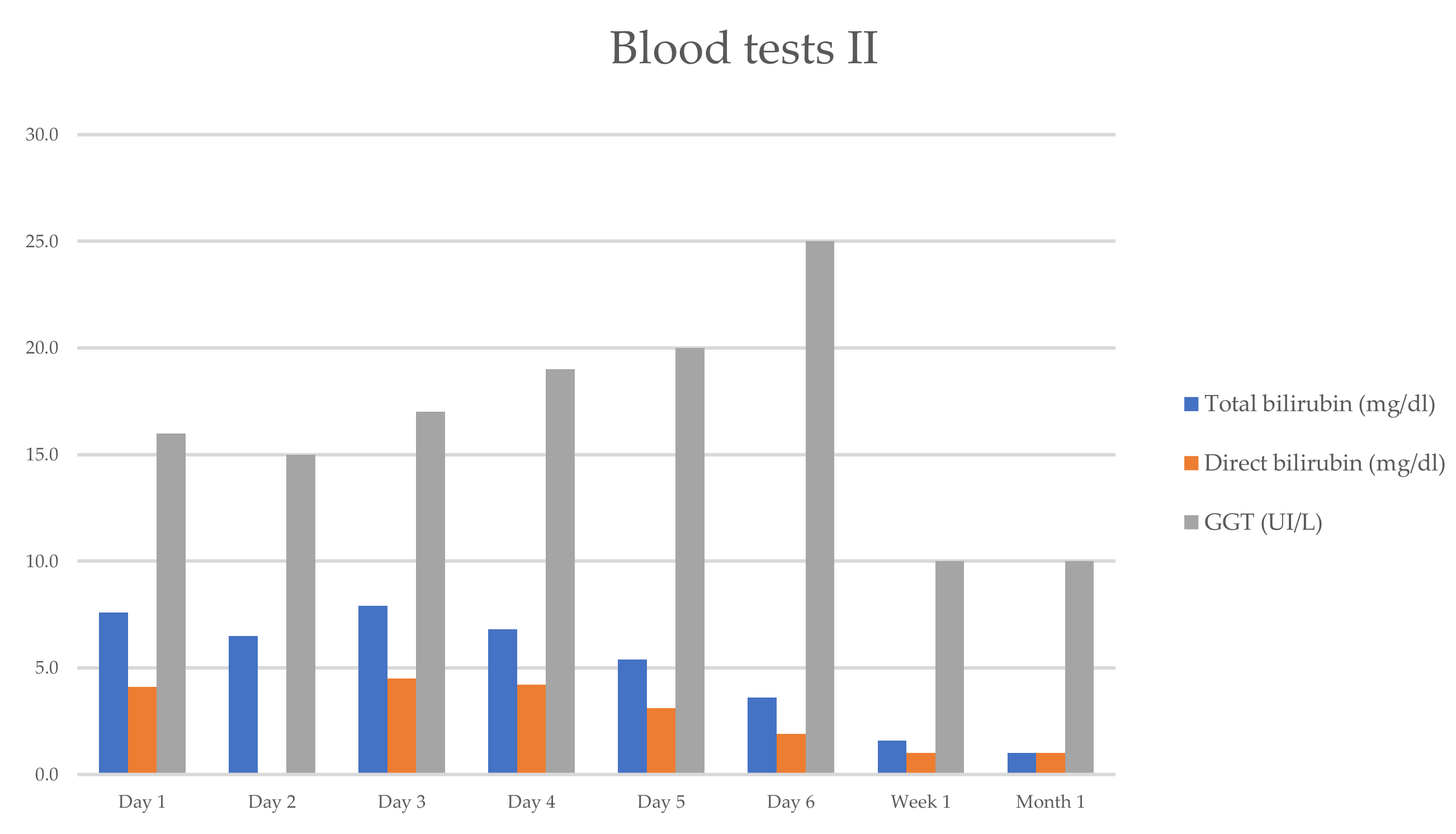
3/mL (segmented neutrophils 68.5%, lymphocytes 25%, eosinophil 0.3%), hemoglobin count of 12.3 g/dL, hematocrit count of 31.1%, and platelet count of 368,000/mL. The serum biochemical assay showed the following results: creatinine, 0.46 mg/dL; Na, 139 mEq/L; and K, 4.0 mEq/L. The laboratory results revealed aminotransferase levels higher than 1000 UI/L (ALT-2120 UI/L, AST—1208 UI/L), and higher total bilirubin (TB—7.9 mg/dL), direct bilirubin (DB—4.1 mg/dL), and alkaline phosphatase (ALP—247 UI/L). The changes in the hepatic enzymes reflected a mixed pattern of liver injury (hepatocellular and cholestatic), with a ratio of serum ALT to serum ALP of 4 (between 2 and 5) (
Table 1).
