A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis–Has It Surpassed the Need for a Biopsy?
Abstract
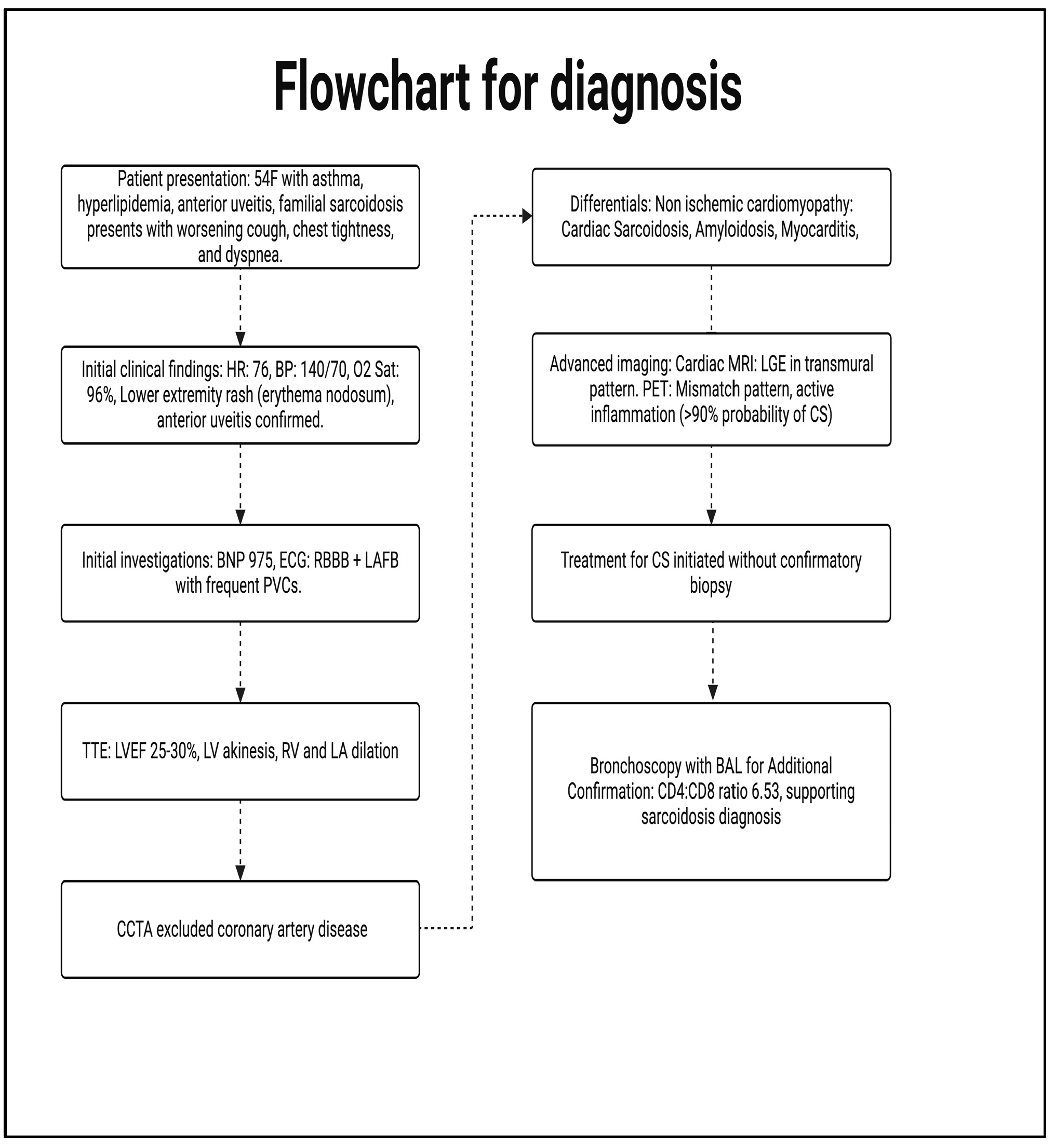
1. Introduction and Clinical Significance
2. Case Presentation
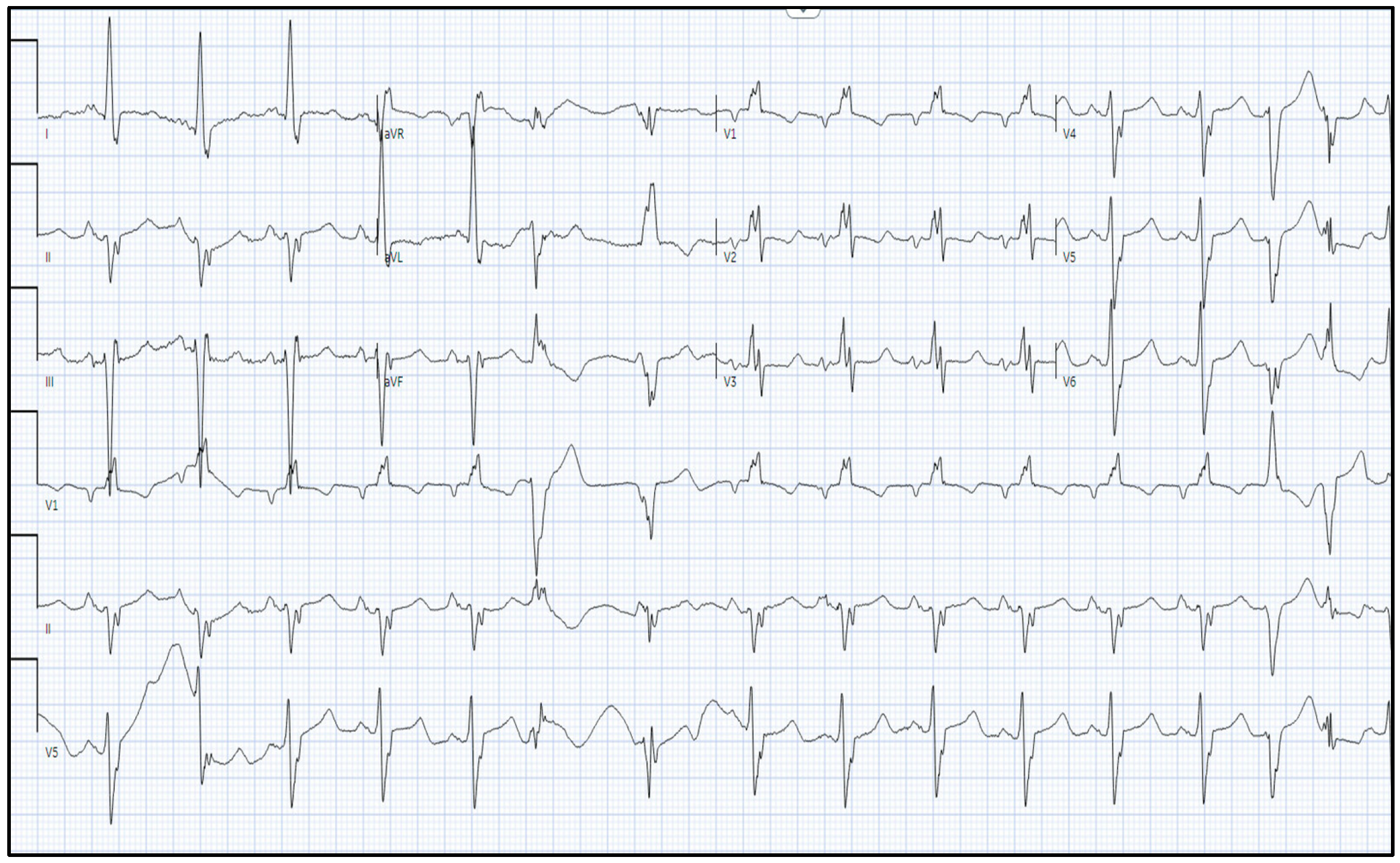
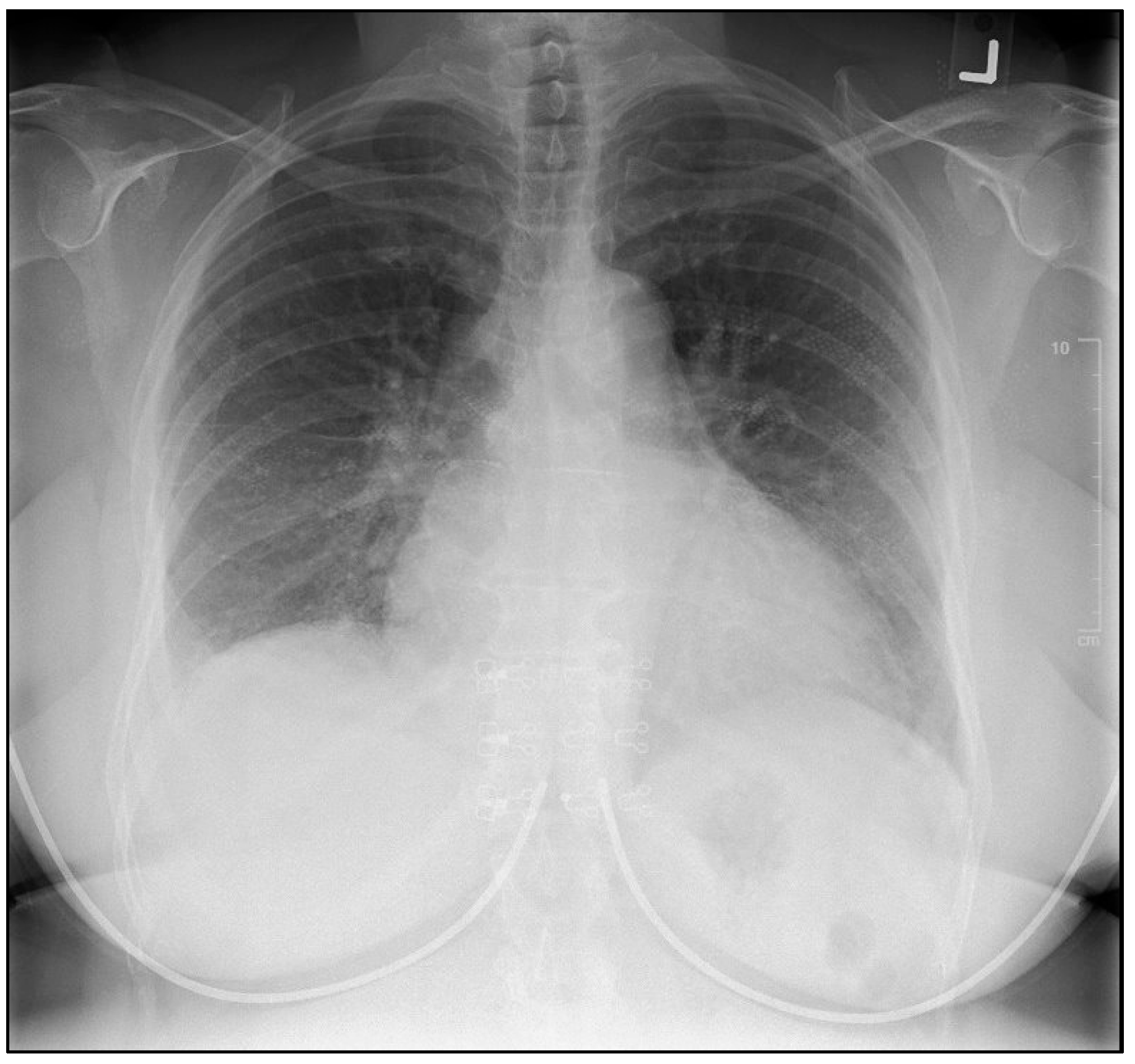
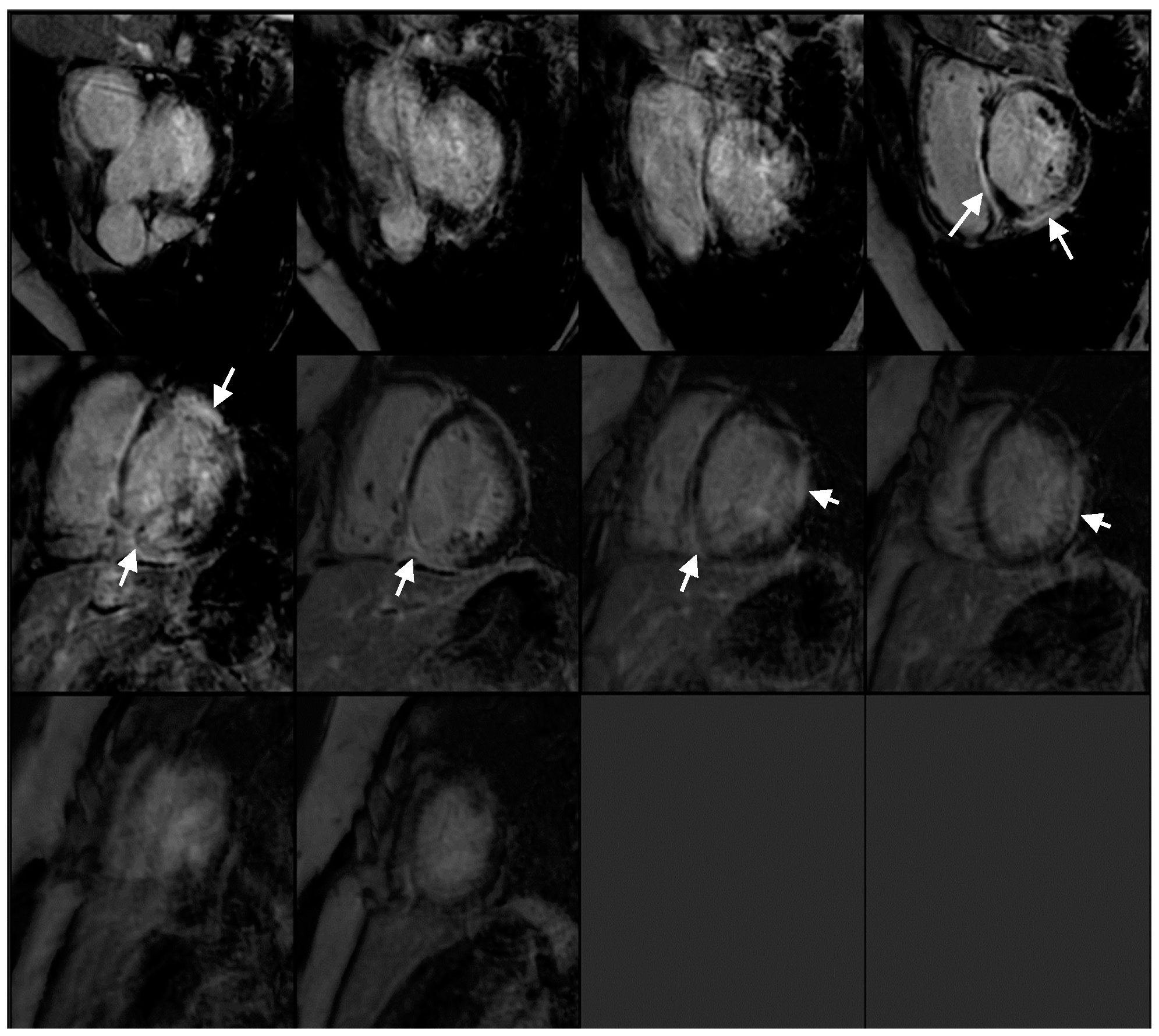
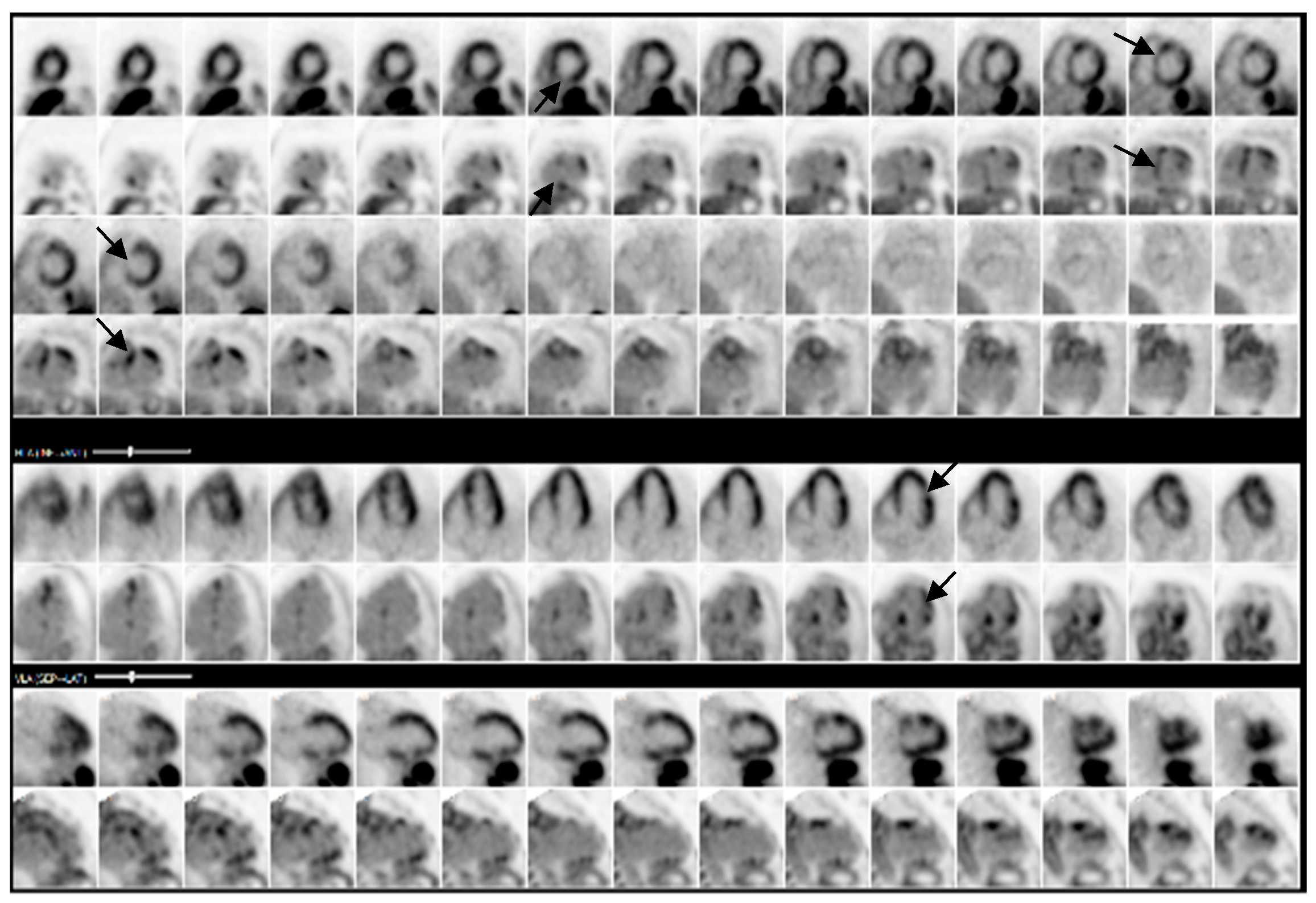
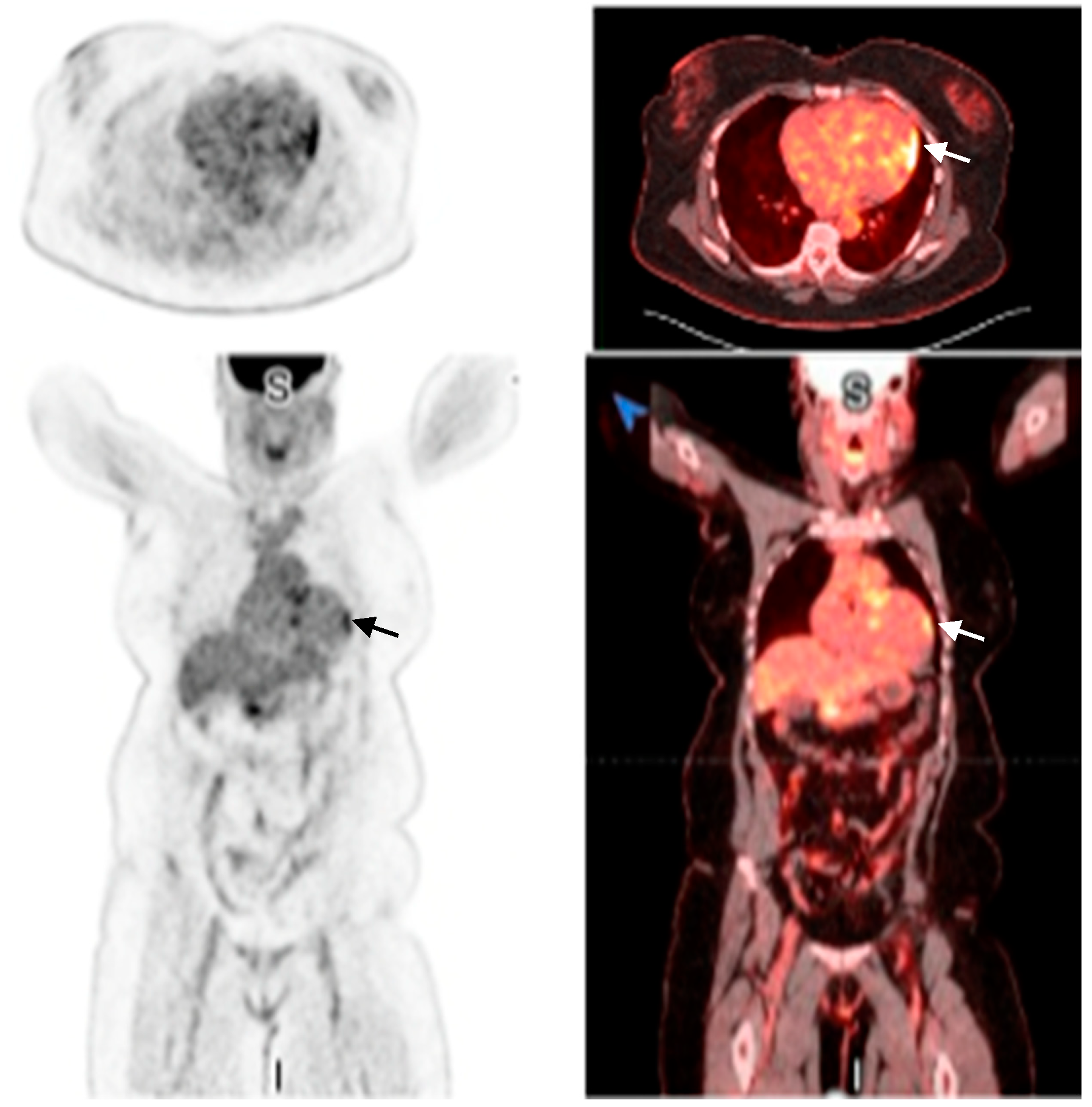
2.1. Investigations
2.2. Management
| Date | Event |
|---|---|
| 4 September 2024 | Patient presents to the emergency department
|
| 13 September 2024 | PET shows >90% probability of cardiac sarcoidosis |
| 14 September 2024 | ICD implantation
|
| 22 October 2024 | Bronchioalveolar lavage supports diagnosis of sarcoidosis |
