The Relationship between Proinflammatory Molecules and PD-L1 in Patients with Obesity Who Underwent Gastric Sleeve Surgery—A Pilot Study
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection and Follow-Up
2.2. Sample Collection, Processing, and Storage Molecular Analysis
2.3. Quantification of Circulating Proinflammatory Cytokines with Immunoenzymatic Testing (ELISA)
2.4. Statistical Analysis
3. Results
3.1. Clinical Parameters and Molecular Analysis
3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.; Lara, J.; Hill, J.O. ABC of obesity. Strategies for preventing obesity. BMJ 2006, 333, 959–962. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Zinman, B.; Brunzell, J.D.; Group, D.E.R. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 2013, 127, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Apolzan, J.W.; Venditti, E.M.; Edelstein, S.L.; Knowler, W.C.; Dabelea, D.; Boyko, E.J.; Pi-Sunyer, X.; Kalyani, R.R.; Franks, P.W.; Srikanthan, P.; et al. Long-Term Weight Loss with Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study. Ann. Intern. Med. 2019, 170, 682–690. [Google Scholar] [CrossRef]
- Tse, L.A.; Wang, C.; Rangarajan, S.; Liu, Z.; Teo, K.; Yusufali, A.; Avezum, A.; Wielgosz, A.; Rosengren, A.; Kruger, I.M.; et al. Timing and Length of Nocturnal Sleep and Daytime Napping and Associations with Obesity Types in High-, Middle-, and Low-Income Countries. JAMA Netw. Open 2021, 4, e2113775. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Cucoreanu, C.; Tigu, A.B.; Nistor, M.; Moldovan, R.C.; Pralea, I.E.; Iacobescu, M.; Iuga, C.A.; Szabo, R.; Dindelegan, G.C.; Ciuce, C. Epigenetic and Molecular Alterations in Obesity: Linking CRP and DNA Methylation to Systemic Inflammation. Curr. Issues Mol. Biol. 2024, 46, 7430–7446. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose Tissue-Liver Cross Talk in the Control of Whole-Body Metabolism: Implications in Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Almuraikhy, S.; Kafienah, W.; Bashah, M.; Diboun, I.; Jaganjac, M.; Al-Khelaifi, F.; Abdesselem, H.; Mazloum, N.A.; Alsayrafi, M.; Mohamed-Ali, V.; et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia 2016, 59, 2406–2416. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Astrom, G.; Sjolin, E.; Wahlen, K.; Carlberg, C.; Laurencikiene, J.; et al. Differential effects of 1alpha,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2012, 51, 335–342. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Madani, R.; Karastergiou, K.; Ogston, N.C.; Miheisi, N.; Bhome, R.; Haloob, N.; Tan, G.D.; Karpe, F.; Malone-Lee, J.; Hashemi, M.; et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1262–E1268. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Bader, J.E.; Wolf, M.M.; Lupica-Tondo, G.L.; Madden, M.Z.; Reinfeld, B.I.; Arner, E.N.; Hathaway, E.S.; Steiner, K.K.; Needle, G.A.; Hatem, Z.; et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 2024, 630, 968–975. [Google Scholar] [CrossRef]
- Tysoe, O. PDL1 reduces adipose inflammation in obesity. Nat. Rev. Endocrinol. 2022, 18, 334. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Sakaue, H. Adipocyte Death and Chronic Inflammation in Obesity. J. Med. Investig. 2017, 64, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Shimi, G.; Sohouli, M.H.; Ghorbani, A.; Shakery, A.; Zand, H. The interplay between obesity, immunosenescence, and insulin resistance. Immun. Ageing 2024, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/ Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Daigle, C.R.; Arterburn, D.E. Long term outcomes of metabolic/bariatric surgery in adults. BMJ 2023, 383, e071027. [Google Scholar] [CrossRef]