In order to exclude the possible causes of acute liver injury, a screening for autoimmune and infectious hepatitis was performed.
The screening for autoimmune hepatitis was negative, with negative autoimmune autoantibodies (Ab): anti-nuclear Ab, anti-mitochondrial Ab, anti-smooth muscle Ab, and anti-liver kidney microsomal Ab (
Table 2).
Screening for infectious hepatitis was negative. The anti-hepatitis A Virus Ab, Hepatitis B surface Antigen (Ag), anti-hepatitis B e-Ab and Ag, anti-hepatitis C Virus Ab, and anti-hepatitis E Virus Ab test results were all negative. There was no evidence of recent infection with Cytomegalovirus, Epstein–Barr Virus, or Herpes Simplex Virus (
Table 3).
Overall, taking into account the clinical and paraclinical investigations, there was a scenario of mixed toxic hepatitis induced by recently used drugs (Acetaminophen and Ashwagandha herb dietary supplement). Treatment with oral prednisone at a dose of 40 mg/day was initiated.
The patient was followed up as an outpatient, with the corticosteroid dosage gradually reduced.
She progressively improved in clinical and laboratory test results
Table 1 and
Figure 1 and
Figure 2, with the normalization of the serum levels of liver enzymes and bilirubin 1 month after starting the oral prednisone. The level of liver enzymes decreased after the discontinuation of corticosteroid therapy and dietary supplements.
Far from the normalization of the liver enzymes and from stopping the consumption of dietary herb supplements, the patient resumed the immunomodulatory treatment with natalizumab under 8 weeks after her last infusion, without changes in hepatic function in the following months (
Table 1).
3. Discussion
We report the case of a female patient diagnosed with multiple sclerosis, currently receiving natalizumab (infusion no. 81) as immunomodulatory treatment. The patient developed drug-induced liver injury (DILI) related to Ashwagandha, which was precipitated by the use of the anti-pyretic drug Acetaminophen. The liver injury pattern was mixed—hepatocellular and cholestatic, with jaundice as the primary clinical presentation. A comprehensive diagnostic evaluation was performed to exclude other potential causes of the acute liver injury. Fortunately, the drug-induced liver injury was self-limiting and resolved with treatment and supportive care.
Natalizumab-induced liver injury is rare and may occur at any point during treatment, after the first or second infusion. Typically, it presents as mild acute hepatitis, which resolves spontaneously [
9].
According to the phase III trials (AFFIRM, SENTINEL, and ASCEND), abnormal liver function tests were reported in 5% of patients receiving natalizumab as immunomodulatory treatment, with severe cases occurring in less than 1% of them. The data from these trials were collected after 2 years of treatment. However, extension studies up to 10 years confirmed that severe hepatobiliary adverse effects occurred in less than 1% of patients [
13,
14,
15].
Since 2009, over 30 cases of patients with severe liver injury induced by natalizumab have been reported in the adverse event system, with an estimated incidence of liver injury from 17 to 100,000 exposed patients [
9].
Ashwagandha offers many benefits, including increased exercise capacity, improved physical performance, better sleep, and reduced stress and anxiety.
Although generally considered safe, Ashwagandha has been associated with a high incidence of drug-induced liver injury. The mechanism of damage is believed to be related to the herb-specific compounds called withanolides, which can induce irreversible damage to hepatocellular DNA [
16,
17].
Reports of liver injury induced by Ashwagandha have been published in medical literature since 2011. The first case reports came from Japan, followed by multiple reports from the United States, India, Germany, and Poland. While the largest cohorts of Ashwagandha-related liver injuries have been reported, the actual number of cases may be higher.
Most herbal drugs contain multi-ingredient compounds, making it difficult to pinpoint the exact substance responsible for liver injury. In most cohorts, patients used only Ashwagandha supplements, establishing with certainty that Ashwagandha can cause severe liver injury and death in some cases [
16,
18,
19,
20,
21,
22,
23,
24]. In the case of our patient, she used only the Ashwagandha supplement.
Patients with chronic liver disease can develop more severe DILI, often resulting in a poor prognosis. Some reports have linked DILI in patients with a history of liver disease to a higher mortality rate, up to 16%, compared to almost 5% in patients without liver disease [
16,
18,
19,
20,
21,
22,
23,
24].