| 25 November 2024 | Brief atrial fibrillation episode noted: initiated on apixaban |
| Present | Patient reports improvement in HF symptoms
|
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAL | Bronchoalveolar Lavage |
| BNP | B-type Natriuretic Peptide |
| CCTA | Coronary Computed Tomography Angiography |
| CS | Cardiac Sarcoidosis |
| ECG | Electrocardiogram |
| GDMT | Guideline-Directed Medical Therapy |
| HF | Heart Failure |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| ICD | Implantable Cardioverter-Defibrillator |
| JCS | Japanese Circulation Society |
| LAFB | Left Anterior Fascicular Block |
| LGE | Late Gadolinium Enhancement |
| LV | Left Ventricle |
| LVEF | Left Ventricular Ejection Fraction |
| MRI | Magnetic Resonance Imaging |
| MTX | Methotrexate |
| PJP | Pneumocystis Jirovecii Pneumonia |
| PVCs | Premature Ventricular Complexes |
| PET | Positron Emission Tomography |
| RBB | Right Bundle Branch |
| TTE | Transthoracic Echocardiography |
| WASOG | World Association of Sarcoidosis and Other Granulomatous Disorders |
References
- Birnie, D.H.; Kandolin, R.; Nery, P.B.; Kupari, M. Cardiac manifestations of sarcoidosis: Diagnosis and management. Eur. Heart J. 2017, 38, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, F.; Kusano, K.; Nakajima, T.; Yazaki, Y.; Morimoto, S.-I.; Culver, D.A.; Isobe, M. The characteristics of Japanese guidelines on diagnosis and treatment of cardiac sarcoidosis compared with the previous guidelines. Sarcoidosis Vasc. Diffuse Lung Dis. 2022, 39, e2022028. [Google Scholar] [CrossRef] [PubMed]