- Ding, L.; Fan, Y.; Li, H.; Zhang, Y.; Qi, D.; Tang, S.; Cui, J.; He, Q.; Zhuo, C.; Liu, M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes. Rev. 2020, 21, e13030. [Google Scholar] [CrossRef]
- Roomy, M.A.; Hussain, K.; Behbehani, H.M.; Abu-Farha, J.; Al-Harris, R.; Ambi, A.M.; Abdalla, M.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Therapeutic advances in obesity management: An overview of the therapeutic interventions. Front. Endocrinol. 2024, 15, 1364503. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Olaniyi, P.; Isarinade, T.; Yusuf, I.A. Recent advances in bariatric surgery: A narrative review of weight loss procedures. Ann. Med. Surg. 2023, 85, 6091–6104. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Newhall, K.J.; Diemer, G.S.; Leshinsky, N.; Kerkof, K.; Chute, H.T.; Russell, C.B.; Rees, W.; Welcher, A.A.; Patterson, S.D.; Means, G.D. Evidence for endotoxin contamination in plastic Na+-heparin blood collection tube lots. Clin. Chem. 2010, 56, 1483–1491. [Google Scholar] [CrossRef]
- Ruck, L.; Wiegand, S.; Kuhnen, P. Relevance and consequence of chronic inflammation for obesity development. Mol. Cell Pediatr. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Scheau, C.; Constantin, C.; Neagu, M. Recent Advances in Signaling Pathways Comprehension as Carcinogenesis Triggers in Basal Cell Carcinoma. J. Clin. Med. 2020, 9, 3010. [Google Scholar] [CrossRef]
- Bowers, L.W.; Doerstling, S.S.; Shamsunder, M.G.; Lineberger, C.G.; Rossi, E.L.; Montgomery, S.A.; Coleman, M.F.; Gong, W.; Parker, J.S.; Howell, A.; et al. Reversing the Genomic, Epigenetic, and Triple-Negative Breast Cancer-Enhancing Effects of Obesity. Cancer Prev. Res. 2022, 15, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Harman-Boehm, I.; Bluher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Kloting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Michailidou, Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr. Opin. Physiol. 