Our patient history had no history of chronic liver disease or alcohol use. However, she was taking the hepatotoxic drug Acetaminophen concomitantly.
In all the cohorts reporting Ashwagandha-related liver injury, comprehensive viral screening for hepatitis was performed, which was deemed highly unlikely to be the cause of the described liver injury mixed pattern [
16,
18,
19,
20,
21,
22,
23,
24]. Autoimmune hepatitis screening revealed positive autoantibodies in one cohort, but without the classical features of autoimmune hepatitis [
16]. Mild elevations in autoimmune markers are commonly observed in patients with DILI, particularly in those with herb-induced liver injury [
16].
Cases of liver injury or autoimmune hepatitis induced by natalizumab have been published in the literature, often presenting overlapping conditions, with autoantibodies and histological patterns of plasma cell infiltration. These cases showed no recurrence after cortisone withdrawal [
9].
In our case, a thorough diagnostic was conducted to exclude other causes of the acute liver injury. The screening for viral and autoimmune hepatitis was negative, with no positive autoantibodies. The blood test results with changes in the hepatic enzymes reflected a mixed pattern of liver injury (hepatocellular and cholestatic).
In all the cohorts, the Ashwagandha supplement was used to reduce stress and anxiety in young or middle-aged people. Most patients consumed the herb according to the label instructions or as prescribed [
16,
18,
19,
20,
21,
22,
23,
24]. In our case, the patient used the Ashwagandha supplement as part of her diet program. The withdrawal of the supplement and treatment with immunosuppressive cortisone resolved the jaundice and the liver injury.
4. Conclusions
The case report highlights the challenges in establishing multiple etiologies and managing acute hepatitis in an MS patient receiving natalizumab as immunomodulatory treatment, a drug known to potentially induce liver injury, particularly after the first infusions, along with a recent history of ingesting hepatotoxic drugs.
The diagnosis of DILI is often one of the most challenging. A wide range of drugs can cause liver injury, including DMTs approved for MS treatment, which can have potential hepatotoxic effects, especially after the first administration. Simple anti-pyretic drugs like Acetaminophen and dietary herb supplements can induce liver injury. Ashwagandha, a herb extensively used in traditional Indian medicine, is a dietary supplement and a potential liver-injury factor.
Given the many documented cases of liver damage caused by Ashwagandha or other drugs, with unknown mechanisms behind the substances it contains, there should be high awareness among healthcare providers about patients using various drugs and herbs, who can present with symptoms and signs of liver injury.
Public education is essential to avoid the use of unrecommended and potentially harmful drugs and herb supplements, aiming to reduce the incidence of DILI.
Author Contributions
Conceptualization, M.-M.D.-M. and S.P.; methodology, M.-M.D.-M., S.P. and C.A.P.; software, M.-M.D.-M.; validation, S.P. and C.A.P.; formal analysis, M.-M.D.-M. and S.P.; investigation, M.-M.D.-M., S.P. and C.A.P.; resources, M.-M.D.-M., S.P. and C.A.P.; data curation, M.-M.D.-M. and S.P.; writing—original draft preparation, M.-M.D.-M. and S.P.; writing—review and editing, M.-M.D.-M.; visualization, M.-M.D.-M.; supervision, S.P. and C.A.P.; project administration, C.A.P. We, the authors, are the only persons responsible for the content of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. In accordance with local standards, our institution (Elias Emergency and University Hospital) does not require ethical approval or institutional review board approval for reporting individual cases or case series when informed consent is provided by the patient.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dijmărescu, I.; Guță, O.M.; Brezeanu, L.E.; Dijmărescu, A.D.; Becheanu, C.A.; Păcurar, D. Drug-Induced Hepatitis in Children: The Experience of a Single Center in Romania. Children 2022, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Hosack, T.; Damry, D.; Biswas, S. Drug-induced liver injury: A comprehensive review. Ther. Adv. Gastroenterol. 2023, 16, 17562848231163410. [Google Scholar] [CrossRef] [PubMed]
- Clinical Manifestations and Diagnosis of Acetaminophen (Paracetamol) Poisoning in Children and Adolescents—UpToDate. Available online: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-acetaminophen-paracetamol-poisoning-in-children-and-adolescents (accessed on 3 August 2024).