- Ueberham, L.; Hagendorff, A.; Klingel, K.; Paetsch, I.; Jahnke, C.; Kluge, T.; Ebbinghaus, H.; Hindricks, G.; Laufs, U.; Dinov, B. Pathophysiological Gaps, Diagnostic Challenges, and Uncertainties in Cardiac Sarcoidosis. J. Am. Heart Assoc. 2023, 12, e027971. [Google Scholar] [CrossRef] [PubMed]
- Munoz, C.; Schneider, A.; Botnar, R.M.; Prieto, C. Recent advances in PET-MRI for cardiac sarcoidosis. Front. Nucl. Med. 2022, 2, 1032444. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Shetty, M. Cardiac Sarcoidosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Santulli, G. Cardiac Sarcoidosis: Updated Insights on Epidemiology and Diagnostic Criteria. Am. J. Cardiol. 2023, 204, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Shaikh, F.; Abtin, F.; Fishbein, M.C.; Saggar, R.; Tsui, E.; Lynch, J.P., 3rd. Extrapulmonary sarcoidosis with a focus on cardiac, nervous system, and ocular involvement. EClinicalMedicine 2021, 37, 100966. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Manabe, O.; Oyama-Manabe, N.; Aikawa, T.; Tsuneta, S.; Tamaki, N. Advances in diagnostic imaging for cardiac sarcoidosis. J. Clin. Med. 2021, 10, 5808. [Google Scholar] [CrossRef] [PubMed]
- Torelli, V.A.; Sivalokanathan, S.; Silverman, A.; Zaidi, S.; Saeedullah, U.; Jafri, K.; Choi, J.; Katic, L.; Farhan, S.; Correa, A. Role of Multimodality Imaging in Cardiac Sarcoidosis: A Retrospective Single-Center Experience. J. Clin. Med. 2024, 13, 7335. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Ryu, J.H.; Matteson, E.L. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Malkonen, H.; Lehtonen, J.; Poyhonen, P.; Uusitalo, V.; Mayranpaa, M.I.; Kupari, M. Endomyocardial biopsy in the diagnosis of cardiac sarcoidosis. Eur. J. Heart Fail. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Mactaggart, S.; Ahmed, R. ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications. J. Respir. 2024, 4, 102–111. [Google Scholar] [CrossRef]
- Giblin, G.T.; Murphy, L.; Stewart, G.C.; Desai, A.S.; Di Carli, M.F.; Blankstein, R.; Givertz, M.M.; Tedrow, U.B.; Sauer, W.H.; Hunninghake, G.M.; et al. Cardiac Sarcoidosis: When and How to Treat Inflammation. Card. Fail. Rev. 2021, 7, e17. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.; Ippolito, P.; Kundur, S.P.; Sivalokanathan, S. A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis–Has It Surpassed the Need for a Biopsy? Reports 2025, 8, 28. https://doi.org/10.3390/reports8010028
Malik A, Ippolito P, Kundur SP, Sivalokanathan S. A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis–Has It Surpassed the Need for a Biopsy? Reports. 2025; 8(1):28. https://doi.org/10.3390/reports8010028
Chicago/Turabian StyleMalik, Ali, Paul Ippolito, Sukruth Pradeep Kundur, and Sanjay Sivalokanathan. 2025. "A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis–Has It Surpassed the Need for a Biopsy?" Reports 8, no. 1: 28. https://doi.org/10.3390/reports8010028
APA StyleMalik, A., Ippolito, P., Kundur, S. P., & Sivalokanathan, S. (2025). A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis–Has It Surpassed the Need for a Biopsy? Reports, 8(1), 28. https://doi.org/10.3390/reports8010028