2019, 12, 39–43. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Sinclair, P.; Docherty, N.; le Roux, C.W. Metabolic Effects of Bariatric Surgery. Clin. Chem. 2018, 64, 72–81. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentic, S.; Sestan, M.; Wensveen, T.T.; Polic, B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin. Immunol. 2015, 27, 322–333. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Bronneke, H.S.; et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R. Inflammatory markers and bariatric surgery: A meta-analysis. Inflamm. Res. 2012, 61, 789–807. [Google Scholar] [CrossRef]
- Askarpour, M.; Khani, D.; Sheikhi, A.; Ghaedi, E.; Alizadeh, S. Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2631–2647. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Du, J.X.; Zhao, J.; Shi, M.T.; Jin, M.W.; Liu, H. Adipocytes promote tumor progression and induce PD-L1 expression via TNF-alpha/IL-6 signaling. Cancer Cell Int. 2020, 20, 179. [Google Scholar] [CrossRef]
- Surmi, B.K.; Hasty, A.H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul. Pharmacol. 2010, 52, 27–36. [Google Scholar] [CrossRef]
- Mallipedhi, A.; Prior, S.L.; Barry, J.D.; Caplin, S.; Baxter, J.N.; Stephens, J.W. Changes in inflammatory markers after sleeve gastrectomy in patients with impaired glucose homeostasis and type 2 diabetes. Surg. Obes. Relat. Dis. 2014, 10, 1123–1128. [Google Scholar] [CrossRef]
- Suarez-Cuenca, J.A.; Ruiz-Hernandez, A.S.; Mendoza-Castaneda, A.A.; Dominguez-Perez, G.A.; Hernandez-Patricio, A.; Vera-Gomez, E.; De la Pena-Sosa, G.; Banderas-Lares, D.Z.; Montoya-Ramirez, J.; Blas-Azotla, R.; et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur. J. Clin. Investig. 2019, 49, e13085. [Google Scholar] [CrossRef] [PubMed]