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Shen, T.; Liu, Y.; Shang, J.; Xie, Q.; Li, J.; Yan, M.; Xu, J.; Niu, J.; Liu, J.; Watkins, P.B.; et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology 2019, 156, 2230–2241.e11. [Google Scholar] [CrossRef]
- Philips, C.A.; Paramaguru, R.; Joy, A.K.; Antony, K.L.; Augustine, P. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury-A single-center experience from southern India. Indian. J. Gastroenterol. 2018, 37, 9–17. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Biolato, M.; Bianco, A.; Lucchini, M.; Gasbarrini, A.; Mirabella, M.; Grieco, A. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs 2021, 35, 861–880. [Google Scholar] [CrossRef]
- Comi, G.; Radaelli, M.; Soelberg Sørensen, P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017, 389, 1347–1356. [Google Scholar] [CrossRef]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.; Lacruz, F.; Bolado-Concejo, F.; Rodríguez-Pérez, I.; Ayuso, T.; Garaigorta, M.; Urman, J. Natalizumab-induced autoimmune hepatitis in a patient with multiple sclerosis. Mult. Scler. 2013, 19, 1234–1235. [Google Scholar] [PubMed]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [PubMed]
- Rudick, R.A.; Stuart, W.H.; Calabresi, P.A.; Confavreux, C.; Galetta, S.L.; Radue, E.-W.; Lublin, F.D.; Weinstock-Guttman, B.; Wynn, D.R.; Lynn, F.; et al. Natalizumab plus Interferon Beta-1a for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 911–923. [Google Scholar]
- Kapoor, R.; Ho, P.R.; Campbell, N.; Chang, I.; Deykin, A.; Forrestal, F.; Lucas, N.; Yu, B.; Arnold, D.L.; Freedman, M.S.; et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018, 17, 405–415. [Google Scholar]
- Philips, C.A.; Valsan, A.; Theruvath, A.H.; Ravindran, R.; Oommen, T.T.; Rajesh, S.; Bishnu, S.; Augustine, P.; on behalf of The Liver Research Club India. Ashwagandha-induced liver injury-A case series from India and literature review. Hepatol. Commun. 2023, 7, e0270. [Google Scholar]
- Siddiqui, S.; Ahmed, N.; Goswami, M.; Chakrabarty, A.; Chowdhury, G. DNA damage by Withanone as a potential cause of liver toxicity observed for herbal products of Withania somnifera (Ashwagandha). Curr. Res. Toxicol. 2021, 2, 72–81. [Google Scholar]
- Lubarska, M.; Hałasiński, P.; Hryhorowicz, S.; Mahadea, D.S.; Łykowska-Szuber, L.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Liver Dangers of Herbal Products: A Case Report of Ashwagandha-Induced Liver Injury. Int. J. Environ. Res. Public Health 2023, 20, 3921. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S.; Avula, B.; Khan, I.A.; Jonasson, J.G.; Ghabril, M.; Hayashi, P.H.; Navarro, V. Liver Injury due to Ashwagandha. A Case Series from Iceland and the U.S. Drug-Induced Liver Injury Network. Liver Int. 2020, 40, 825. [Google Scholar]
- Weber, S.; Gerbes, A.L. Ashwagandha-Induced Liver Injury: Self-Reports on Commercial Websites as Useful Adjunct Tools for Causality Assessment. Am. J. Gastroenterol. 2021, 116, 2151–2152. [Google Scholar]
- Ireland, P.J.; Hardy, T.; Burt, A.D.; Donnelly, M.C. Drug-induced hepatocellular injury due to herbal supplement ashwagandha. J. R. Coll. Physicians Edinb. 2021, 51, 363–365. [Google Scholar]
- Rattu, M.; Maddock, E.; Espinosa, J.; Lucerna, A.; Bhatnagar, N. An Herbal Liver Effect: Ashwagandha-Induced Hepatotoxicity; Poster, Rowan-Virtua Research Day; Department of Emergency Medicine: Glassboro, NJ, USA, 2022; Available online: https://rdw.rowan.edu/cgi/viewcontent.cgi?article=1194&context=stratford_research_day (accessed on 16 March 2025).
- Ali, S.M.J.; Suresh, M.G.; Sanchez-Cruz, J.; Anand, C. S2976 Cry Me a Liver: Ashwagandha-Induced Liver Toxicity. Am. J. Gastroenterol. 2022, 117, e1931. [Google Scholar]
- Tóth, M.; Benedek, A.E.; Longerich, T.; Seitz, H. Ashwagandha-induced acute liver injury: A case report. Clin. Case Rep. 2023, 11, e7078. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).