- AziziKia, H.; Shojaei, S.; Mousavi, A.; Salabat, D.; Shaker, F.; Dolama, R.H.; Radkhah, H.; Alilou, S. Periprocedural Changes of Serum Biomarkers in Predicting Complications Following Bariatric Surgery for Obesity: Systematic Review and Meta-analysis. Obes. Surg. 2024, 34, 2198–2215. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells 2022, 11, 1883. [Google Scholar] [CrossRef]
- de Sousa, A.R.T.; Freitas Junior, W.R.; Perez, E.A.; Ilias, E.J.; Silva, A.S.; Alves, V.L.S.; Afonso, J.P.R.; Oliveira, M.C.; Fonseca, A.L.; da Silva, M.M.; et al. Surgery for Obesity and Weight-Related Diseases Changes the Inflammatory Profile in Women with Severe Obesity: A Randomized Controlled Clinical Trial. Obes. Surg. 2021, 31, 5224–5236. [Google Scholar] [CrossRef]
- Salminen, P.; Gronroos, S.; Helmio, M.; Hurme, S.; Juuti, A.; Juusela, R.; Peromaa-Haavisto, P.; Leivonen, M.; Nuutila, P.; Ovaska, J. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients with Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2022, 157, 656–666. [Google Scholar] [CrossRef]
- Cho, J.M.; Kim, H.J.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: Systemic review and meta-analysis. Surg. Obes. Relat. Dis. 2015, 11, 1273–1280. [Google Scholar] [CrossRef]
- McLean, C.; Mocanu, V.; Birch, D.W.; Karmali, S.; Switzer, N.J. Hypoalbuminemia Predicts Serious Complications Following Elective Bariatric Surgery. Obes. Surg. 2021, 31, 4519–4527. [Google Scholar] [CrossRef]
- Mosli, R.H.; Mosli, H.H. Obesity and morbid obesity associated with higher odds of hypoalbuminemia in adults without liver disease or renal failure. Diabetes Metab. Syndr. Obes. 2017, 10, 467–472. [Google Scholar] [CrossRef]
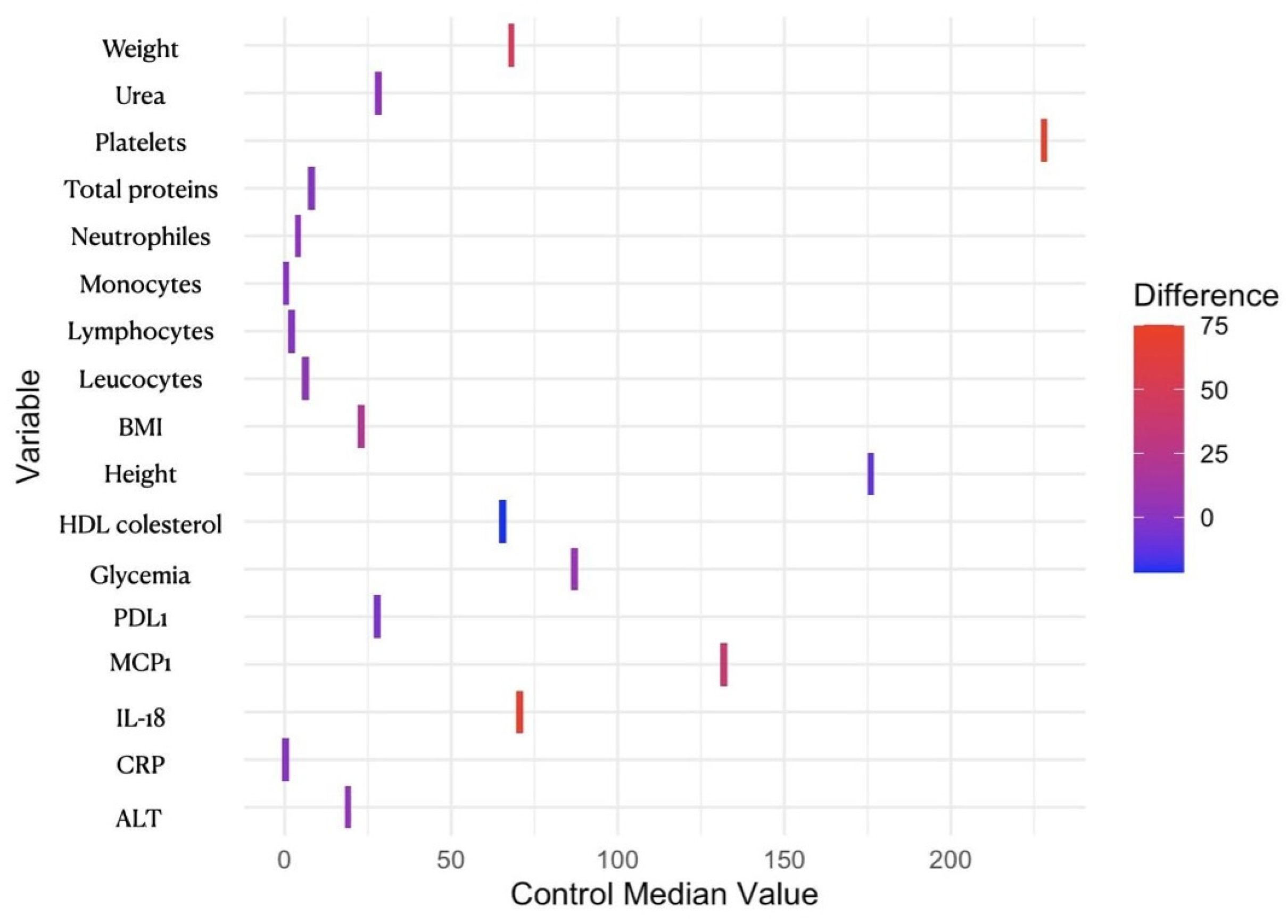
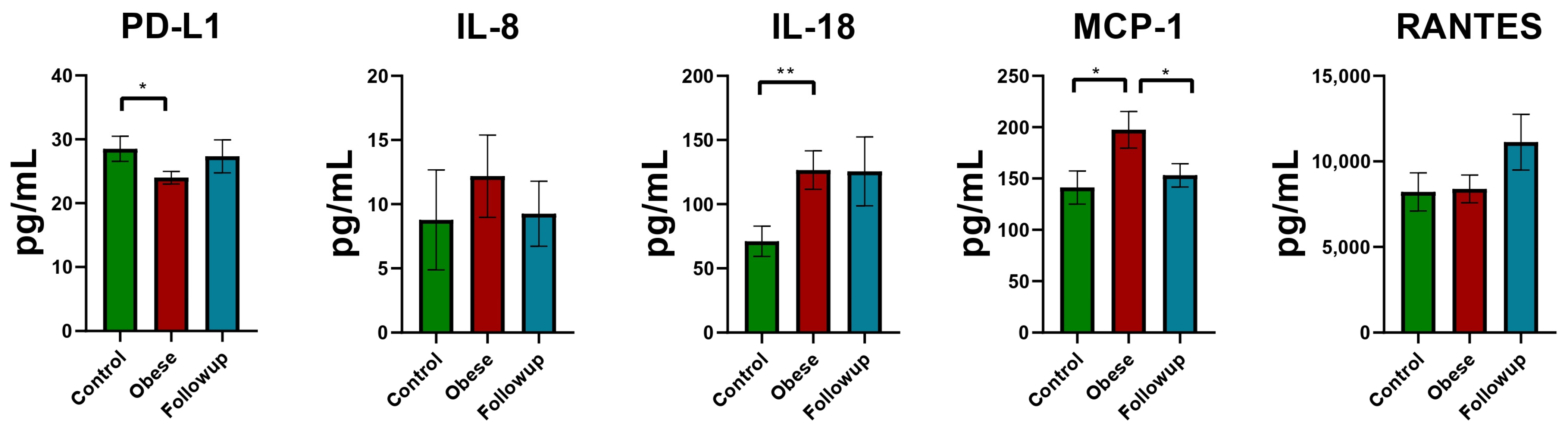
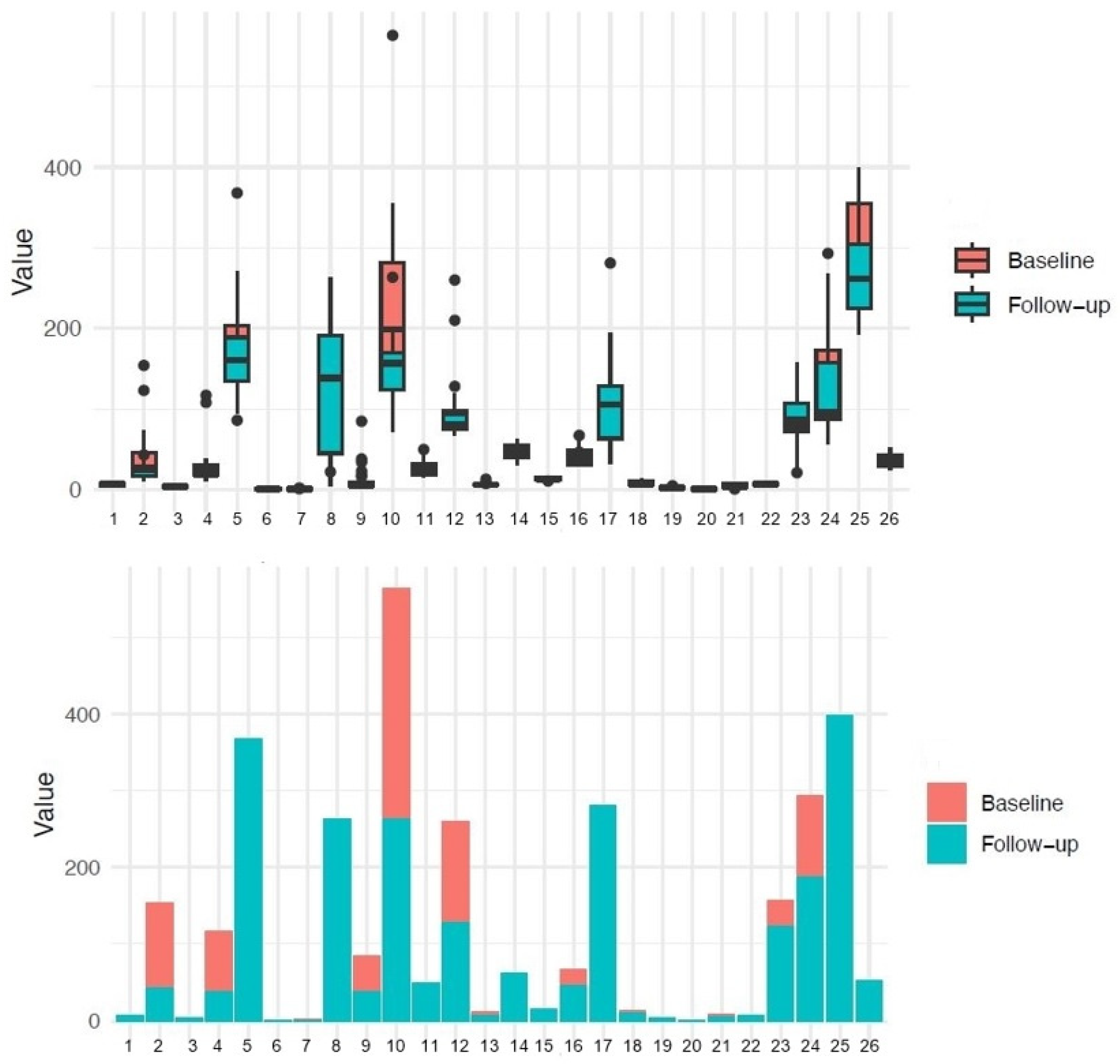
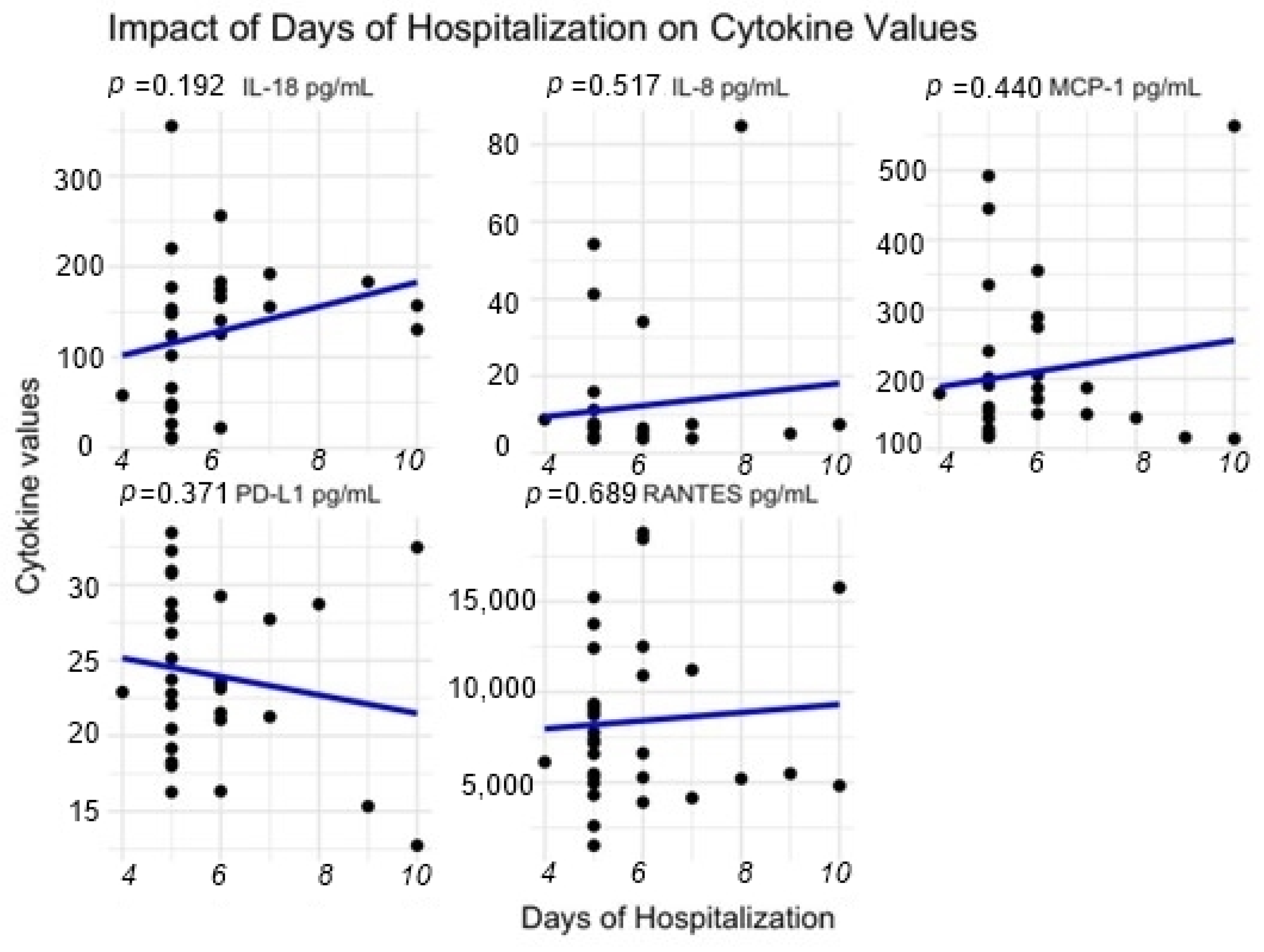
| Patient Characteristics by Cohort | |||
|---|---|---|---|
| Obese vs. Control Patients | |||
| Patient Characteristics | Obese, N = 31 1 | Control, N = 23 1 | p-Value |
| Age | 42 (30, 54) | 36 (28, 50) | 0.300 |
| Sex | 0.052 | ||
| Females | 24 (77%) | 12 (52%) | |
| Males | 7 (23%) | 11 (48%) | |
| Weight (kg) | 117 (106, 140) | 68 (62, 74) | <0.001 *** |
| Height (cm) | 164 (160, 172) | 176 (164, 180) | 0.011 * |
| BMI (kg/m2) | 45 (40, 47) | 23 (21, 24) | <0.001 *** |
| IL-8 (pg/mL) | 7 (4, 8) | 4 (4, 5) | 0.500 |
| IL-18 (pg/mL) | 140 (48, 174) | 70 (25, 102) | 0.006 ** |
| RANTES (pg/mL) | 7175 (5244, 11,070) | 7231 (5939, 9166) | >0.900 |
| PD-L1 (pg/mL) | 23 (21, 28) | 28 (22, 35) | 0.049 * |
| MCP-1 (pg/mL) | 171 (127, 222) | 132 (99, 191) | 0.035 * |
| Hemoglobin (g/dL) | 13.90 (13.30, 14.65) | 13.90 (12.65, 15.10) | 0.900 |
| Leukocytes (109/L) | 9.59 (8.23, 10.72) | 6.19 (5.44, 6.65) | <0.001 *** |
| Neutrophils (109/L) | 6.27 (5.15, 7.12) | 4.04 (3.54, 4.65) | <0.001 *** |
| Lymphocytes (109/L) | 2.52 (2.19, 3.05) | 1.99 (1.74, 2.29) | 0.001 *** |
| Monocytes (109/L) | 0.59 (0.44, 0.65) | 0.38 (0.33, 0.43) | <0.001 *** |
| Platelets (109/L) | 303 (254, 346) | 228 (193, 246) | <0.001 *** |
| CRP (mg/dL) | 0.73 (0.58, 0.95) | 0.26 (0.13, 0.38) | <0.001 *** |
| Albumin (g/dL) | 4.11 (3.90, 4.28) | 4.56 (4.38, 4.63) | 0.300 |
| Total protein (g/dL) | 6.94 (6.73, 7.41) | 7.94 (7.92, 7.97) | 0.022 * |
| Glucose (mg/dL) | 94 (82, 104) | 87 (78, 96) | 0.022 * |
| ALT (U/L) | 23 (18, 36) | 19 (15, 30) | 0.031 * |
| AST (U/L) | 22 (18, 28) | 21 (16, 25) | 0.200 |
| Urea (mg/dL) | 31 (26, 39) | 28 (25, 33) | 0.046 * |
| Creatinine (mg/dL) | 0.69 (0.61, 0.83) | 0.81 (0.76, 0.95) | 0.063 |
| Uric acid (mg/dL) | 5.93 (5.05, 6.47) | 3.75 (3.75, 3.75) | 0.150 |
| HDL cholesterol (mg/dL) | 44 (40, 52) | 66 (57, 72) | <0.001 *** |
| LDL cholesterol (mg/dL) | 105 (78, 122) | 112 (94, 123) | 0.300 |
| Total cholesterol (mg/dL) | 183 (141, 205) | 180 (164, 210) | 0.300 |
| Triglycerides (mg/dL) | 131 (103, 179) | 86 (62, 120) | 0.068 |
| Iron (ug/dL) | 79 (61, 100) | 77 (62, 86) | >0.900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucoreanu, C.; Muresan, X.M.; Tigu, A.-B.; Nistor, M.; Moldovan, R.-C.; Pralea, I.-E.; Iacobescu, M.; Iuga, C.-A.; Constantinescu, C.; Dindelegan, G.-C.; et al. The Relationship between Proinflammatory Molecules and PD-L1 in Patients with Obesity Who Underwent Gastric Sleeve Surgery—A Pilot Study. Reports 2024, 7, 74. https://doi.org/10.3390/reports7030074
Cucoreanu C, Muresan XM, Tigu A-B, Nistor M, Moldovan R-C, Pralea I-E, Iacobescu M, Iuga C-A, Constantinescu C, Dindelegan G-C, et al. The Relationship between Proinflammatory Molecules and PD-L1 in Patients with Obesity Who Underwent Gastric Sleeve Surgery—A Pilot Study. Reports. 2024; 7(3):74. https://doi.org/10.3390/reports7030074
Chicago/Turabian StyleCucoreanu, Ciprian, Ximena Maria Muresan, Adrian-Bogdan Tigu, Madalina Nistor, Radu-Cristian Moldovan, Ioana-Ecaterina Pralea, Maria Iacobescu, Cristina-Adela Iuga, Catalin Constantinescu, George-Calin Dindelegan, and et al. 2024. "The Relationship between Proinflammatory Molecules and PD-L1 in Patients with Obesity Who Underwent Gastric Sleeve Surgery—A Pilot Study" Reports 7, no. 3: 74. https://doi.org/10.3390/reports7030074
APA StyleCucoreanu, C., Muresan, X. M., Tigu, A.-B., Nistor, M., Moldovan, R.-C., Pralea, I.-E., Iacobescu, M., Iuga, C.-A., Constantinescu, C., Dindelegan, G.-C., & Ciuce, C. (2024). The Relationship between Proinflammatory Molecules and PD-L1 in Patients with Obesity Who Underwent Gastric Sleeve Surgery—A Pilot Study. Reports, 7(3), 74. https://doi.org/10.3390/reports7030074










